Adlard E.R. (ed.) Chromatography in the Petroleum Industry
Подождите немного. Документ загружается.

22
Chapter
I
0
min
4
min
8
hin
Fig.
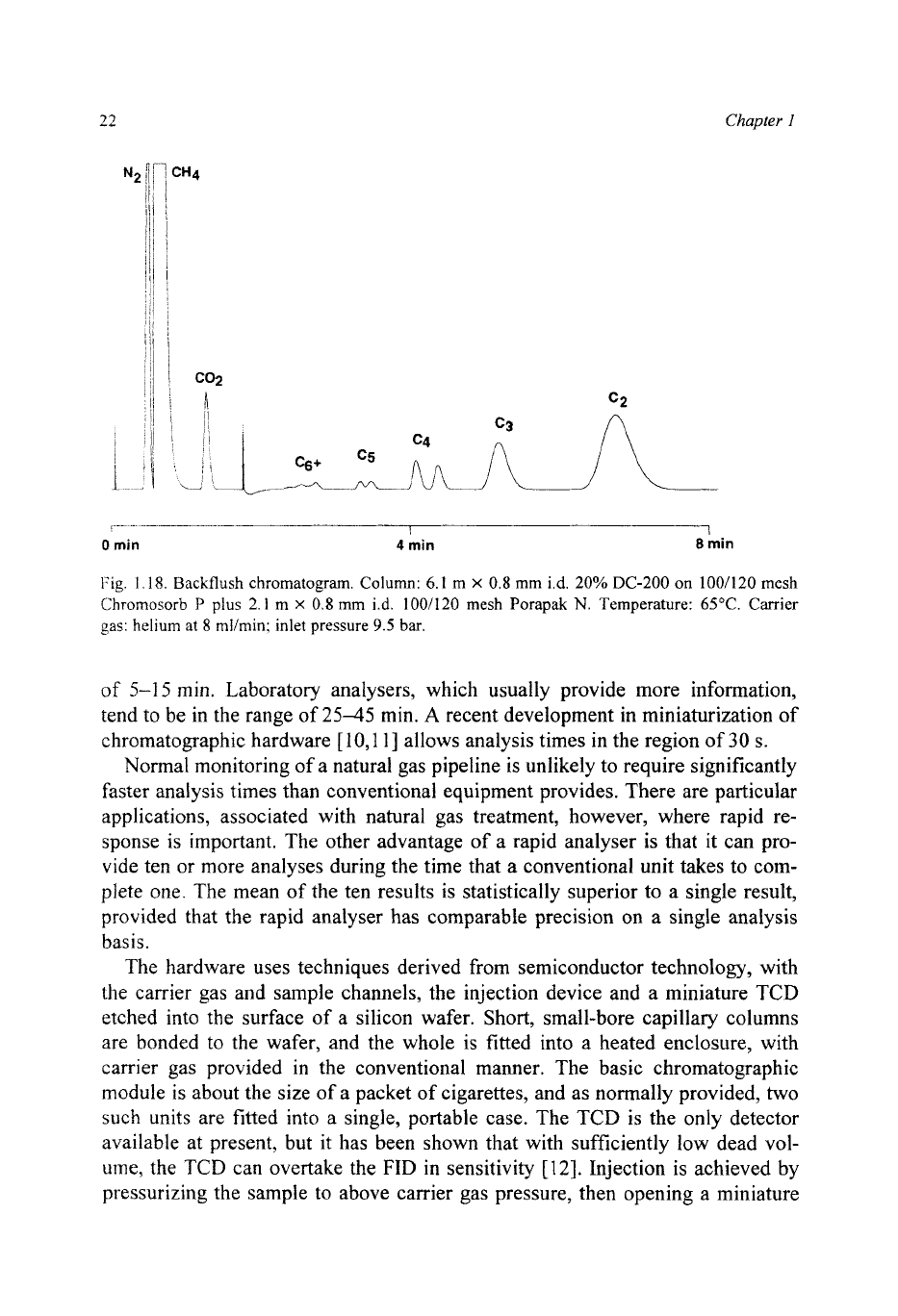
1.18. Backflush chromatogram. Column:
6.
I
m
X
0.8
mm
id.
20%
DC-200 on
100/120
mesh
Chromosorb
P
plus
2.1 m
x
0.8
mm
i.d.
1001120 mesh Porapak
N.
Temperature:
65°C.
Carrier
gas:
helium
at
8 mlimin: inlet pressure
9.5
bar.
of
5-1
5
rnin. Laboratory analysers, which usually provide more information,
tend to be in the range
of
25-45
min. A recent development in miniaturization of
chromatographic hardware
[
10,11]
allows analysis times in the region of
30
s.
Normal monitoring of a natural gas pipeline is unlikely to require significantly
faster analysis times than conventional equipment provides. There are particular
applications, associated with natural gas treatment, however, where rapid re-
sponse
is
important. The other advantage of a rapid analyser
is
that it can pro-
vide ten
or
more analyses during the time that
a
conventional unit takes to com-
plete one. The mean
of
the ten results is statistically superior to a single result,
provided that the rapid analyser has comparable precision on a single analysis
basis.
The hardware uses techniques derived from semiconductor technology, with
the carrier gas and sample channels, the injection device and a miniature TCD
etched into the surface of a silicon wafer. Short, small-bore capillary columns
are
bonded to the wafer, and the
whole
is fitted into
a
heated enclosure, with
carrier gas provided
in
the conventional manner. The basic chromatographic
module
is
about the size
of
a
packet
of
cigarettes, and as normally provided,
two
such units are fitted into a single, portable case. The TCD is the only detector
available at present, but it has been shown that with sufficiently low dead vol-
ume,
the TCD can overtake the FID in sensitivity
[12].
Injection
is
achieved by
pressurizing the sample to above carrier gas pressure, then opening a miniature
The
anafysis
of
hydrocarbon gases
23
diaphragm valve between the two for a defined time; sample size is adjusted by
selecting a different opening time.
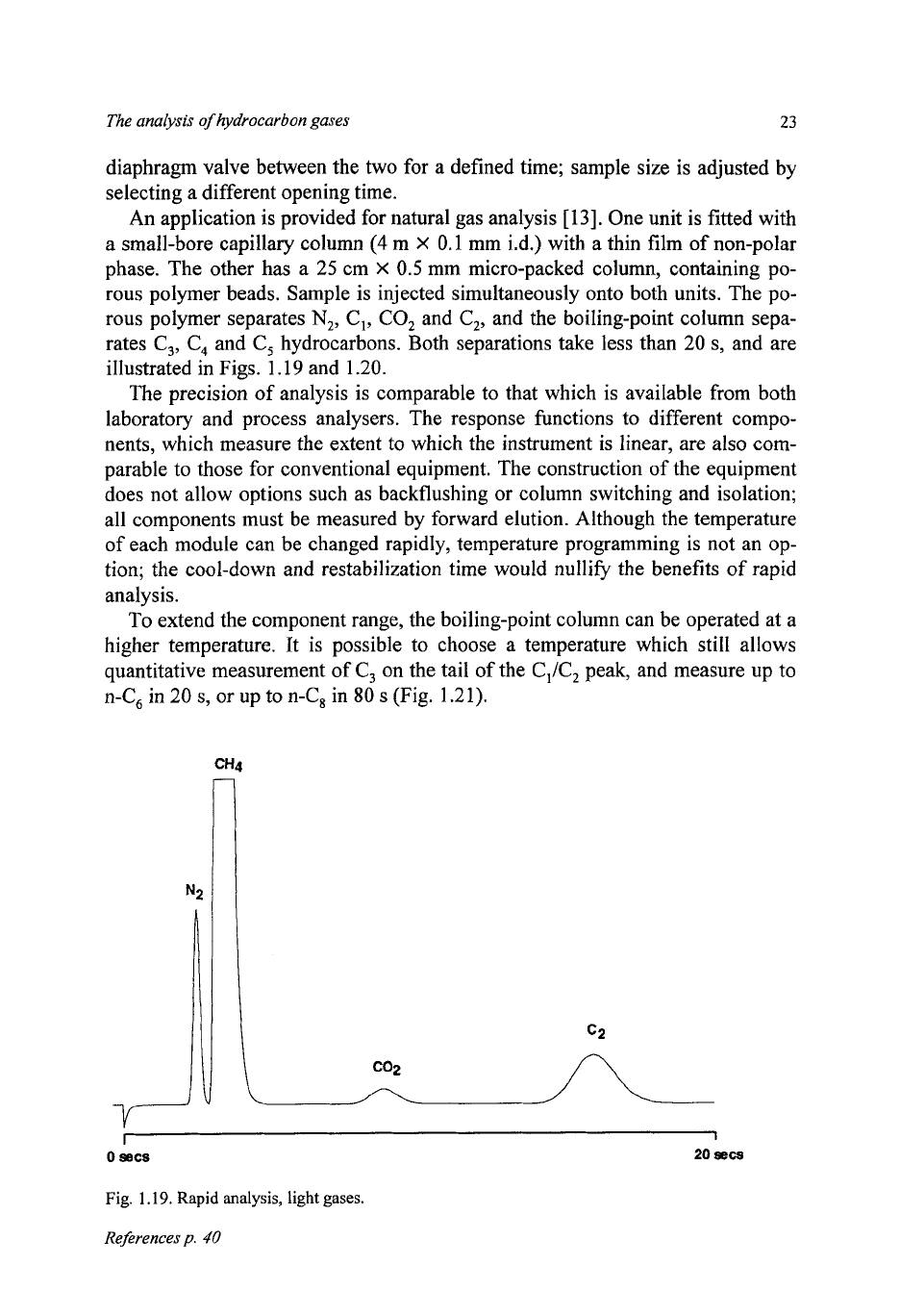
An application
is
provided for natural gas analysis
[
131.
One unit is fitted with
a small-bore capillary column
(4
m
X
0.1
mm i.d.) with
a
thin film of non-polar
phase. The other has a
25
cm
X
0.5
mm micro-packed column, containing po-
rous polymer beads. Sample is injected simultaneously onto both units. The po-
rous polymer separates
N,,
C,, CO, and C,, and the boiling-point column sepa-
rates C,,
C,
and C, hydrocarbons. Both separations take less than
20
s,
and are
illustrated in Figs.
1.19
and
1.20.
The precision
of
analysis is comparable to that which is available from both
laboratory and process analysers. The response functions to different compo-
nents, which measure the extent to which the instrument is linear, are also com-
parable to those for conventional equipment. The construction
of
the equipment
does not allow options such as backflushing or column switching and isolation;
all components must be measured by forward elution. Although the temperature
of each module can be changed rapidly, temperature programming is not an op-
tion; the cool-down and restabilization time would nullify the benefits of rapid
analysis
.
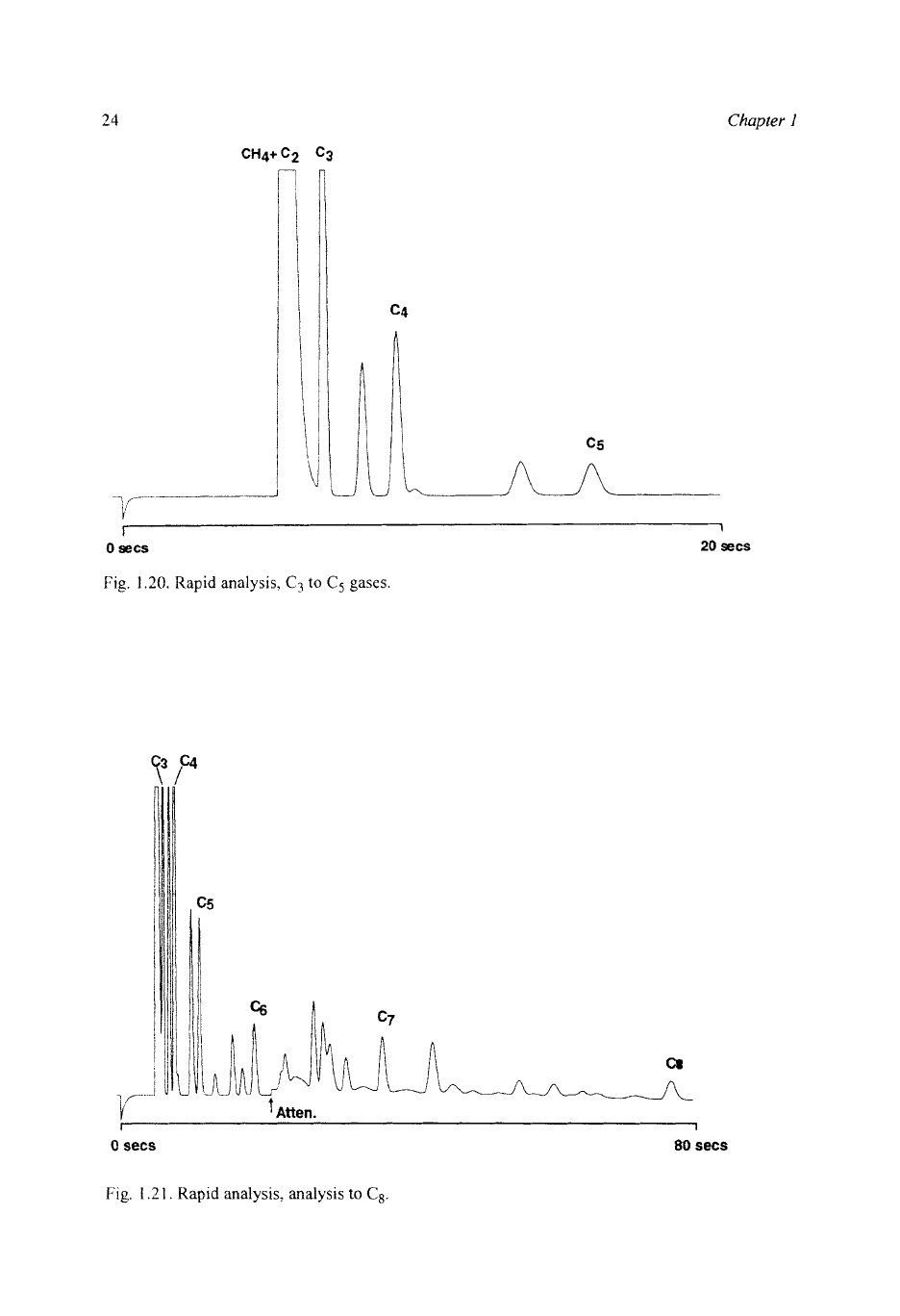
To extend the component range, the boiling-point column can be operated at a
higher temperature. It is possible to choose a temperature which still allows
quantitative measurement of
C,
on the tail of the C,/C, peak, and measure up to
n-C, in
20
s,
or
up to n-C, in
80
s
(Fig.
1.21).
I
0
s0cs
Fig.
1.19.
Rapid analysis, light gases.
References
p.
40
24
Chapter
I
r
I
1
0
Secs
20
secs
Fig.
1.20.
Rapid analysis,
C3
to
C5
gases
0
sees
Fig.
1.2
1.
Rapid
analysis,
analysis
to
C8.
80
secs

The analysis ofhydrocarbon gases
25
1.2.3
Quantitative measurement
Natural gas analysis has probably received more attention than any other type
of sample in respect of quantitative measurement. This is mainly in respect of
CV determination, because of the very large financial implications
of
the huge
volumes of natural gas which are traded. Other properties, important for engi-
neering or safety reasons, can be derived from the same set of composition data.
To be able to quote the value of a property with a defined level of uncertainty
requires that the entire procedure be considered, from sampling through calibra-
tion and analysis to calculation of composition, and thence to calculation
of
properties, using the correct procedure and accurate base data on pure com-
pounds. Kenter
et
al.
[
141
have described the uncertainty calculations for natural
gas CV measurement.
Considering the analytical procedure alone, the following criteria must be
met.
1.
It must be comprehensive, with no significant components or groups ig-
nored. Detailed judgement as to what is significant depends upon the appli-
cation.
2.
Unless only intended for on-line use, it should recognize air contamination
of the sample.
3.
All significant components or groups should be resolved
so
that there is no
mutual interference.
4.
It must be precise.
5.
The response for each component must be a consistent and predictable
The requirement for accuracy is not included here,
as
the accuracy of chroma-
tographic analysis is principally determined by the accuracy of the calibration
standards used. The analytical method can, if the above criteria are satisfied,
transfer that accuracy to the sample.
IS0
10723
[15],
currently at committee
draft stage, describes tests
of
analyser performance broadly covering the above
headings.
function of concentration.
1.2.3.
I
Comprehensive
analysis
The analytical requirement, and further details such as the desired frequency
of analysis and the availability of equipment will allow the user to choose from
the options described above.
1.2.3.2 Air contamination
Air can contaminate a sample when it is taken into a container or when it is
subsequently transferred to the analyser, or both. If the analysis requires differ-
ent injections of sample for the measurement of different groups of components,
air contamination may be present in some injections and not in others, and may
References
p.
40

26
Chapter
1
or may not be recognized. Wherever possible, samples subject to air contamina-
tion should be analysed using a method in which both air and normal natural
gas
components are measured from the same injection.
1.2.3.3
Resolution
The ideal of baseline resolution is not possible for
all
components in a com-
plex mixture such as natural gas. Components to
C,
can be effectively fully re-
solved with packed columns, and most
C,
and
C,
isomers separated on a capil-
lary. Again the degree of resolution required for minor components depends on
the application.
I.
2.3.4
Precision
‘The approach to measuring the purity of a nominally pure substance
is
to
measure the impurities, and calculate the concentration of the substance by dif-
ference. Since methane is the dominant component in natural gas, one approach
has been to measure all other components and to calculate the methane by differ-
ence. Taking this approach, however, means that the calculated methane value is
also the sink for the errors involved in the determination of
all
the other compo-
nents. It
is
therefore preferable to measure all components, including methane.
Errors
in
chromatographic measurement can be regarded as being of
two
types;
those which affect each component differently, and those which influence
all components, or a substantial group of components to the same extent. An ex-
ample of the former is the effect of random electrical baseline noise on the deci-
sion as to where peaks begin and end. An example of the latter is variation in
sample size or a change
in
detector sensitivity. Sample size variation is quite
common in gas analysis, where the injection device uses a defined volume, but
the molar injected quantity depends upon the pressure and temperature of the
sample at the time
of
injection.
If all components are measured from a single injection, then sample size
variation has the same proportional influence on all of them. If they are meas-
ured with a single detector, then the results, which should add up to
loo%,
can
be normalized to that value provided that the necessary correction is quite small.
It
is
quite common to normalize data which add to between, for example,
98
and
102%, but to investigate larger variations. Different detectors, even if used in
series
on
the same column system, can and do show changes in sensitivity of
different magnitudes and sometimes in different directions. Consider the popular
combination of TCD and FID; the FID sensitivity depends, among other factors,
upon the carrier gas
to
hydrogen ratio, whereas the TCD is quite indifferent to
the FID hydrogen flow. It is common
to
find that normalization of data from the
two detectors gives results which are more precise for those components meas-
ured by TCD, but less precise for the FID. The TCD, even if
only
being used to
measure
Nz,
CO,,
C,
and
Cz,
accounts for those components which comprise the

The
analysis
of
hydrocarbon
gases
21
great majority of the molar quantity, and hence are most influential
on
the nor-
malization correction. The FID may be measuring many more components, but
their total is small and so, therefore, is their influence
on normalization.
For this reason, where
two
detectors are used, a bridge component should be
selected which gives a good and interference-free signal with each detector
[5,16].
The ratio of detector responses for this component is then measured at
each analysis and compared with the ratio that was found at the time of calibra-
tion. Small deviations in relative detector response can then be allowed for by
adjusting the data from the second detector.
This approach can also be used if the analysis
is
split up into different parts,
some components being measured on one analyser and some
on
another. The
bridge component, measured on each,
is
used to allow for changes in the re-
sponse of the different instruments.
1.2.3.5
Response
function
Because most chromatographs give a larger signal for what is reckoned to be a
larger concentration of component, it is convenient and comforting to assume
that the response to that component is linear, i.e. that it is represented by a
straight line through the origin when plotting instrument response against com-
ponent concentration. This assumption means that the response factor is inde-
pendent of concentration, and justifies calibration with one mixture, at a single
point. Unfortunately, the assumption is not necessarily true, particularly for
methane, the inevitable major component.
Chromatography consists of a series of compromises, including the choice
of
sample size in order to allow detection of minor components and simultaneous
quantitative measurement of major components. By using a series of test gases,
of carefully selected and accurately defined compositions, response functions
can be evaluated and curves plotted. It is common to find that methane shows
significant deviations from linear behaviour, and other components, such as ni-
trogen and ethane, show lesser but still noticeable deviations. These deviations
will give rise to bias errors if they are not allowed for, with the size of the
errors
depending upon the size of the deviations and upon the concentration differences
between calibration standard and sample.
Having quantified the component response function, typically as a polynomial
expression, these coefficients can then be used in place of the assumption of lin-
ear response for subsequent quantitative measurements of samples. There re-
mains the uncertainty of the long-term stability of these functions, and hence the
frequency with which they should be re-checked. If possible, a preferable course
would be to adjust the analytical conditions in order to ensure that linear re-
sponses are available for all components.
Deviations from linear response are of more concern for the TCD than for the
FID. This is not to suggest that the FID is free from such problems, but that the
References
p.
40

28
Chapter
1
TCD is more likely to be used for measurement of major components. The TCD
is an admirably simple device, and deviations from linearity should only occur
when the concentration of component in carrier gas within the detector exceeds
a
certain value. Above this concentration, the incremental signal increase for an
increment of extra concentration diminishes, and the response, which had been
following a straight line, starts to curve.
This overload effect
is
only dependent upon the instantaneous concentration
of component in the detector.
For
the same amount of component,
a
peak that
is
narrow and tall may give a non-linear response, whereas one that is broad and
shallow will
not.
As
has been stated, methane is both the most likely candidate
and the most frequently observed component to give non-linear response. At the
same time, the different tactics used in isothermal analysis have a greater effect
upon the peak shape for methane than for other components, and hence will be
likely to influence the nature
of
its response. When eluted directly to the detec-
tor, as
in
Figs.
1.6
and
1.8,
the peak
is
narrow and tall, promoting non-linear be-
haviour. Intermediate storage in and elution from a porous polymer column, as
in
Fig.
1.10,
produces a wider and less tall peak. Figure
1.12
shows the even wider
methane peak which results from storage
on
a
molecular sieve, which should be
the most favourable in terms of linearity of response.
It
is
therefore evident that the benefit
of
the more complex configuration when
moving from Fig.
1.7
to Fig.
1.9
to Fig.
1.11
is
not only extra detail and better
separation, but also improved linearity of response. The penalty
is
the extra time
required for the analysis.
1.3
REFINERY
GAS
The refining of crude oil starts with distillation, converting the crude into
a
series of fractions which will themselves form products or feedstocks. The light-
est fraction consists of propane and butanes, with small amounts of ethane and
pentanes. Subject to further separation, this forms product streams of propane
(typically
95%
pure) and butane (typically
40%
isobutane and
60%
n-butane),
generally referred to as liquefied petroleum gas
or
LPG.
The ethane content is
too
low to form a useful feedstock, and is used as fuel gas around the plant.
The light gases can also be fed to the catalytic cracker, where
the
simple
mixture of saturated hydrocarbons is converted to a more complex mixture
which also contains unsaturates and inorganic gases. The more reactive unsatu-
rated components are the key to further processes, such as polymerization and
the production of oxygenates for gasoline.
Refinery gas is the name given to this catalytic cracker product, and it con-
tains hydrogen, oxygen, nitrogen, carbon monoxide, carbon dioxide, hydrogen
sulphide, and saturated and unsaturated hydrocarbons in the range
C,
to
C,.
It

The analysis
of
hydrocarbon gases
29
may contain small amounts of higher hydrocarbons, such as
C,,
and may also
pick up, during the course of the processes for which it
is
used, traces of aromat-
ics or other higher hydrocarbons. Among the hydrocarbons, saturates and mono-
unsaturates (alkanes and alkenes) form the main components, with dienes and
acetylenic compounds (alkynes) normally present only as traces. As the gas is
subjected to different fractionations and reactions,
so
the relative proportions of
components obviously change.
Those hydrocarbon components which are not used in processing, or as fuel
gas, may be added to propane or butane product streams. These products may,
therefore, be totally saturated hydrocarbons, containing relatively small amounts
of each other, or mainly saturated, but with significant concentrations of unsatu-
rates.
1.3.1
Analytical
requirements
Most gas streams measured only exist within the refinery boundaries, being
made, fractionated, reacted and combusted according to the process requirements
at any particular time. Analysis of these streams is aimed at optimizing the proc-
ess for which they are used, and it can be argued that consistency of measure-
ment between inlet and outlet streams is more important than absolute accuracy
of composition. (The alternative argument, that medium or long term consistency
can only be guaranteed by ensuring accuracy, is probably a better one.)
Product streams, such as propane, propene and butane, which are to be sold to
customers, require analysis for quality measurement, usually against a specifica-
tion. The specification, as for natural gas, will detail certain properties, but will
also require that there are not more than defined amounts of specific impurities.
Properties specified will be quite different from those for natural gas. This is
largely because the composition of the refinery product is dictated by the proc-
esses through which it is generated, which are controlled, whereas the composi-
tion of natural gas is subject to as little alteration from that found at the produc-
tion separator as the supplier can achieve. Although propane, for example, is
sold very widely for heating, the combustion characteristics of the product vary
very little between batches, whereas natural gas can show large differences. On
the other hand,
LPG
is
sold and handled as a liquid, and
so
its vapour pressure is
an important property.
If an analytical method can be found that is capable of measuring the complex
mixture which
is
refinery gas, it should also be usable for simpler mixtures, such
as product streams. Although the mixture does not have as wide a boiling range
as natural gas, it contains extra components (hydrogen, carbon monoxide, un-
saturated hydrocarbons) which add their own complexity. It is, therefore, as with
natural gas, likely to need a complex analytical system, involving multiple col-
umns, or temperature programming, or both.
References
p.
40
3
0
Chapter
I
1.3.2
Analytical
procedures
Separation
of
the inorganic gases, and
of
methane and the
C,
hydrocarbons
can be achieved by a molecular sieve/porous polymer combination such as is
shown
in
Fig.
1.16.
Carbon monoxide elutes after methane on an activated mo-
lecular sieve, and the C, unsaturates can be separated from
CO,
and from each
other on an appropriate porous polymer. This
is
a well-established column com-
bination, and there is really no practicable alternative for the inorganic gases.
The temperature programmed porous polymer column shown in Fig.
1.15
would
be incapable
of
sensibly distinguishing
0,,
N,
and CO. Because
of
the presence
of
CO,
it
is
unlikely that the configuration would be exactly the same,
or
the
or-
der
of
elution similar to that
in
Fig. 1.17; this is discussed further below.
'The hydrocarbons in
Fig.
1.17
are separated according to boiling point, using
a capillary column. The boiling points of refinery gas hydrocarbons are given in
Table
1.1.
In the table, component names are given in both IUPAC and the more
commonly used trivial
form.
The symbols are used to annotate the chroma-
tograms.
As
Table
1.1
shows, there are significant boiling point differences, except for
the
C,
hydrocarbons
(l-C4-,
b.p. 443°C and i-C4-, b.p.
-7.08"C).
It is possible
to
separate all components using a boiling point capillary column with
a
sub-
ambient temperature programme, but the method
is
not advisable where compo-
nent concentrations vary widely.
TABLE
1.1
REFINERY
GAS
NAMES,
SYMBOLS
AND
BOILING-POINTS
Component Symbol Boiling point
("C)
Methane
Ethane
Ethene (ethylene)
Ethyne (acetylene)
Propane
Propene (propylene)
Propyne (Me-acetylene)
Propadiene
2-Me-propane (I-butane)
Butane (n-butane)
1
-Butene
cis-2-Rutene
rmns-2-Butene
2-Me-propene (1-butene)
1.3-Butadiene
2-Me-butane (1-pentane)
Pentane (n-pentane)
c1
c2
c,-
c,=
c3
c3-
c3=
c3-
i-C,
n-C4
1
-c4-
c2-c4-
t2-C4-
i-C,-
i-C5
n-CS
1,3-C,--
-161.78
-88.78
-103.88
-84.93
-42.24
-47.88
-23.62
-35.18
-12.1
1
4.73
-6.43
3.45
0.70
-7.08
-4.80
27.59
35.83

The analysis of hydrocarbon gases
31
There have been many references to columns and column combinations for
separation of all C, saturates and unsaturates
[17-191.
One of the earliest [20]
referred to the use of activated alumina, the surface of which was strongly ad-
sorptive to unsaturated hydrocarbons. When fully activated, retention times for
unsaturated hydrocarbons were excessive, and their peak shapes were poor,
so
a
degree of deactivation was recommended, using water or a combination of water
and silicone oil. This gave good separation, but was only consistent over a few
hours, as the water was stripped off by the dry carrier gas and the relative reten-
tions
of
saturated and unsaturated hydrocarbons changed significantly. It was
usually necessary to precede a day’s work with the injection of a relatively large
amount of liquid water, which would distribute itself over the alumina and allow
several hours of reasonably consistent separations.
Capillary columns packed with alumina were demonstrated [21] shortly after
the first reference, and later applied to refinery gas analysis [22]. In this applica-
tion, a humidification device was used for the carrier gas. This consisted of a
length of tubing packed with copper sulphate crystals, which, at ambient tem-
perature, assured a constant water content in the carrier gas, and hence a constant
polarity. In fact, with this device, there is the mechanism for fine-tuning the col-
umn behaviour. The vapour pressure of water above copper sulphate crystals is
constant at a constant temperature, and
so
the partial pressure of water in carrier
gas is controlled by adjustment of the total pressure in the humidifier. With the
arrival of porous layer open tubular (PLOT) capillary columns, alumina was
used in this form. The separations which had been incomplete with packed col-
umns were now baseline to baseline.
These columns were rapidly seen to give superior performance to any of the
alternatives, and became the state-of-the-art solution to the problem. The need to
maintain a constant level of moisture in the carrier gas was inconvenient, and
so
alternative methods for deactivation were studied. The use of inorganic salts
proved to be effective, and potassium chloride treatment was the first such to be
commercially successful. KCI deactivated alumina, used with dry carrier gas,
gives excellent separation, but still has an affinity for moisture, which can alter
both absolute and relative retentions. When not in use, therefore, it is recom-
mended that the column should be kept
at
a relatively high temperature (e.g.
150OC) to avoid adventitious moisture. Columns are available with 0.32 mm in-
ternal diameter, with a 5pm layer, or with 0.53 mm internal diameter, with a
10pm layer.
Figure 1.22 is a chromatogram of hydrocarbons on KCI-deactivated alumina.
The separations are good, such that most components could be measured even if
they were trace impurities. The separations which could be improved are those
of acetylene and propadiene from the C, saturates, and of 1-butene from trans-2-
butene. Further studies of alternative deactivating agents [23] showed that the
use of sodium sulphate rather than potassium chloride gave a phase with rather
References
p.
40
