All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.

Sr
total
¼
86
Sr 1 þ
88
Sr
86
Sr
þ
87
Sr
86
Sr
þ
84
Sr
86
Sr
atomic mass of Sr:
If we wish to make a precise calculation, we must recalculate the atomic mass of strontium
because the
87
Sr/
86
Sr ratio is not exactly that of the standard samples, which is 0.709. This is
not an easy operation because we must calculate the atomic mass of each isotope (or look it
up in tables).
5
We shall ignore this difficulty. The standard isotope ratios are:
85
Rb=
87
Rb ¼ 2: 5926;
88
Sr=
86
Sr ¼ 8:3752;
84
Sr
86
Sr ¼ 0:056 584:
The atomic mass of Sr is 87.613 and of Rb is 85.35.
We therefore find:
Rb
Sr
mass
¼
87
Rb
86
Sr
0:341;
which gives:
Rb
Sr
Earth
¼ 0:031
Exercise
Calculate the relation between the
147
Sm/
144
Nd ratio and the Sm/Nd gravimetric chemical
ratio.
Answer
Read on!
6.2.4 Position of granites in the (Sr, Nd) diagram
Analysis ofgranites ofvarious ages initiallyby teams in Paris and Cambridge (1978^80) (see
Alle
'
gre and Ben Othman,1980; O’Nions et al., 1983; Ben Othman et al.,1984) showed, as
expected,muchwiderdispersionofisotoperatiosthanforthemantle.Insteadofhavingasim-
plealmostlinearcorrelation asforoceanicbasalts,continentalgranites, gneisses, andgranu-
lites de¢ne a fairlybroad domain, but in a‘‘symmetrical’’positionto thebasalts relativeto the
reference point of the primitive mantle (Figure 6. 12). This pattern of distribution of points
representing the continental crust is the outcome of two combined e¡ects: for one thing, the
continentalcrustismadeupoftectonicsegmentsofdi¡erentages¢ttedtogetherlikeamosaic;
for another, in the complex pro cesses occurring in the continents (erosion, sedimentation,
metamorphism, hydrothermalism, anatexis, folding), fractionation between Rb and Sr and
b etween Sm andNd aredi¡erent andsotheisotopicresultsarevaried.
5
The precise atomic mass of an isotope is not just the number of neutrons neutron mass þnumber of
protons proton mass, expressed as a unit of mass (1/12 of
12
C), because the bonding energy
E ¼mc
2
, related to the energy of formation of the atomic nucleus, must be subtracted.
239 Strontium–neodymium isotopic coupling
6.2.5 Back to the isotope evolution diagrams
We can now plotthe isotope evolution diagram for the
87
Sr/
86
Sr ratio versus time for basalt
and granite.The novelty is that we can now plot the straight line of isotope evolution of th e
Earth, that is, of the primitive mantle as the reference since none of Rb, Sr, Nd, or Sm is
incorporated i n th e Earth’s core.
6
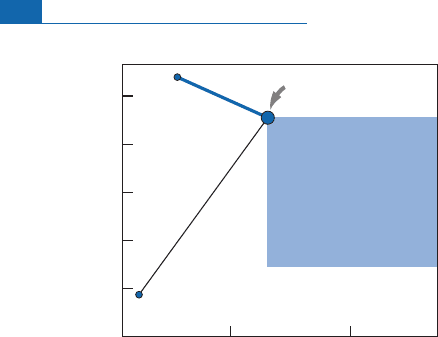
Figure 6. 13 shows that granites, which constitute most of
the continental crust, are situated above the primitive mantle, and oceanic basalts below,
withthe MORB being the mostextreme.
BymeasuringtheRband Srcontents ofthe continentalcrust,we canestimatetheslopeof
the straight line of evolution for the continents as equal to l
87
Rb=
87
Sr
.The
87
Rb/
86
Sr
ratios of continental rocks range from 0.5 to 3.The value1is a good average. Conversely, the
87
Rb/
86
Sr ratios of MORBs are very low (0.001). The straight line of evolution of the
MORBsource medium is virtuallyhorizontal.
As said, the continents are made up of segments of tectonic provinc es that are pieced
together and formed at di¡erent times. It is th erefore a seri es of vectors of evolution that
must be considered and which lie behind the wide dispersion of the present-day
87
Sr/
86
Sr
isotope ratios (Figure 6. 1).
Let us try to construct an analogous diagram for the
143
Nd/
144
Nd ratio. To do this, we
mustmeasurethe comp osition ofgraniteswhichturn outtohaveverynegative"parameters
(the Bulk Earthbeing taken as the referencewith "
Earth
¼0).Whenwe plotthe resultson the
Nd isotope evolution diagram, we observe a similar distribution as for Sr but in revers e.
The values of the granites are below those of the pr imitive mantle and the basalt values
above,withtheMORBlyingfurthestout,withthehighest "parameters (Figure6. 13).
143
Nd/
144
Nd
Granites
Present-day
Bulk Earth
Oceanic
basalts
0.7050.700
0.513
0.512
0.511
0.510
0.509
0.710
87
Sr/
86
Sr
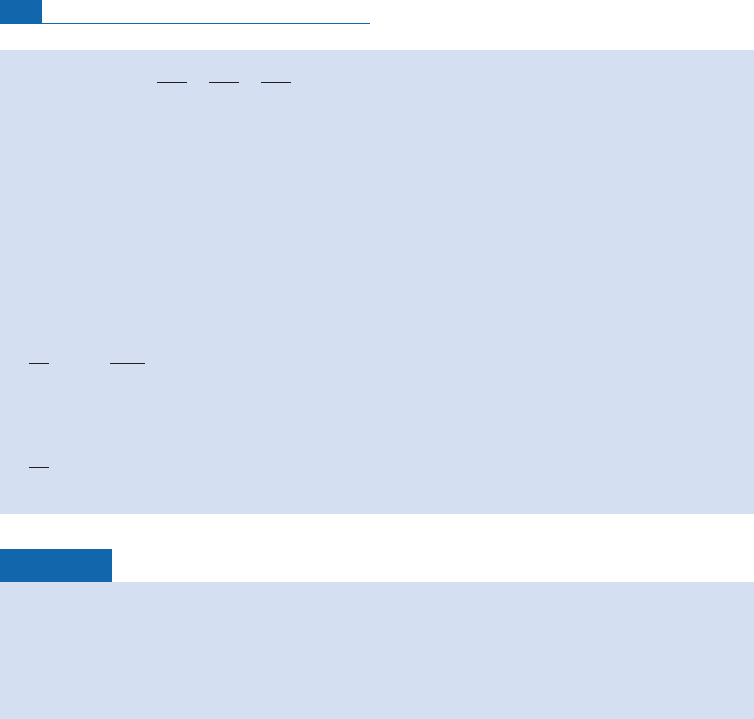
Figure 6.12 Position of granites in the (Sr, Nd) isotope correlation diagram. Notice the domain is much
broader than that of basalt. The straight lines of evolution of oceanic basalts and of the Earth’s primitive
mantle are shown.
6
We shall use the terms ‘‘Earth,’’ ‘‘Bulk Earth,’’ and ‘‘primitive mantle’’ interchangeably when speaking
of Rb/Sr or Sm/Nd ratios, since the corresponding ratios are all identical.
240 Radiogenic isotope geochemistry
Topin downthe quantitative evolutionsof
143
Nd/
144
Nd ratios,wehavesoughtto evaluate
the
147
Sm/
144
Nd ratios of th e two‘‘symmetrical’’ reservoirs: the continental crust and the
mantle, which is the MORB source. For the crust, the
147
Sm/
14 4
Nd ratios are close
(0.10^0.12). For the MORB source, the
147
Sm/
144
Nd ratios range from 0.15 to 0.3. Unlike
with
87
Sr/
86
Sr, the evolutionofthe MORBsources intermsof
147
Sm/
144
Ndis notahorizon-
tal line.
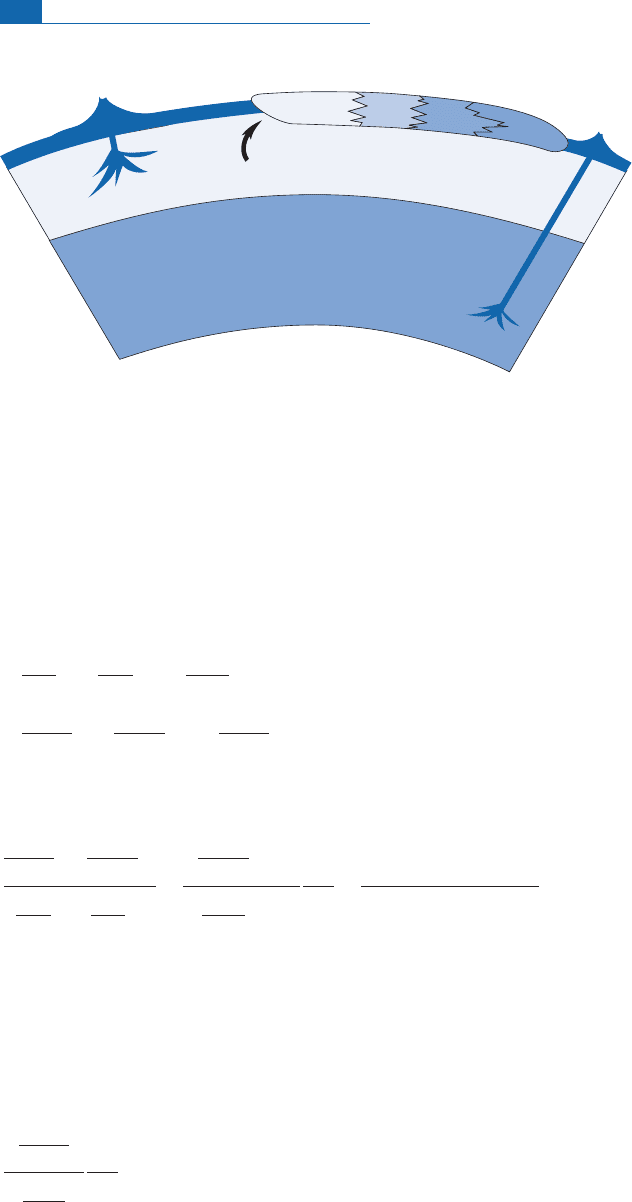
On this basis, a si mple geo dynam ic model can be developed.The continental crust has
di¡erentiated at the expense of the m antle probably through volcanism or magmatic pro -
cesses related to subduction.T he continental crust is enriched (compared with the mantle)
in elements such as potassium, rubidium, cesium, rare earths, thorium, and uranium.
According to the plate tectonics paradigm, the continents ‘‘£oat’’ on the mantle and are
never swallowed up again by it: the enriche d elements of the continental crust aretherefore
stored atthe Earth’s surface.Thus theyare removed from theupper mantle, which is conse-
quentlydepletedin these elements.
Measurements of concentrations reveal that the continental crust is greatly enriched in
Rb an d less so in Sr.Therefore, the Rb/Sr ratio of the continental crust is far higher than
that remaining in the mantle after extraction (residual mantle). Likewise, the continental
cru st is much more enrich ed in Nd than in Sm, and so the Sm/Nd ratio of the continental
cru st is lowerthan inthe residual m antle.T his explains the origin ofthe inverseisotope cor-
relation.T he di¡erences in chemicalbehavior between the Rb^SrandSm^Nd syste ms are
important in dispersion. As Figure 6.1 4 shows, the Sm/Nd ratio is just as widely dispersed
in MORBs as it is in granite, whereas the dispersion of Rb/Sr is very di¡erent in the two
media (MORB values showing little dispersion). This is because of the properties of rare
earths, which areverysimilar to eachother.
Extraction ofelements by the conti nental crust atthe expense of the mantle is a complex
geologicalprocessthat mustbe evaluatedbutwhichprobablyinvolves magmaticprocesses.
MORB
Bulk
Earth
Continental
crust
MORB
+10
0.7025
0.7050
0.7100
0.7125
0.7150
0.7175
0.7200
–20
0
87
Sr/
86
Sr
Earth
Earth
4.55 Ga present day
Time
4.55 Ga present day
Time
ε
Nd
Continental
crust
Figure 6.13 Isotope evolution diagrams inferred directly from the (Sr, Nd) isotope correlation. These
diagrams show how we can imagine the surface part of the Earth evolved, with continental crust having
preferentially extracted some elements and a depleted oceanic mantle (MORB source) as a result of that
extraction. Positions in the isotope evolution diagrams are ‘‘symmetrical’’ relative to the Bulk Earth.
241 Strontium–neodymium isotopic coupling
Geological mapping(seePlate5) andthe isotopic dispersion ofcontinentalrockssuggest
that this di¡erentiation of the continents has been going on throughout geological time in
successive episod es.The upper mantle fromwhich the continental cr ust has been extracted
and which is termed ‘‘deplete d’’ mantle (that is, depleted in certain elements such as potas-
sium, uranium, thorium, rubidium, etc.) is therefore the residue of this extraction process.
Buttheupper mantle is alsoinvolved inthe gigantic process ofplate tectonics.It is therefore
a medium in which convection processes occur, with transport of matter over great dis-
tan ces but also with vigorous mixing. This medium is th erefore relatively homogeneous,
which explains thenarrowisotopic spreadof MORBs in Figure 6. 1.
It seems that MORBs are samples from the part of the mantle that is most a¡ected by
extraction of the contin ental crust. As MORBs crop out at the ocean £oor along oceani c
ridges, itis assumed thatthis reservoir is the upper part ofthe mantle (as con¢rmed nowby
tomographic imageryobtained byseismology).
In contrast, OIB comes from the more primitive, deeper mantle; it is c ontaminated as it
crosses the upper mantle and so its isotopic compositions lie midway between those of
MORBandthose ofthe more primitive mantle (Figure 6. 15).
6.2.6 Evolution of time in the (Nd, Sr) isotope diagram and the
question: geochemical differentiation or mixing?
Intheforegoingreasoning,toillustrate changesinthe Srand Nd isotope ratios, we returned
to the diagrams of isotope ratios versus time, adding an essential component, the reference
constituted by the straight lines of evolution of the Bulk Earth (Alle
'
gre et al., 1979). Can
these evolutionsnotbe shown di rectlyon the (Sr, Nd) isotope diagram? Ofcourse they can,
and this presentation has the advantage of clearly situating the isotope domains observed.
20
30
10
0
135
87
Rb/
86
Sr
N
Granitoids
10
5
0
0.1 0.2 0.3
147
Sm/
144
Nd
15
10
20
30
MORB
40
20
MORB
Granitoids
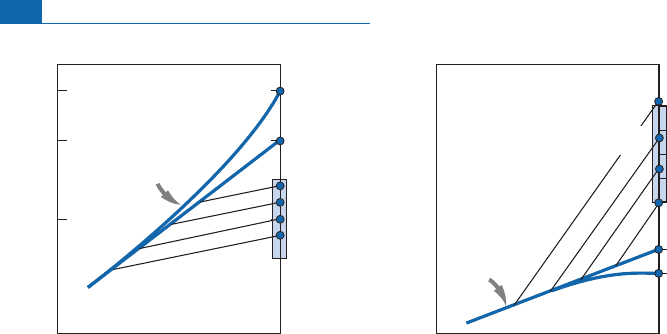
Figure 6.14 Statistical distribution of
87
Rb/
86
Sr and
147
Sm/
144
Nd ratios in MORB and granitoids.
242 Radiogenic isotope geochemistry
ThestraightlineofevolutionoftheEarthsystem canbe drawn.Thetwoevolutionequations
are:
87
Sr
86
Sr
¼
87
Sr
86
Sr
0
þ
87
Rb
86
Sr
Earth
l
Rb
t
143
Nd
144
Nd
¼
143
Nd
144
Nd
0
þ
147
Sm
144
Nd
Earth
l
Sm
t
8
>
>
>
<
>
>
>
:
Eliminatingtgives:
143
Nd
144
Nd
143
Nd
144
Nd
0
87
Sr
86
Sr
87
Sr
86
Sr
0
¼
147
Sm
144
Nd
Earth
87
Rb
86
Sr
Earth
l
Sm
l
Rb
¼
0:196 6:54 10
12
0:091 1:42 10
11
¼ 0:9919:
Therefore in the
143
Nd=
144
Nd;
87
Sr=
86
Sr
diagram, the evolution is a straight line with
a slope very close to unity. This straight line is graduated in time, of course. The two
straight lines of evolution leading to the average of the continental crust and of the resi-
dual mantle, the MORB source, can be drawn. Their slopes can be easily calculated
and are equal to :
147
Sm
144
Nd
87
Rb
86
Sr
l
Sm
l
Rb
:
Depleted
upper mantle
Continental crust
MORB
OIB
Primitive
deep mantle
T
4
T
3
T
2
T
1
Figure 6.15 The standard model of the mantle. The mantle is separated into two layers: the MORB
derived from the upper layer and the OIB from the deeper layer. The upper layer is depleted in some
elements by extraction of the continental crust. Thedeeperlayerismoreprimitive (that is, closer
to the value of the Bulk Earth). The continents are made up of a series of provinces of varied ages
T
1
,T
2
,T
3
,T
4
.
243 Strontium–neodymium isotopic coupling
With the approximate values for the continental crust and the primitive mantle, we ¢nd the
slope of the straight line of evolution of the d epleted mantle is very, very steep (more than
100) and thatofthe c ontinental crustis closeto0.05.
Exercise
Given that the slope of the straight line of evolution of the depleted mantle is very high and
the extreme value of MORB is
143
Nd/
144
Nd ¼0.513 50 and
87
Sr/
86
Sr ¼0.7021, what is the
minimum age of differentiation of the crust–mantle?
Answer
The age is greater than 2 Ga.
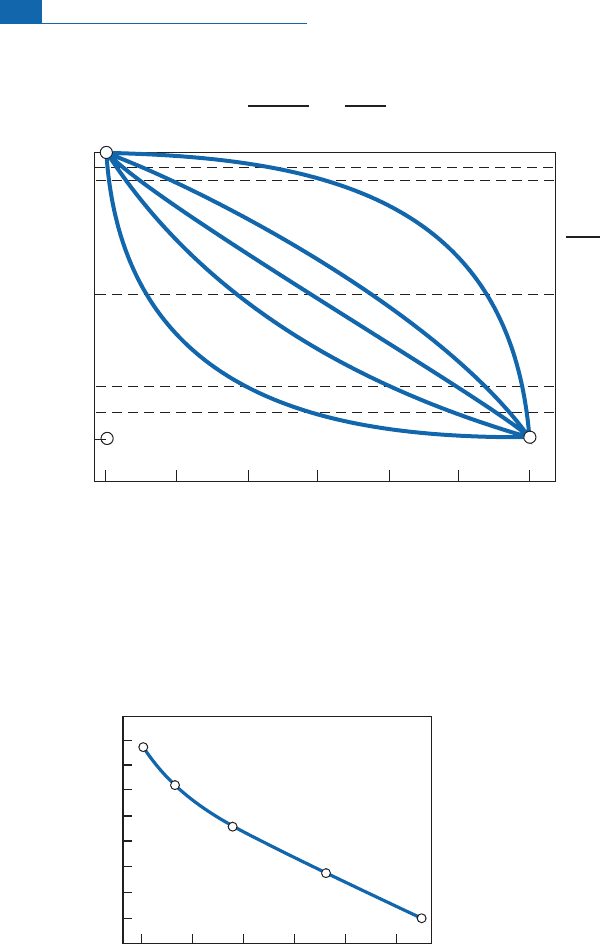
On the basis of this reasoning, we can trace the curves of evolution of the depleted mantle
and the continental crust (Figure 6. 1 6), assuming that the continental crust has been
extracted in a series of episodes.When the theoretical models are compared with experi-
mentaldata, a questionarises.Howcanwe explainthe dispersionofvaluesfor the continen-
tal crust andthelesse r dispe rsion ofbasalts?
A priori, there are two hypotheses: di ¡erentiation or mixing ?(ora combination of the
two ?).Were there multiple crust^mantle di ¡erentiations giving rise to di¡erent pieces of
cru st and leaving di¡erent pieces of depleted mantle? Or, on the contrary, are all the inter-
mediate points the result of mixing between two extreme components? Before discussing
these questions, we need to know how a mixture is represented in the (
143
Nd/
14 4
Nd,
143
Nd/
144
Nd
Granites
of
continental
crust
Granites
of
continental
crust
Present-day
Bulk Earth
Bulk Earth
Mixing
Depleted
mantle
1 Ga
2 Ga
3
Ga
Oceanic
basalts
0.7050.700
0.513
0.512
0.511
0.510
0.509
143
Nd/
144
Nd
0.710
87
Sr/
86
Sr
0.7050.700
0.513
0.512
0.511
0.510
0.509
0.710
Basalts
MORB
a
b
Figure 6.16 Diagram of (Nd–Sr) isotope evolution over geological time. (a) We have supposed that the
continental crust was extracted in a series of episodes and that the residual mantle reflected these
extractions in a continuous, gradual manner. (b) We have supposed the extraction episodes of pieces of
continents were followed by mixing phenomena both in the residual mantle to the left of the straight
line of evolution of the Earth and in the continental pieces (one case is shown, to the right of the straight
line of evolution of the Earth). After Alle
`
gre
et
al
.(1979).
244 Radiogenic isotope geochemistry

87
Sr/
86
Sr) diagram.We have spoken of mixing in the isochron diagrams but not yet in the
caseofpaired isotope ratios.
6.2.7 Mixing in isotope ratio correlation diagrams
Im agine we have two reser voirs characterized by their isotope ratios noted here R and
(e.g.,
87
Sr/
86
Sr and
143
Nd/
144
Nd). How is a mixture represented in the (R , )diagram?Ifwe
write the equations of the mixture M between two extreme reservoirs (1) and (2), where A
and B areth e two chemical elementswhose ratios areunderc onsideration, weget:
R
M
¼ R
A
x
1
þ R
B
ð1 x
1
Þ
M
¼
A
y
1
þ
B
ð1 y
1
Þ
where x
1
¼ m
1
C
1
A
=m
1
C
1
A
þ m
2
C
2
A
and y
1
¼ðm
1
C
1
B
Þ=ðm
1
C
1
B
þ m
2
C
2
B
Þ; C
A
1
and C
A
2
being the concentration s of element A in poles 1 and 2, and C
B
1
and C
B
2
being the concen-
trations for element B. Let us calculate the mixing equation in the (R, )diagram.
Combining thetwo equations for th e tworatiosgives:
R
1
R
M
R
M
R
2
¼ K
1
M
M
2
;
with K ¼ðC
1
A
=C
1
B
Þ
ðC
2
A
=C
2
B
Þ. If the denominator of the two ratios is identical (as is the
case, for example, with l ead isotopes whose denominator is common lead,
204
Pb), the m ix-
ing equation is a st raight l in e.The same is tr ue ifthe denominators are di¡erent, but K ¼1,
thatis, ifthe chemical ratios ofthe two elements are identical for1and 2 (for example i f Nd/
Sr is constantinbothsystems).
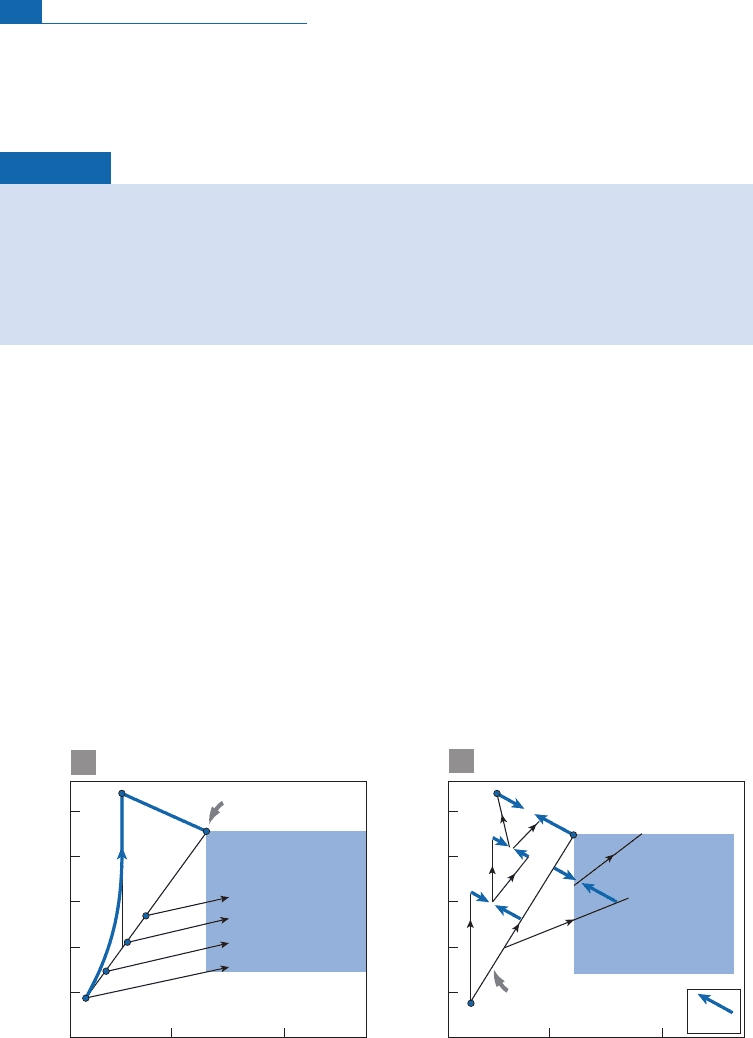
Generally, when K is di¡erent from1, the mixing curve is a hyperbola whose shape varies
with K (Figure 6. 17). However, if K is not too di¡erent from 1, the mixing curve is almost a
straight l i n e. (We shall make much use ofthisproperty in what follows.) This is generally the
casefor mixturesbetween mantlerocksorbetween continentalrocksfor the Nd^Sr system.
Exercise
We suppose a granite has been formed from a mixture of a basalt intrusion and pre-existing
acidic rock. The characteristics of the basalt magma are "
Nd
¼þ10,
C
Nd
¼7ppm,
87
Sr/
86
Sr ¼0.7025, and
C
Sr
¼300 ppm; those of the pre-existing rock are: "
Nd
¼20,
C
Nd
¼20 ppm,
87
Sr/
86
Sr ¼0.725, and
C
Sr
¼100 ppm. Calculate the mixing curve for the various
granite facies, supposing the
m
crust
/
m
magma
ratio varies from 0.1 to 1. (See Figure 6.18.)
Answer
Table 6.3 Mixing curve
Number
m
crust
/
m
mantle
"
Nd
87
Sr/
86
Sr
1 0.1 –18.98 0.719 00
2 0.25 –17.58 0.715 200
3 0.5 –15.53 0.711 50
4 0.75 –13.75 0.709 33
5 1 –12.2 0.708 12
245 Strontium–neodymium isotopic coupling
Di¡erentiation results in straight lines, while the mixtures in the Nd^Sr isotope dia-
grams are almost straight lines. By virtue of a powerful mathematical theorem by
which, in a linear (or near-linear) system, when there are two solutions, any combina-
tion of the two solutions are also solutions, there is no means of distinguishing by
ε
Nd
87
Sr/
86
Sr
K =
= 0.1
(Sr/Nd)
1
1
C
Nd
2
C
Nd
(Sr/Nd)
2
0.5
1
10
50
100
+10
+5
K = 10
2
0.705
m
1
m
2
K = 2
K
= 1
K
= 0.5
K
= 0.1
1
0
0.703 0.7040.702
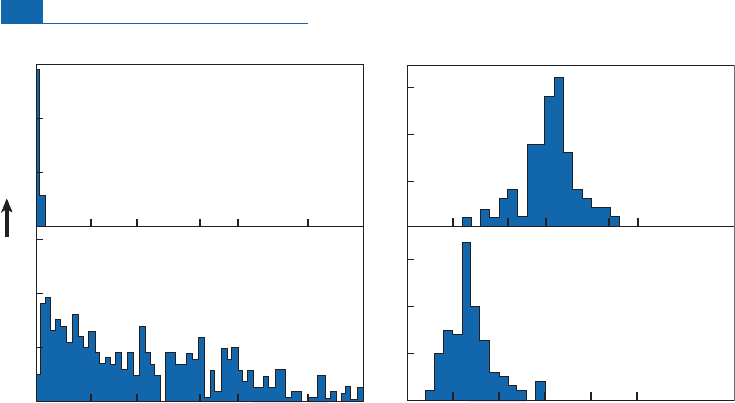
Figure 6.17 Theoretical mixing diagram between two poles 1 and 2 in the (Nd, Sr) isotope diagram for
various values of relative concentrations of Nd–Sr of 1 and 2. Parameters are shown in the figure:
K
is
the ratio of Sr/Nd chemical ratios, the ratio of Nd concentrations is taken as 0.1, and the result is
graduated in mass ratio
m
1
/
m
2
.
0.708
–12
–14
–16
–18
0.710
5
4
3
2
1
0.712 0.714 0.716 0.718
87
Sr/
86
Sr
ε
Nd
0
Figure 6.18 Representation of the various granite facies resulting from the crust–mantle mixture in the
exercise above.
246 Radiogenic isotope geochemistry

analyzing the exper imental data which of di¡erentiation or mixing predominate in
explaining th e Sr^Nd isotope correlation diagram as a whole. Geological considera-
tions suggest that both phenomena are involved in the dispersion of the points
observed. In the mantle, magma arises from di¡erentiation which separates a magma
composition from that of a residue. Conversely, convection in the mantle results in
mechanical mixing. In the continental crust, the formation of granites as well as the
erosion cycle, chemical sedimentation, an d metamorphism also correspond to chemical
di¡erentiation. Sedimentation and tectonic folding are mixing pro cesses. These two
phenomena probably occurred in the past and in recent periods (genesis and transfer
of magma). We asked the question: di¡erentiation or mixing ? The answer seems to b e
di¡erentiation and mixing. There’s a simple example of a non-uni que solution, or, if
you like, of quantitative uncertainty.
6.2.8 The Sr–Nd–Hf system
Wehavejust explored the coherence within the Nd^Sr isotope systems.We aregoing toadd
tothis the
176
Lu ^
176
Hfsystem, whichwi ll strengthen the cohesion withoutcontributingany
fundamental new information.
Lutetium is a heavy rare earthwhile hafnium hasgeochemical proper ties closetothose
of light rare earths. Lutetium-176 disintegrates into
176
Hf at a de cay rate l ¼1.94 10
11
yr
1
. It was only natural, then, to try to connect the variations in
176
Hf/
177
Hf isotope
composition w ith the variations observed in
143
Nd/
144
Nd. But this endeavor was
long unsuccessful because of di⁄culties in correctly analyzing the isotope composition
of Hf in the low amounts in which it occurs in rocks. This di⁄culty was overcome in
1980 by Patchett and Ta t s u m o t o (1980), working for the U.S. Geological Survey
in Denver. Since th en, a substantial amount of rock has been me asured by teams at
the Un iversity of Arizona at Tucson an d the E
¤
cole Normale Supe
¤
rieure in Lyon and the
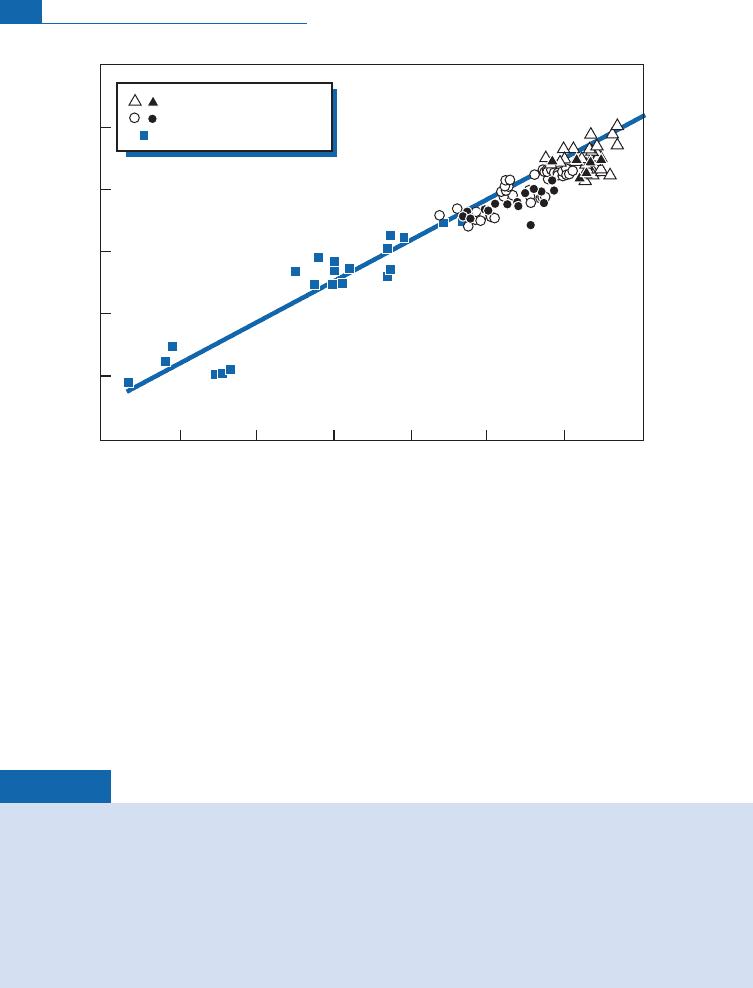
result is an extraordinarily coherent correlation between the isotope compo sitions of Nd
and Hf (Figure 6. 1 9). This correlation is important because it strengthens cohesion
(Bl ichert-Toft and Albare
'
de, 1997; se e also Patchett,1983;Ve r v o o r t and Bl ichert-Toft,
1999). It shows that the isotopic variations ob served for Nd are not the outcome of s ome
property peculiar to Nd but that they obey more general rules. As we did w ith Nd, we
de¢n e:
"
Hf
ð0Þ¼
176
Hf
177
Hf
176
Hf
177
Hf
p
176
Hf
177
Hf
p
6
6
6
6
6
6
4
7
7
7
7
7
7
5
10
4
:
The c onstants required for these calculations are:
Ratio at 4:55 Ga ð
176
Hf=
177
HfÞ
0
¼ 0:279 78
Present-day Bulk Earth ð
176
Hf=
177
HfÞ
p
¼ 0:282 95:
247 Strontium–neodymium isotopic coupling
The value of (
176
Lu/
177
Hf)
p
can be c alculated from the growth of the (
176
Hf/
177
Hf)
p
ratios,
giving
Lu
p
¼ 0:036.
Exercise
Three rocks (1, 2, and 3) are measured with
176
Hf/
177
Hf isotope compositions of 0.2835,
0.2832, and 0.281, respectively. Calculate "
Hf
(0). Can you identify the rocks from the Nd–Hf
correlation?
Answer
"
1
¼þ19, "
2
¼þ8.8, and "
3
¼68.9.
1 ¼MORB, 2 ¼OIB, and 3 ¼very old granitoid rock.
6.3 The continental crust–mantle system
Examination of the regularities and c orrelations in Sr^Nd^Hf system s sparked the idea
thatthefunda mentalprocessbehind chemicaldi¡erentiationoftheupper partoftheplanet
was the extraction of continental cr ust and its repercussions on the mantle.We shall try to
look more closely at this process in quantitative terms using the model developed by
Alle
'
gre,Hart,andMinster(1983a).
MORB
BE
OIB
0 Ma
T
> 0.09 Ma
T
< 0.8 Ma
T
> 1.15 Ma
0.2835
0.2830
0.2825
0.2820
0.2815
0.5110 0.5114 0.5118 0.5122 0.5126 0.5130
MORB
OIB
Continental material
143
Nd/
144
Nd
176
Hf/
177
Hf
Figure 6.19 Modern (Nd, Hf) isotope correlations after data from Patchett and Tatsumoto (1980) and
Vervoort and Blichert-Toft (1999). The correlation is excellent and extends linearly to rocks of the
continental crust for which only a few points are shown.
248 Radiogenic isotope geochemistry
