All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.

Exercise
The temperature of the very high atmosphere is typically 700–900 K. Calculate the escape
velocity of atomic hydrogen and then compare it with that of the molecule H
2
.
Answer
The mass of an atom or molecule is the molar mass divided by Avogadro’s number,
6.023 10
23
. The mass of a hydrogen atom is 0.001/6.023 10
23
, which is 1.65 10
27
kg.
For H,
ffiffiffiffiffiffiffiffiffi
3
kT
m
r
¼
3 1:381 10
23
800
1:65 10
27
1=2
therefore
V
e
¼4.48 km s
1
.
For H
2
,
V
e
¼3.16 km s
1
.
The two velocities calculated in the exe rcise above are less than11.2 km s
1
.They seemto
indicate that hydrogen does not escape from the Earth. Now, this is not so, as observations
and measurements prove! W here is the mistake? In fact, the velocities ofthevarious atoms
or molecules do not have constant values: theyobey B olt z man n’s stat i st ical d is t ri bu t ion.
And foravelocityof 4.48 km s
1
,some10%of
2
H particles escape everysecond.
For
4
He ,V
e
¼2.24 km s
1
.Some10
15
atoms have the necessary velocity to escape per
second.That represents a substantial quantity of ato ms in geological terms, when the time
measured is multiplied by millions ofyears. However, for the othe r rare gases, the nu mber
of particles attaining escape velocity is too low to be e¡e ctive: they remain trapped by the
gravitational ¢eld.
Theupshotfor us is that,as the atmosphere derives from degassingofthe mantle and not
(as for the major planets) from retention ofa primarygas envelope, the atmosphere maybe
considered as the complement of the mantle (as continental crust was for strontium and
Table 6.7 Decay systems leading to rare gas isotopes
Radioactivity Radioactiveproduct Isotope ratios studied
238
U,
235
U,
237
Th radioactive
chains
!
4
He
!
4
He
3
He
18
O(,n)
21
Ne !
21
Ne
!
21
Ne
20
Ne
40
Ke
cap !
40
Ar
!
40
Ar
36
Ar
129
I(extinct)
!
129
Xe
!
129
Xe
130
Xe
238
Uspontaneous ¢ssion
131
Xe,
132
Xe,
134
Xe,
136
Xe
!
131;132;134;136
Xe
130
Xe
244
Pu extinctspontaneous
¢ssion
131
Xe,
132
Xe,
134
Xe,
136
Xe
!
131;132;134;136
Xe
130
Xe
279 Isotope geochemistry of rare gases

n eodymium) for argon, krypton, and xenon, but not for helium and probably not for neon
either, if we consider all of geological time. For argon, kry pton, and xenon, we can write a
balance equation:
atmosphere þ mantle ¼ whole Earth:
The isotope composition of the rare gases of the atmosphere has been known for about
50 years. However, it was for a long time di⁄cult to obtain a measurement of the isotope
compositions ofthe raregases ofthe mantle.This wasbecau se itwastoo easy for samples to
be contaminated by theatmosphere, whichskewed theresults. Raregases occur in lowcon-
centrationsin rocksfromthe mantle (basalts)andanycontactwiththeatmosphere contam-
inates them.Whe re molten lava is in contact with the air, contamination is catastrophic. It
is much the same for subm arine contact, as rare gases are soluble in sea water, which con-
taminates the gas itself (hence the importance of knowing the solubility of rare gases in
water).The geoche mistry of raregasesbegan with the discoveryofpillow lavas, whose rims
turn to glass at th e contact of sea water, preventing the sea water containing dissolved rare
gas es from contaminating the lava. Moreover, when pillow lavas are emplaced, the rare
gas es migrate and concentrate in gaseous i nclusions concentrating the rare gases 1000
times compared with magmas. More recently it has been possible to analyze He and Ne in
gas eous inclus ions inolivine phenocrysts.
Thesecond factor making this analysis di⁄cultis thelow abundanceofraregases,which
decreases with their mass. The atmosphere does not retain He and Ne quantitatively, as
said, so He and Ne con centrations are relatively low in the atmosphere (and in sea water).
As the concentrations of rare gases are higher in magmas, magmas are the less di⁄cult to
analyz ebecausetheyarelesslikely tobe contaminatedbythe atmosphere.
Measu ring rare gases with a mas s spectrometer is a di⁄cult but very sensitive business.
Special equipment is required to extract gases without them being contaminated by the
atmosphereorbyprevioussampling (see Figure6.3 7).
Exercise
The rare gas composition of the atmosphere is expressed in cubic centimeters at standard
temperature and pressure in Table 6.8 below.
(1) What is the composition of the atmosphere in rare gases expressed in moles?
(2) What is the composition of the atmosphere in rare gases expressed in grams?
(3) What is the composition in
3
He and
40
Ar in moles and grams, given that
40
Ar/
36
Ar ¼296.8
and that
3
He/
4
He ¼1.4 10
6
?
(4) What is the concentration of these gases if related to the mass of the Earth?
Answer
Under standard conditions, 1 mole of an ideal gas occupies 22.4 liters. The table below shows
the answers to questions (1) and (2).
4
He
20
Ne
36
Ar
84
Kr
130
Xe
Composition
(mole)
0.0926 10
16
0.29098 10
16
0.0555 10
17
0.1149 10
15
0.06263 10
13
Composition (g) 0.3704 10
16
5.8196 10
16
1.998 10
17
9.6516 10
15
8.1449 10
13
280 Radiogenic isotope geochemistry
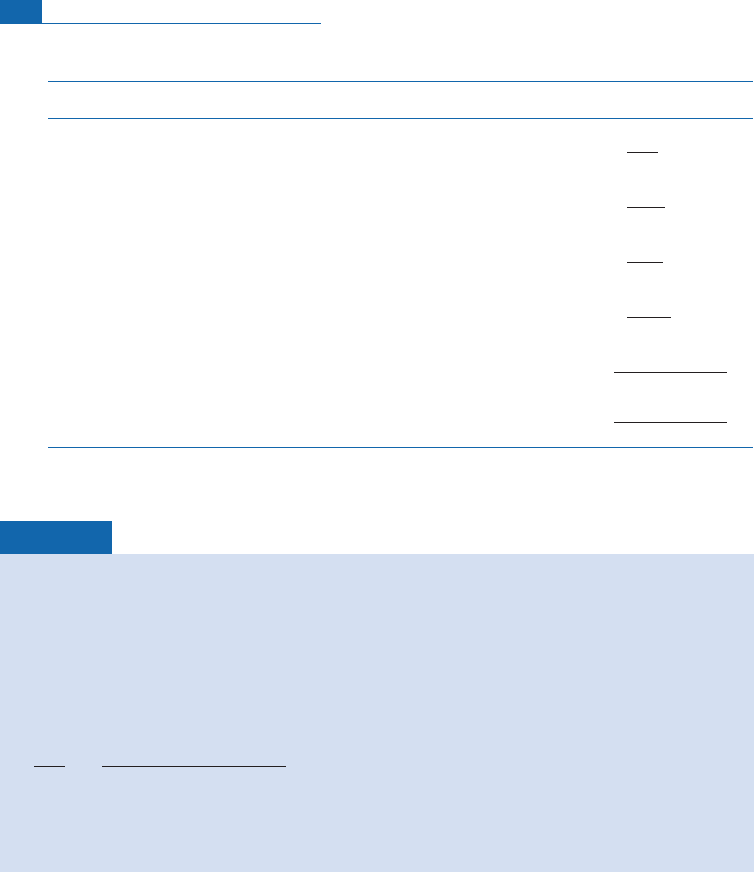
Crucible
V 8
V 7
Sample storage
Ti 1
SAES
getter trap
CH 2
V 0
CH3
V 1V 2V 3V 4
V 5
Turbo-molecular
pump
CH 1
Air
Ion
pump
to
mass spectrometer
to cold
head
Ti 2
Extraction line
Mass spectrometer
ElectromagnetIon source
SAES getter
trap
Thermo-ionization
gauge
Gas inlet to
ion pump
Collection system:
- Faraday collector
- Electron multiplier
- Ion counter
Figure 6.37 Laboratory equipment for measuring the isotope composition of rare gases. The
fundamental point is that the measurement enclosure is at very low pressure of 10
9
mm Hg. It
comprises two parts: (i) the extraction line made of special glass (or metal). This is a circuit where the
much more abundant gases (H
2
O, O
2
,N
2
,CO
2
,CH
4
) are captured because their presence in the mass
spectrometer would lower the partial pressure of the rare gases too much for them to be measured.
(ii) The mass spectrometer. The purified gases are fed one at a time into the mass spectrometer with its
electron bombardment source and where the gases are enclosed (we speak of static measurement):
helium, neon, argon, krypton, and xenon are measured in turn. Concentrations are very low. The signal
is measured either with a Faraday cup or by an ion counter.
281 Isotope geochemistry of rare gases

(3) The results are shown in the table below.
40
Ar
3
He
Concentration 65.8 10
18
1.647 24 10
18
Composition (mole) 3.8892 10
9
1.296 10
8
(4) The mass of the Earth is 6.057 10
27
kg. The results are shown in the table below.
4
He
36
Ar
Concentration 3.47 10
8
2.083 10
8
6.4.1 Isotope geochemistry of helium
Helium-3 was ¢rst discovered by Alvarez and Co rnog (1939). After the Second World War,
Aldrichand Nier (1948)discoveredthevariationsinterrestrialabundances,butheliumisotope
geochemistry wasinitiatedbythe Sovietteamunderthe imp etus of IgorTolstikhi n (Mamyrin
et al.,1969;Tolstikhin et al.,1974)andbyBrian Clarke and Harmon Craig (Clarkeet al.,1969)
attheScripps Institution ofOceanographyandtheirstudents andlaterbyMark Kurz and Bill
Jenkins (1981)attheWoodsHoleOceanographicInstitution.
The principles are as follows. Helium-4 is the product of collateral disintegration of
radioactive chains ( particles): 8 for the
238
U chain,7 for the
235
Uchain, and 6 for the
232
Th chain. Helium-3 is a stable isotope.The
4
He/
3
He ratio in a closed systemvaries with
the equation:
4
He
3
He
¼
4
He
3
He
0
þ
238
U
3
He
fðtÞ:
Thefunction (t) is de¢ned asbelow:
Iftis small,fðtÞ¼ 8l
8
þ
7l
5
137:8
þ 6
Th
U
l
2
t 2:47t,ift is in Ga.
Iftislarge, ftðÞ¼ 8e
l
8
t
1
þ
7
137:8
e
l
5
t
1
þ 6
Th
U
e
l
2
t
1
.
In keeping with an odd practice introduced by Harmon Craig, the helium isotope ratio in
basaltrocks is often expressed‘‘upside down’’:
3
He
4
He
¼ R
3
He
4
He
atmosphere
with
3
He
4
He
atmosphere
¼ 1:4 10
6
:
Table 6.8 Rare gas composition of the atmosphere
4
He
20
Ne
36
Ar
84
Kr
130
Xe
Composition (cm
3
)2.07610
19
6.5 18 10
19
1.245 10
20
2.245 10
18
1.4 03 10
16
282 Radiogenic isotope geochemistry
When R ¼8, the value is 8 times that of the atmosphere. If R ¼30, it is 30 times that of
the atmosphere, and so on. In what fol lows, we shall give the results in
4
He/
3
He ratios
but we shall add the R(
3
He) notation for comparison w ith papers and books using this
notation.
Measurements of
4
He/
3
He on ocean basalts display a very di¡erent distribution for
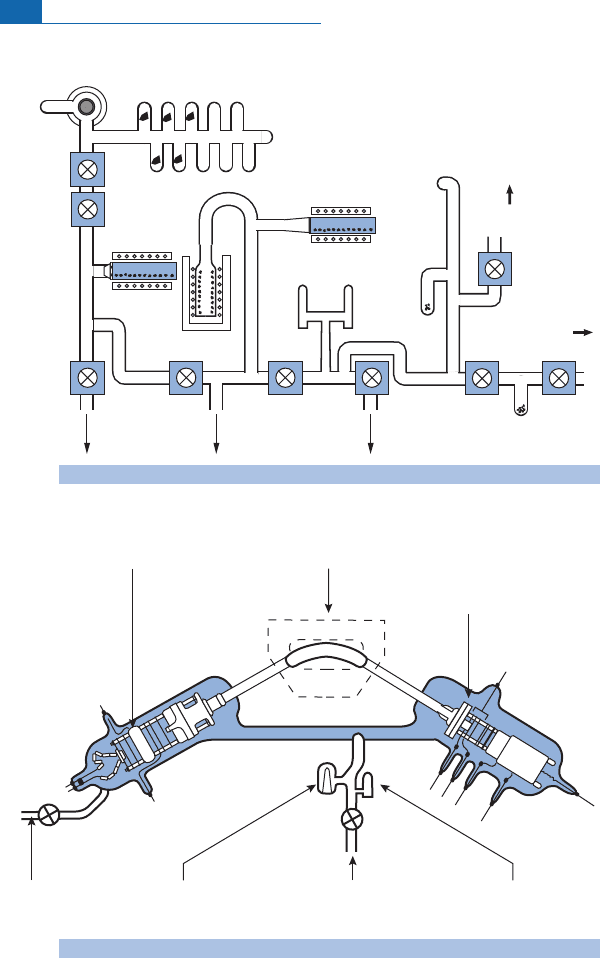
MORBandOIB(Figure6.38).
The MORB distribution is tightly clustered around
4
He/
3
He ¼90 00 0whereas the OIB
distribution is verydispersed with ratios rang ing from13 000 to130 000. However, looking
more closely, large islands like Hawaii or Iceland, with large volumes of bas alt, havevalues
between13 000 and36 000(Figure6.38).
The simplest i nterp retation resulting from the standard model is to say that MORB
derives from the upper- mantle reservoir with a high
238
U/
3
He ratio and so by radioactive
decayahigh
4
He/
3
Heratio;OIBderivesfrom a moreprimitivereservoir wherethe
3
He con-
tentishigher, and sothe
238
U/
3
Heratiois small eras is the
4
He/
3
Heratio.This isbecausethe
upper reservoir, which is directly involved in the tectonic plate mechanism, is highly
degassed, whereas the deep reservoir is much less degassed (Figures 6.39 and 6.40). This
dual origin is con¢ rmed by the existence ofthe Schilling e¡e ct, that is a mixing of OIB and
MORB along some mid-ocean ridges. Thus, the mid-Atlantic ridge southwards from
Iceland displays avariation in
4
He/
3
He ratios, which increase from Ic eland (14 000) south-
wardswheretheyreach120 000.Thisis analogoustowhatwesaw for Sr isotope composition
(Kurz andJenkins,1981).
Exercise
Accepting that the lower mantle is a closed system for U and He, calculate the
238
U/
3
He
ratio ¼
1
He
given that
4
He/
3
He ¼15 000 and that (
4
He/
3
He)
initial
¼2500. If the
4
He/
3
He
60
OIB
40
20
100 000
"High
3
He" "Low
3
He"
Tristan, Gough, São Miguel
Loihi
60
MORB
N
4
He/
3
He
40
200 000 300 000
20
Figure 6.38 Distribution of
4
He/
3
He ratios in MORB and OIB. The difference in dispersion can be
observed.
283 Isotope geochemistry of rare gases
ratio ¼100 000 (still the hypothesis of a closed system), calculate
2
He
. (We take Th/
U 4.)
Answer
We use the dating formulae established in Chapter 2 and recalled above. We find:
1
He
¼684
and
2
He
¼5300.
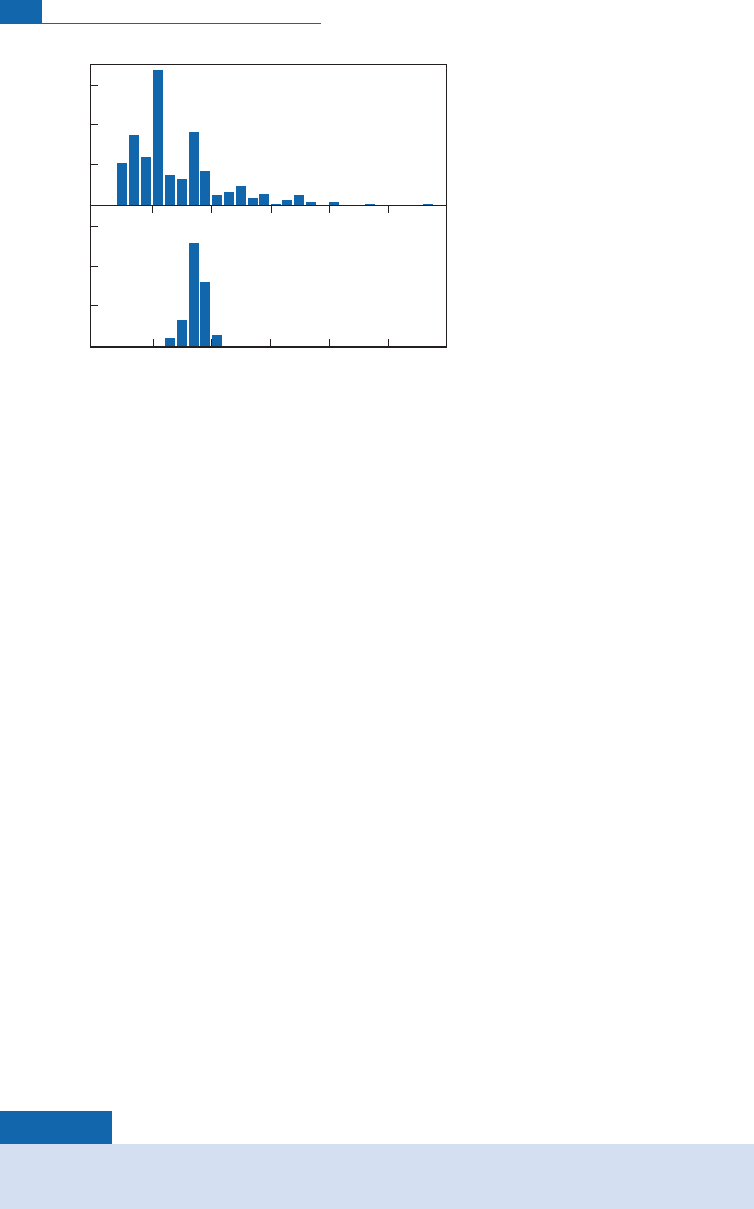
Little degassed
lower mantle
Highly
degassed
upper mantle
AtmosphereHe
Ne Ar, Kr, Xe
Figure 6.39 The standard model developed from the results for helium isotope analysis and extended
to all rare gases. With an atmosphere that retains argon, krypton and xenon but lets helium (and neon
in the past) diffuse, the upper mantle is highly degassed but not so the lower mantle.
80
70
60
50
40
30
20
10
4.5
4.05.0 3.0
Time (Ga)
4
He /
3
He x 10
3
2.0 1.0 0
OIB
MORB
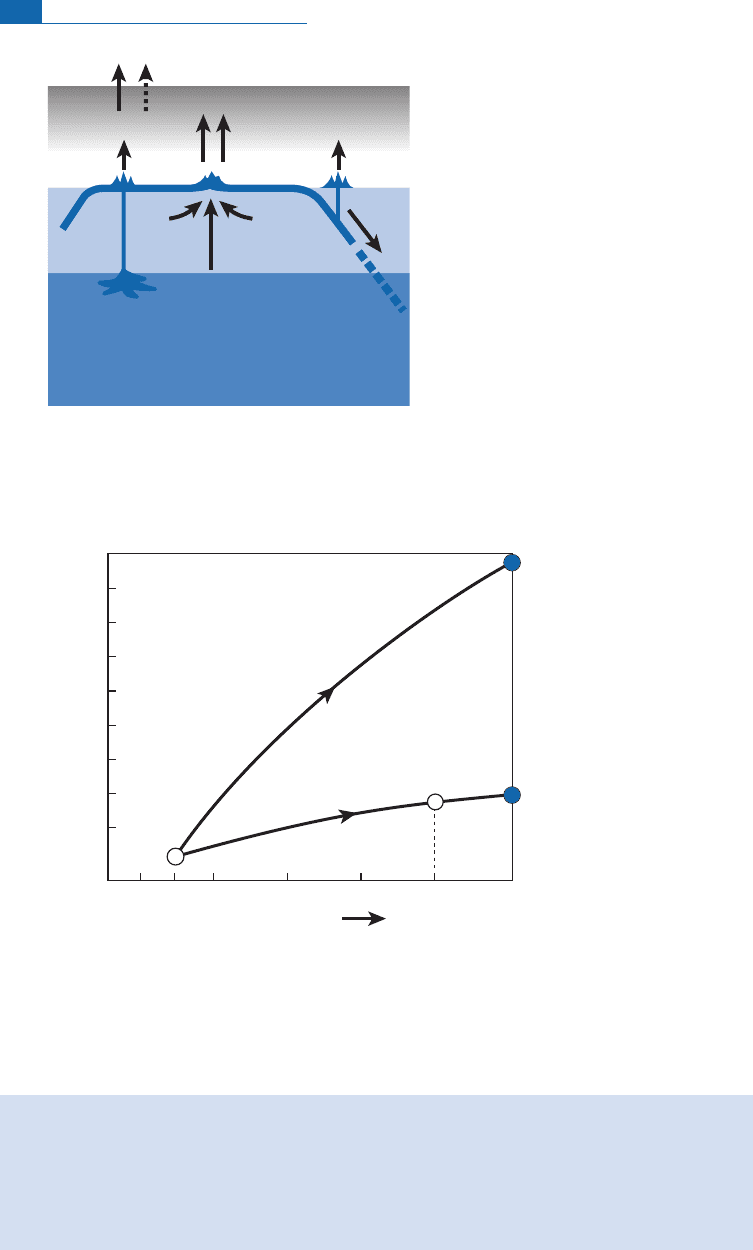
Figure 6.40 Possible changes in
4
He/
3
He ratios in the MORB (upper mantle) and OIB source reservoirs. It
is assumed they evolved in a closed system over 4.5 10
9
years in both cases, which is undoubtedly an
extreme oversimplification, but gives an order of ideas. The values of
238
U/
3
He ¼m
U, He
considered are
4900 for the upper mantle and 800 for the lower mantle.
284 Radiogenic isotope geochemistry
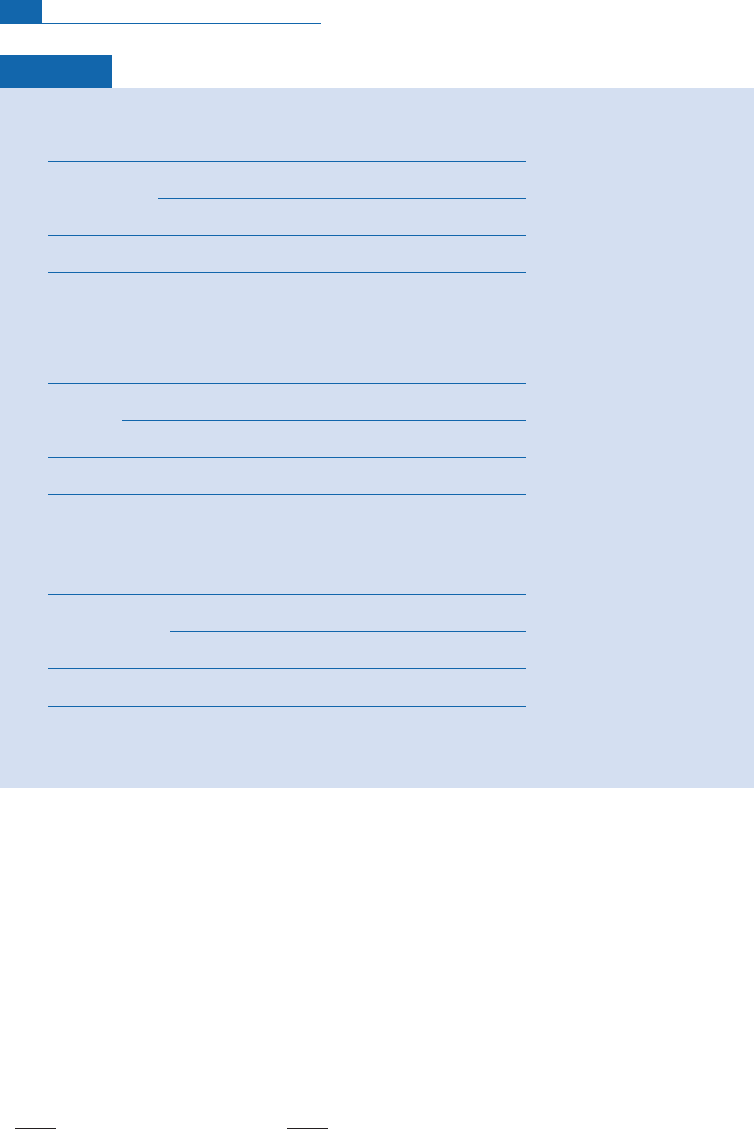
Exercise
For four basalts, we give the helium isotope composition with Craig’s
3
He/
4
He ¼
NR
A
notation
in the table below.
(1) Calculate the
4
He/
3
He composition of the four samples and plot the curve
NR
A
¼(
4
He/
3
He).
(2) Suppose we have measured the Sr isotope ratios in the same samples as set out below.
Draw the Sr–He isotope correlation using both notations (see Figure 6.41). What do you conclude?
Answer
(1)
(2) The three curves for the abundances of the two radiogenic isotopes
4
He and
87
Sr are shown
below. Craig’s
R
A
notation destroys perfect linear correlation.
6.4.2 Isotope geochemistry of neon
Neon has three isotopes:
20
Ne,
21
Ne, and
22
Ne. The abundance of
21
Ne varies with nuclear
reactions caused by particlesemittedbyuranium andthorium chains
18
O(,n)
21
Ne or, for
17 % ,
24
Mg(n,)
21
Ne.Thesevariationsaresaidtobenucleogenic,although in facttheyfollow
themathematicallawsofradiogenicproduction(We t h e r i l l ,1954).Weshow theisotopevaria-
tions observed in nature in a plot of (
20
Ne/
22
Ne,
21
Ne/
22
N e) where the tw o ratios vary in the
basaltsbut for two di¡erent reasons.The
21
Ne/
22
Ne ratios vary for nucleogenic reasons.The
20
Ne/
22
Ne ratios vary in nature because the Earth’s atmosphere has a di¡erent value from
thatofthe Earth’s mantle(which is closer tothatoftheSun)(Craigand Lupton,1976).
20
Ne
22
Ne
atmosphere
¼ 9:5
20
Ne
22
Ne
Sun
¼ 13:5:
8
Basalt
B
1
B
2
B
3
B
4
N
40 5 10 20
Basalt
B
1
B
2
B
3
B
4
N
0.7035 0.7022 0.7029 0.7033
Basalt
B
1
B
2
B
3
B
4
(
4
He/
3
He) 17 800 142 000 71 400 35 710
8
Terrestrial values can vary by only
20
Ne/
22
Ne from the Sun’s values but may be very different from the
atmospheric value.
285 Isotope geochemistry of rare gases
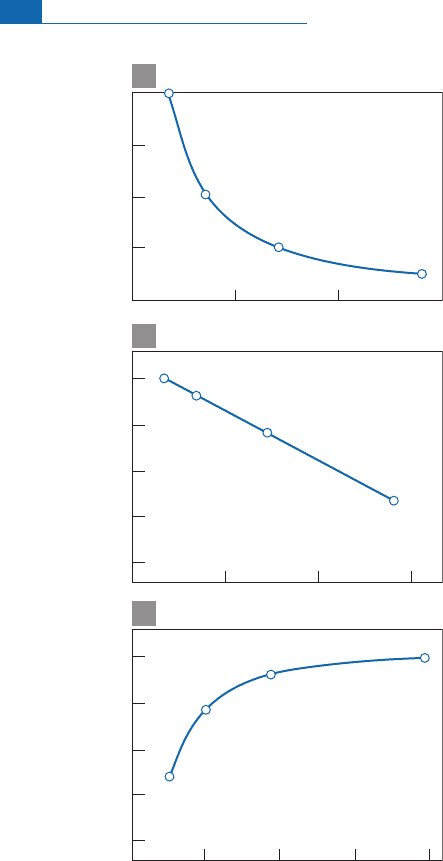
This circumstance is not fully elucidated
9
but is a godsend because, for each isotope ratio
measurement, the proportion contaminated by the Earth’s atmosphere can be calculated
and uncontaminated values obtained. Measurements on MORB and OIB by the Paris
87
Sr/
86
Sr
4
He/
3
He x 10
3
0.7035
10 20
0.7033
0.7029
0.7022
30
0.7030
0.7025
0.7020
0.7015
3
He/
4
He = NR
A
4
He/
3
He x 10
3
50 100
10
20
30
87
Sr/
86
Sr
3
He/
4
He = NR
A
0.7035
10 20 30 40
0.7030
0.7025
0.7020
0.7015
a
b
c
Figure 6.41 Results. (a)
4
He/
3
He
3
He/
4
He in
NR
A
. (b)
87
Sr/
86
Sr
4
He/
3
He linear correlation. (c) Same
relations but with
3
He/
4
He ¼
NR
A
notation.
9
It is thought that, early in the Earth’s history, the atmosphere was very hot and neon probably escaped
by isotope fractionation by a process known as hydrodynamic escape.
286 Radiogenic isotope geochemistry
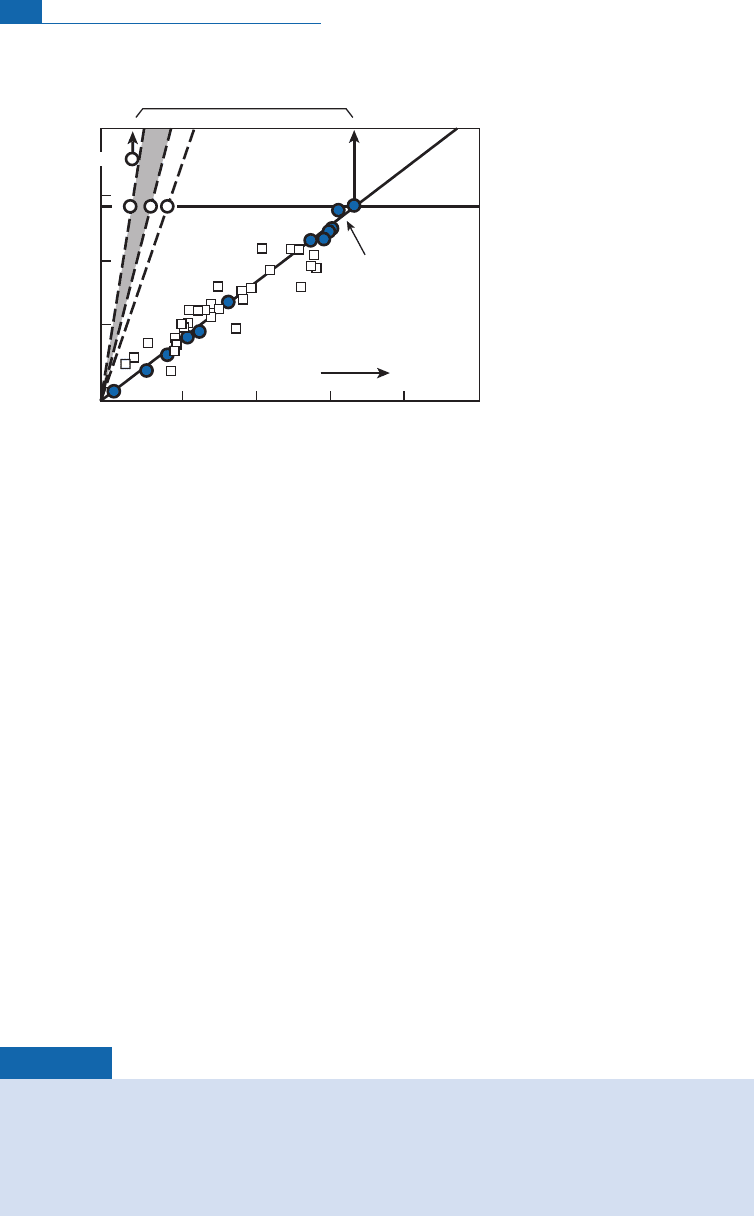
group (Sarda et al.,1988; Moreira and Alle
'
gre, 1998; Mor eira et al.,1998)canbeusedto
de¢ne two clearly distinct distributions (Figure 6.42). The experimental measurements
form straight lines, connecting the pure composition to be measured with the atmospheric
value. The MORB de¢nes a ‘‘low-angle’’ straight line p assing through the atmospheric
value. The OIB de¢nes steeper straight lines, close to the atmosphe re^Sun straight-line
segment.
We de¢ ne (
21
Ne/
22
Ne)* ratios,which areuncontaminate d bythe atmosphereattheinter-
sectofthehorizontallineofthesolar ratio(orslightlylower)withthe straightli nes (sample^
atmosphere). These (
21
Ne/
22
Ne)* ratios are automatically c orrected for atmosph eric
contamination.
Thesevalues are close to7.5 10
2
for MORB and to 3.8 10
2
for OIB. (By taking10
2
as
the unit, we deal with ¢gures like 7.5 or 3.8, which is handier.) Moreover, it can be seen that
intermediate values occur where hot spots underlie mid-oceanic ridges (Schilling e¡ect)
(Figure6.43).
Neon isotopes therefore con¢rm th e idea of the mantle being two reservoirs (an upper
highlydegass ed andlower moreprimitivem antle) describedfrom helium isotopes.
Exercise
Imagine we have a measurement of the raw isotope composition of neon of an oceanic basalt:
20
Ne/
21
Ne ¼11.5 and
21
Ne/
22
Ne ¼5 10
2
. Calculate (
21
Ne/
22
Ne)*.
Answer
(
21
Ne/
22
Ne)* ¼7 10
2
.
(
21
Ne/
22
Ne)*
21
Ne/
22
Ne
20
Ne/
22
Ne
MORB
Hawaii
Iceland
mass fractionation line
13
14
12
11
10
0.04 0.05 0.06
Nucleogenic
0.07
Upper
mantle
Terrestrial line
Air
Lower
mantle
Sun
Figure 6.42 Correlation diagrams for (
20
Ne/
22
Ne,
21
Ne/
22
Ne). Results for oceanic basalts are used to
define what is called [
21
Ne/
22
Ne]*.
287 Isotope geochemistry of rare gases
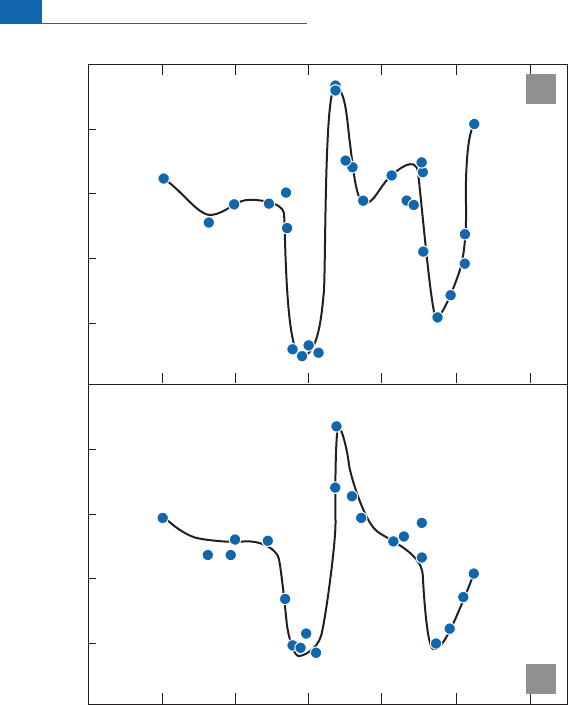
6.4.3 Isotope geochemistry of argon
This is undoubtedly theoldest formofraregasgeochemistry. It was¢rstintroducedbyPaul
Dam o n and Larry Kulp (1958)andbyKarl Ture ki a n (1959) when they were working at
Columbia University but su¡ered greatly from the di⁄culty in correcting the values meas-
ure d for atmospheric contamination. And yet, argon has considerable advantages com-
pared with other rare gases. It is retained by the atmosphere, meaning that a balance
equation canbewritten.Thereisnoinitial
40
Ar,as
40
Ar isnotmadein stellar nucleosynthesis
and is entirely p roduced by
40
K decay. However, the big disadvantage is its extreme
sensitivity to atmospheric pollution.Therefore aerial basalts, for example, are unsuitable for
measurement, as are many submarinebasalts. All of the
40
Ar/
36
Ar isotope ratios published
21
Ne/
22
Ne
extrap
Latitude (S)
ShonaDiscovery
0.04
0.06
0.08
0.10
46°44°42° 50°48° 54°52°
4
He/
3
He
0.04
0.06
0.08
0.10
a
b
Figure 6.43 Variation of
4
He/
3
He and [
21
Ne/
22
Ne]* ratios on the mid-Atlantic ridge between 428 and
548 S. There are two hot spots beneath the ridge in this area, with topographic effects known as the
Discovery and Shona seamounts. They are illustrations of the Schilling effect. In both cases the two hot
spots are clearly detected by helium and neon systematics. After Sarda
et
al
.(2000).
288 Radiogenic isotope geochemistry
