Alligood K., Sauer T., Yorke J.A. Chaos: An Introduction to Dynamical Systems
Подождите немного. Документ загружается.


C HAOS
Corollary 3.30 The logistic map g(x) ⫽ ax(1 ⫺ x), where 0 ⱕ a ⱕ 4,
has at most one periodic sink.
Proof: The map g has negative Schwarzian, so Theorem 3.29 applies. All
orbits that begin outside [0, 1] tend toward ⫺
⬁
, so no points in [0, 1] have an
infinite basin. Since the only critical point of g is x ⫽ 1 2, there can be at most
one attracting periodic orbit.
We found earlier in this chapter that for a ⫽ 4, all periodic orbits are sources.
Here is another way to see that fact, since when a ⫽ 4, the orbit with initial value
x ⫽ 1 2 maps in two iterates to the fixed source 0.
134

C HALLENGE 3
☞ C HALLENGE 3
Sharkovskii’s Theorem
A
CONTINUOUS MAP of the unit interval [0, 1] may have one fixed point and
no other periodic orbits (for example, f(x) ⫽ x 2). There may be fixed points,
period-two orbits, and no other periodic orbits (for example, f(x) ⫽ 1 ⫺ x).
(Recall that f has a point of period p if f
p
(x) ⫽ x and f
k
(x) ⫽ x for 1 ⱕ k ⬍ p.)
As we saw in Exercise T3.10, however, the existence of a periodic orbit of
period three, in addition to implying sensitive dependence on initial conditions
(Challenge 1, Chapter 1), implied the existence of orbits of all periods. We found
that this fact was a consequence of our symbolic description of itineraries using
transition graphs.
If we follow the logic used in the period-three case a little further, we can
prove a more general theorem about the existence of periodic points for a map
on a one-dimensional interval. For example, although the existence of a period-5
orbit may not imply the existence of a period-3 orbit, it does imply orbits of all
other periods.
Sharkovskii’s Theorem gives a scheme for ordering the natural numbers in
an unusual way so that for each natural number n, the existence of a period-n
point implies the existence of periodic orbits of all the periods higher in the
ordering than n. Here is Sharkovskii’s ordering:
3 Ɱ 5 Ɱ 7 Ɱ 9 Ɱ ...Ɱ 2 ⭈ 3 Ɱ 2 ⭈ 5 Ɱ ...Ɱ 2
2
⭈ 3 Ɱ 2
2
⭈ 5 Ɱ ...
...Ɱ 2
3
⭈ 3 Ɱ 2
3
⭈ 5 Ɱ ...Ɱ 2
4
⭈ 3 Ɱ 2
4
⭈ 5 Ɱ ... Ɱ 2
3
Ɱ 2
2
Ɱ 2 Ɱ 1.
Theorem 3.31 Assume that f is a continuous map on an interval and has a
period p orbit. If p Ɱ q, then f has a period-q orbit.
Thus, the existence of a period-eight orbit implies the existence of at least
one period-four orbit, at least one period-two orbit, and at least one fixed point.
The existence of a periodic orbit whose period is not a power of two implies the
existence of orbits of all periods that are powers of two. Since three is the “smallest”
natural number in the Sharkovskii ordering, the existence of a period-three orbit
implies the existence of all orbits of all other periods.
The simplest fact expressed by this ordering is that if f has a period-two
orbit, then f has a period-one orbit. We will run through the reason for this fact, as
it will be the prototype for the arguments needed to prove Sharkovskii’s Theorem.
135
C HAOS
Let x
1
and x
2
⫽ f(x
1
) be the two points of the period-two orbit. Since f(x
1
) ⫽ x
2
and f(x
2
) ⫽ x
1
, the continuity of f implies that the set f([x
1
,x
2
]) contains [x
1
,x
2
].
(Sketch a rough graph of f to confirm this.) By Theorem 3.17, the map f has a
fixed point in [x
1
,x
2
].
The proof of Sharkovskii’s theorem follows in outline form. We adopt the
general line of reasoning of (Block et al.; 1979). In each part, you are expected to
fill in an explanation. Your goal is to prove as many of the propositions as possible.
Assume f has a period p orbit for p ⱖ 3. This means that there is an x
1
such that f
n
(x
1
) ⫽ x
1
holds for n ⫽ p but not for any other n smaller than p.
Let x
1
⬍ ⭈⭈⭈ ⬍ x
p
be the periodic orbit points. Then f(x
1
)isoneofthex
i
,
but we do not know which one. We only know that the map f permutes the
x
i
.Inturn,thex
i
divide the interval [a, b] ⫽ [x
1
,x
p
]intop ⫺ 1 subintervals
[x
1
,x
2
], [x
2
,x
3
],...,[x
p⫺1
,x
p
]. Note that the image of each of these subintervals
contains others of the subintervals. We can form a transition graph with these
p ⫺ 1 subintervals, and form itineraries using p ⫺ 1 symbols.
Let A
1
be the rightmost subinterval whose left endpoint maps to the right
of itself. Then f(A
1
) contains A
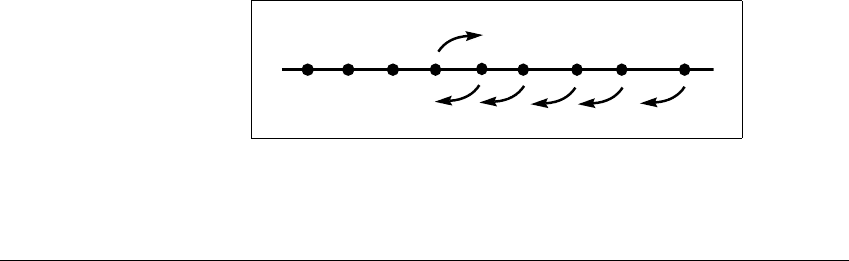
1
(see Figure 3.14 for an illustration of the p ⫽ 9
case).
Step 1 Recall that the image of an interval under a continuous map is an
interval. Use the fact that A 債 B ⇒ f(A) 債 f(B) to show that
A
1
債 f(A
1
) 債 f
2
(A
1
) 債 ....
(We will say that the subintervals A
1
,f(A
1
),f
2
(A
1
),... form an increasing
“chain” of subintervals.)
Step 2 Show that the number of orbit points x
i
lying in f
j
(A
1
)isstictly
increasing with j until all p points are contained in f
k
(A
1
) for a certain k. Explain
why f
k
(A
1
) contains [x
1
,x
p
]. Use the important facts that the endpoints of each
x
1
x
9
A
1
Figure 3.14 Definition of
A
1
.
A
1
is chosen to be the rightmost subinterval whose left-hand endpoint maps to the
right under f.
136

C HALLENGE 3
subinterval are obliged to map to the subinterval endpoints from the partition,
and that each endpoint must traverse the entire period-p orbit under f.
As a consequence of Step 2, the endpoints of A
1
cannot simply map among
themselves under f—there must be a new orbit point included in f(A
1
), since
p ⫽ 2. So at least one endpoint maps away from the boundary of A
1
, implying
that f(A
1
) contains not only A
1
but another subinterval, which we could call A
2
.
Step 3 Prove that either (1) there is another subinterval (besides A
1
)
whose image contains A
1
,or(2)p is even and f has a period-two orbit. [Hints:
By definition of period, there are no periodic orbits of period less than p among
the x
i
. Therefore, if p is odd, the x
i
on the “odd” side of A
1
cannot map entirely
amongst themselves, and cannot simply exchange points with the “even” side of
A
1
for arithmetic reasons. So for some subinterval other than A
1
, one endpoint
must be mapped to the odd side of A
1
and the other to the even side. If p is even,
the same argument shows that either there is another subinterval whose image
contains A
1
,orelsethex
i
on the left of A
1
map entirely to the x
i
on the right of
A
1
, and vice versa. In this case the interval consisting of all points to the left of
A
1
maps to itself under f
2
.]
Step 4 Prove that either (1) f has a periodic orbit of period p ⫺ 2in
[x
1
,x
p
], (2) p is even and f has a period-two orbit, or (3) k ⫽ p ⫺ 2. Alternative
(3) means that f(A
1
) contains A
1
and one other interval from the partition called
A
2
, f
2
(A
1
) contains those two and precisely one more interval called A
3
,andso
on. [Hint: If k ⱕ p ⫺ 3, use Step 3 and the Fixed-Point Theorem (Theorem 3.17)
to show that there is a length p ⫺ 2 orbit beginning in A
1
.]
Now assume that p is the smallest odd period greater than one for which f
has a periodic orbit. Steps 5 and 6 treat the concrete example case p ⫽ 9.
Step 5 Show that the endpoints of subintervals A
1
,...,A
8
map as in
Figure 3.15, or as its mirror image. Conclude that A
1
債 f(A
8
), and that the
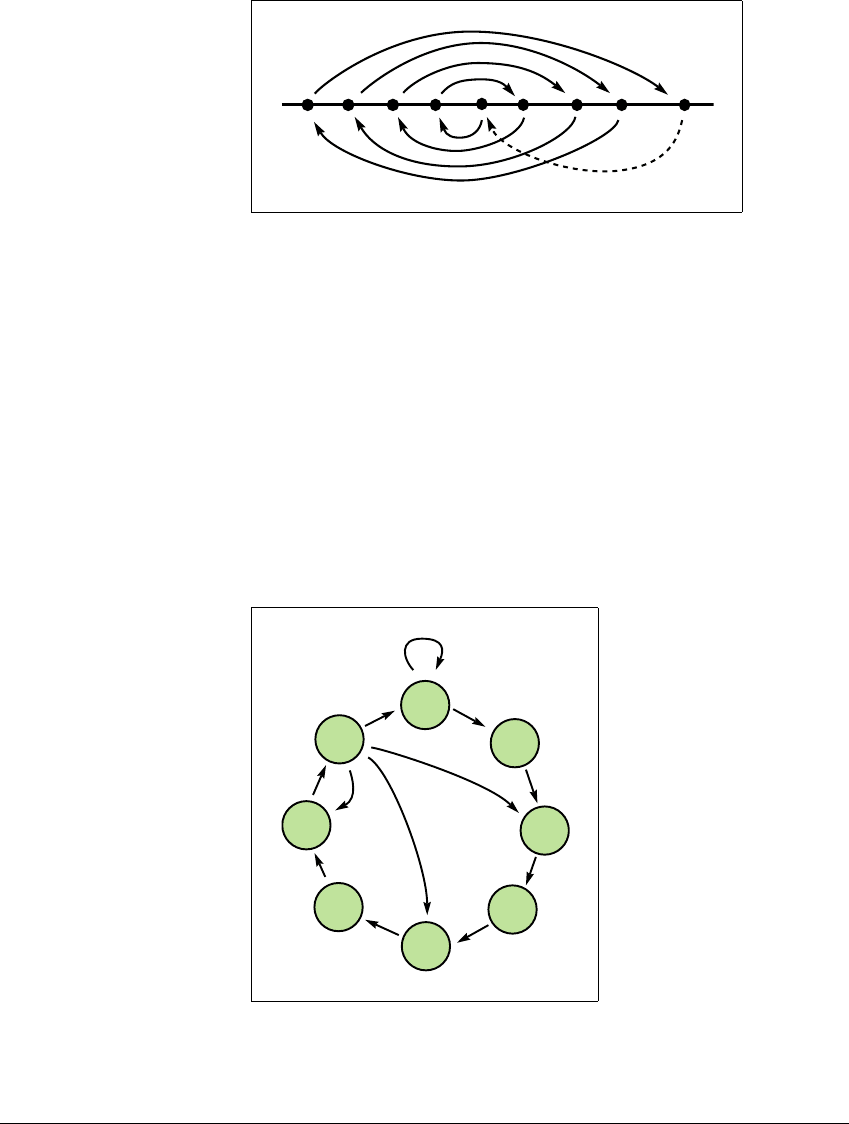
transition graph is as shown in Figure 3.16 for the itineraries of f. In particular,
A
8
maps over A
i
for all odd i.
Step 6 Using symbol sequences constructed from Figure 3.16, prove the
existence of periodic points of the following periods:
(a) Even numbers less than 9;
(b) All numbers greater than 9;
(c) Period 1.
This proves Sharkovskii’s Theorem for maps where 9 is the smallest odd period.
137
C HAOS
A
1
A
2
A
4
A
6
A
8
A
3
A
5
A
7
Figure 3.15 A map with a period-nine orbit and no period-three, -five, or
-seven orbits.
It must map subintervals as shown, or as the mirror image of this picture.
Step 7 In Steps 5 and 6, we assumed that the smallest odd period was 9.
Explain how to generalize the proof from 9 to any odd number greater than 1.
Note that Step 4 is not required for the p ⫽ 3 case.
Step 8 Prove that if f has a periodic orbit of even period, then f has a
periodic orbit of period-two. [Hint: Let p be the smallest even period of f.Either
Step 3 gives a period-two orbit immediately, or Step 4 applies, in which case Steps
5 and 6 can be redone with p even to get a period-two orbit.]
A
1
A
2
A
3
A
4
A
5
A
6
A
7
A
8
Figure 3.16 Transition graph for period-nine map.
The existence of orbits of periods 1, 2, 4, 6, 8, and all numbers greater than 9 is
implied by this graph.
138

C HALLENGE 3
Step 9 Let k be a positive integer and let f be any map such that f
2
k
has
a period r point x. Prove that x is a period 2
j
r point of f for some 0 ⱕ j ⱕ k.
Moreover, prove that if r is an even number, then j ⫽ k. (As always, by period we
mean minimum period.)
Step 10 Prove that if f has a periodic orbit of period 2
k
,thenf has periodic
orbits of periods 2
k⫺1
,...,4, 2, 1. (Since f
2
k⫺2
has a period-four point, it has a
period-two point, by Step 8. Ascertain the period of this orbit as an orbit of f.)
Step 11 Assume p ⫽ 2
k
q is the leftmost number on the list for which f
has a period-p point, where q is an odd number greater than 1. The integers to
the right of p in Sharkovskii’s ordering are either powers of 2 or are of form 2
k
r,
where r is either an even number or a number greater than q.Sincef
2
k
has a
period-q orbit, Step 7 implies that f
2
k
has orbits of this form. Use Step 9 to check
that these orbits are orbits of f of period 2
k
r. Use these periodic orbits and Step
10 to complete the proof of Sharkovskii’s Theorem.
139

C HAOS
E XERCISES
3.1. Let f
a
(x) ⫽ a ⫺ x
2
,wherea is a constant.
(a) Find a value a
1
of the parameter a for which f
a
has exactly one fixed point.
(b) Describe the limit of all orbits of f
a
for a ⬍ a
1
.
(c) The map f
a
has an attracting fixed point for a in the open interval (a
1
,a
2
).
Find a
2
.
(d) The map f
a
has an attracting period-two point for a in the open interval
(a
2
,a
3
). Find a
3
.
(e) Describe the dynamics of f
a
for a ⫽ 2.
3.2. Decide on a partition for the map f(x) ⫽ 2x (mod 1) on [0, 1], and draw its transition
graph and schematic itineraries as in Figure 3.2(a)(b). How do they differ from those
for the tent map?
3.3. (a) Find a conjugacy C between G(x) ⫽ 4x(1 ⫺ x)andg(x) ⫽ 2 ⫺ x
2
.
(b) Show that g(x) has chaotic orbits.
3.4. Show that g(x) ⫽ 2.5x(1 ⫺ x) has no chaotic orbits.
3.5. (a) Sketch a graph of the map f(x) ⫽⫺2x
2
⫹ 8x ⫺ 5.
(b) Find a set of two subintervals that form a partition.
(c) Draw the transition graph for f. What are the possible periods for periodic
orbits?
3.6. Repeat the previous exercise with f(x) ⫽ 2x(1 ⫺ x).
3.7. Let T be the tent map. Prove that the periodic points of T are dense in I.
3.8. Assume that 兵x
1
,x
2
,...,x
9
其 is a periodic orbit for a continuous map f on the real
line, with x
1
⬍ ⭈⭈⭈ ⬍ x
9
. Assume f(x
i
) ⫽ x
i⫹1
if i ⬍ 9andf(x
9
) ⫽ x
1
.
(a) What periods does Sharkovskii’s Theorem guarantee on the basis of the
period-nine orbit?
(b) Draw the transition graph for this map. Which periods are certain to exist?
3.9. Assume that x
1
⬍ x
2
⬍ x
3
⬍ x
4
are points on the real line, and that f is a continuous
map satisfying f(x
1
) ⫽ x
2
,f(x
2
) ⫽ x
4
,f(x
3
) ⫽ x
1
and f(x
4
) ⫽ x
3
. For simplicity,
assume that f is monotonic (increasing or decreasing) except possibly at the four
points mentioned.
140

E XERCISES
(a) Sketch a graph of f.
(b) Draw the transition graph for f.
(c) What periods must exist?
3.10. Assume that x
1
⬍ ⭈⭈⭈ ⬍ x
5
are points on the real line, and that f is a continuous
map satisfying f(x
1
) ⫽ x
3
,f(x
2
) ⫽ x
5
,f(x
3
) ⫽ x
4
,f(x
4
) ⫽ x
2
and f(x
5
) ⫽ x
1
. Assume
that f is monotonic (increasing or decreasing) except possibly at the five points
mentioned.
(a) Sketch a graph of f.
(b) Draw the transition graph for f.
(c) What periods must exist?
3.11. Let f be a continuous map from the unit interval onto itself (that is, such that
f([0, 1]) ⫽ [0, 1]).
(a) Prove that f must have at least one fixed point.
(b) Prove that f
2
must have at least two fixed points. (Hint: Explain why either
f or f
2
must have points 0 ⱕ x
1
⬍ x
2
ⱕ 1suchthatx
1
mapsto0andx
2
maps
to 1.)
(c) If in addition 0 and 1 are not fixed points for f, show that f
2
must have at
least 3 fixed points.
3.12. Let f(x) ⫽ rx(1 ⫺ x), r ⬎ 2 ⫹
5. Show that the Lyapunov exponent of any orbit
that remains in [0, 1] is greater than zero, if it exists.
3.13. Let n be a positive integer, and f(x) ⫽ nx (mod 1) on [0, 1]. Which points are
periodic, eventually periodic, and asymptotically periodic? Which orbits of f are
chaotic orbits?
3.14. (From Seth Patinkin) Let I be an interval in the real line and f a continuous
function on I. The goal of this exercise is to prove the following theorem:
Assume there is an initial value whose orbit is dense in I and assume f
has two distinct fixed points. Then there must be a point of period 3.
The following two facts are to be used as help in proving the theorem.
(i) Suppose there is a point a in I such that a ⬍ f(a)andthereisab ⬍ a in I
such that f(b) ⱕ b and there is a k ⬎ 1suchthatf
k
(a) ⱕ b. Then there is a point of
period 3. (Of course it is possible to reverse the inequalities, f(a) ⬍ a ⬍ b ⱕ f(b),
and b ⱕ f
k
(a), to again get a true statement.)
(ii) Assume there is no period 3 point and that there is some orbit dense in I.
Then there is a unique fixed point c and x ⫺ c and f(x) ⫺ x always have opposite
signs for every x ⫽ c in I.LetA ⫽ 兵x : f(x) ⬎ x其 and B ⫽ 兵x : f(x) ⬍ x其.Use(i)to
show that A and B must each be an interval.
141

C HAOS
Discussion: In Chapters 1 and 3 we discussed that implications of having a
period 3 orbit. It implies the existence of periodic orbits of all other periods and
it implies sensitivity to initial data. This exercise provides a partial converse. The
assumption that some orbit is dense in an interval is quite reasonable. It can be
shown that for any piecewise expanding map F (see Chapter 6), the orbit of almost
every initial point is dense in the union of a finite number of intervals, and for some
k the piecewise expanding map F
k
has the property that the orbit of almost every
point in dense in an interval. The proof of these results is beyond the scope of this
book. We could choose such an interval to be I and f ⫽ F
k
to be our function. It
might or might not have two fixed points.
3.15. (Party trick.) (a) A perfect shuffle is performed by dividing a 52-card deck in half,
and interleaving the halves, so that the cards from the top half alternate with the
cards from the bottom half. The top card stays on top, and so it and the bottom
card are fixed by this operation. Show that 8 perfect shuffles return the deck to its
original order. [Hint: Number the original card order from 0 to 51. Then a perfect
shuffle can be expressed as the map
f(n) ⫽
2n if 0 ⱕ n ⱕ 25
2n ⫺ 51 if 26 ⱕ n ⱕ 51
The goal is to show that all integers are fixed points under f
8
. First show that
f
8
(n) ⫽ 2
8
n ⫺ 51k for some integer k,wherek may be different for different n.]
Caution: when demonstrating at actual parties, be sure to remove the jokers first!
If the deck consists of 54 cards, then 52 perfect shuffles are required.
(b) If the bottom card 51 is ignored (it is fixed by the map anyway), the
above map is f(x) ⫽ 2x (mod 51), where we now consider x to be a real number.
Nonperiodic orbits have Lyapunov number equal to 2, yet every integer point is
periodic with period a divisor of 8. Sharkovskii’s Theorem shows that it is typical for
chaotic maps to contain many periodic orbits. Find all possible periods for periodic
orbits for this map on the interval [0, 51].
3.16. Define the map
f(x) ⫽
1 ⫺ 3x if 0 ⱕ x ⱕ 1 3
⫺1 3 ⫹ x if 1 3 ⱕ x ⱕ 1
Note that x ⫽ 0 is a period 4 point. Let I ⫽ [0, 1 3], J ⫽ [1 3, 2 3] and K ⫽
[2 3, 1].
(a) Draw the transition graph.
(b) For each periodic orbit of period p ⬍ 6, label the points of the orbit as
a
1
⬍ a
2
⬍ ... ⬍ a
p
and list the itinerary of a
1
and show where each point of
the orbit maps. For example, there is a period two point IJ which is the itinerary
including a
1
⬍ a
2
,wheref(a
1
) ⫽ a
2
,andf(a
2
) ⫽ a
1
. (In this case, a
1
⫽ 1 6and
a
2
⫽ 1 2, but you need not list values for the a
i
in general.)
(c) Show that there is no period 4 orbit with the itinerary IJIJ.
142

L AB V ISIT 3
☞ L AB V ISIT 3
Periodicity and Chaos in a Chemical Reaction
I
N ELEMENTARY chemistry classes, a great deal of emphasis is placed on
finding the equilibrium state of a reaction. It turns out that equilibria present only
one facet of possible behavior in a chemical reaction. Periodic oscillations and
even more erratic behaviors are routinely observed in particular systems.
Studies on oscillating reactions originally focused on the Belousov-
Zhabotinskii reaction, in which bromate ions are reduced by malonic acid
in the presence of a catalyst. The mechanism of the reaction is complicated.
More than 20 species can be identified at various stages of the reaction. Experi-
ments on this reaction by a group of researchers at the University of Texas were
conducted in a continuous-flow stirred tank reactor (CSTR), shown schemati-
cally in Figure 3.17. The solution is stirred at 1800 rpm by the impeller. There is
a constant flow in and out of the tank, perfectly balanced so that the total fluid
volume does not change as the reaction proceeds. The feed chemicals are fixed
concentrations of malonic acid, potassium bromate, cerous sulfate, and sulfuric
acid. The flow rate is maintained as a constant throughout the reaction, and the
bromide concentration is measured with electrodes immersed in the reactor. The
output of the experiment is monitored solely through the bromide measurement.
The constant flow rate can be treated as a system parameter, which can be
changed from time to time to look for qualitative changes in system dynamics,
or bifurcations. Figure 3.18 shows several different periodic behaviors of the
bromide concentration, for different settings of the flow rate. The progression
of flow rate values shown here is decreasing in the direction of the arrows and
results in periodic behavior of periods 6, 5, 3, 5, 4, 6, and 5. Each oscillation (an
up and down movement of the bromide concentration) takes around 2 minutes.
Approximately one hour was allowed to pass between changes of the flow rate to
allow the system to settle into its asymptotic behavior.
Roux, J.-C., Simoyi, R.H., Swinney, H.L., “Observation of a strange attractor”. Physica
D 8, 257-266 (1983).
Coffman, K.G., McCormick, W.D., Noszticzius, Z., Simoyi, R.H., Swinney, H.L., “Uni-
versality, multiplicity, and the effect of iron impurities in the Belousov-Zhabotinskii
reaction.” J. Chemical Physics 86, 119-129 (1987).
143
