ASM Metals HandBook Vol. 8 - Mechanical Testing and Evaluation
Подождите немного. Документ загружается.


31. P.F. Thomason, Ductile Fracture of Metals, Pergamon Press, Oxford, 1990
32. J.R. Rice and M.A. Johnson, Inelastic Behavior of Solids, M.F. Kanninen, Ed., McGraw-Hill, New
York, 1970, p 641
33. J. R. Rice, Fracture—An Advanced Treatise, H. Liebowitz, Ed., Academic Press, New York, 1968, p
191
Introduction to the Mechanical Behavior of Metals
Todd M. Osman, U.S. Steel Research; Joseph D. Rigney, General Electric Aircraft Engines
Selected References
Structure of Metals
• D.A. Porter and K.E. Easterling, Phase Transformations in Metals and Alloys, Van Nostrand Reinhold,
Birkshire, UK, 1987
• C.R. Barrett, W.D. Nix, and A.S. Tetelman, The Principles of Engineering Materials, Prentice-Hall,
Inc., Englewood Cliffs, New Jersey, 1973
• W. Hume-Rothery and G.V. Raynor, The Structure of Metals and Alloys, The Institute of Metals,
London, 1956
• D. Hull and D.J. Bacon, Introduction to Dislocations, Pergamon Press, London, 1984
• P.B. Hirsch, Ed., Defects, Vol 2, The Physics of Metals, Cambridge University Press, Cambridge, 1975
• U.F. Kocks, C.N. Tome, and H.-R. Wenk, Texture and Anisotropy, Cambridge University Press,
Cambridge, 1998
• D. Hull, An Introduction to Composite Materials, Cambridge University Press, 1975
Deformation of Metals and Strength of Metals
• M.A. Meyers and K.K. Chawla, Mechanical Metallurgy: Principles and Applications, Prentice Hall,
Inc., 1984
• J.M. Gere and S.P. Timoshenko, Mechanics of Materials, 2nd ed., PWS Publishers, 1984
• T.H. Courtney, Mechanical Behavior of Materials, McGraw-Hill, New York, 1990
• J.W. Martin, Micromechanisms in Particle Hardened Alloys, Cambridge University Press, 1980
• P.F. Thomason, Ductile Fracture of Metals, Pergamon Press, Oxford, 1990
• R.W.K. Honeycombe, The Plastic Deformation of Metals, 2nd ed., Edward Arnold, London, 1984
Special Conditions in Flow and Fracture
• J.F. Knott, Fundamentals of Fracture Mechanics, Butterworths, 1981
• B.R. Lawn and T.R. Wilshaw, Fracture of Brittle Solids, Cambridge University Press, 1975
• H.L. Ewalds and R.J.H. Wanhill, Fracture Mechanics, Edward Arnold, London, 1985
• D. Broek, Elementary Engineering Fracture Mechanics, Martinus Nishoff Publishers, 4th ed.,
Dordrecht, Netherlands, 1987
Introduction to the Mechanical Behavior of
Nonmetallic Materials
M.L. Weaver and M.E. Stevenson, The University of Alabama, Tuscaloosa

Introduction
MANY DIFFERENT types of materials are used in applications where a resistance to mechanical loading is
necessary. The type of material used depends strongly upon a number of factors including the type of loading
that the material will experience and the environment in which the materials will be loaded. Collectively known
as engineering materials (Ref 1), they can be pure elements, or they can be combinations of different elements
(alloys and compounds), molecules (polymers), or phases and materials (composites). All solid materials are
typified by the presence of definite bonds between component atoms or molecules. Ultimately, it is the type of
bonding present that imparts each class of materials with distinct microstructural features and with unique
mechanical and physical properties.
Crystalline solids exhibit atomic or molecular structures that repeat over large atomic distances (i.e., they
exhibit long-range-ordered, LRO, structures) whereas noncrystalline solids exhibit no long-range periodicity.
The atomic and molecular components of both crystalline and noncrystalline solids are held together by a series
of strong primary (i.e., ionic, covalent, and metallic) and/or weak secondary (i.e., hydrogen and Van der Waals)
bonds. Primary bonds are usually more than an order of magnitude stronger than secondary bonds. As a result,
ceramics and glasses, which have strong ionic-covalent chemical bonds, are very strong and stiff (i.e., they
have large elastic moduli). They are also resistant to high temperatures and corrosion, but are brittle and prone
to failure at ambient temperatures. In contrast, thermoplastic polymers such as polyethylene, which have weak
secondary bonds between long chain molecules, exhibit low strength, low stiffness, and a susceptibility to creep
at ambient temperatures. These polymers, however, tend to be extremely ductile at ambient temperatures.
In this article, some of the fundamental relationships between microstructure and mechanical properties are
reviewed for the major classes of nonmetallic engineering materials. The individual topics include chemical
bonding, crystal structures, and their relative influences on mechanical properties. The present article has been
derived in structure and content from the article “Fundamental Structure-Property Relationships in Engineering
Materials,” in Materials Selection and Design, Volume 20 of ASM Handbook (Ref 2). In light of the
bewildering number of different engineering materials within each class, discussions were limited to a number
of general examples typifying the general features of the major classes of nonmetallic materials.
References cited in this section
1. N.E. Dowling, Mechanical Behavior of Materials: Engineering Methods for Deformation, Fracture,
and Fatigue, 2nd ed., Prentice Hall, 1999, p 23
2. T.H. Courtney, Materials Selection and Design, Vol 20, ASM Handbook, ASM International, 1997, p
336–356
Introduction to the Mechanical Behavior of Nonmetallic Materials
M.L. Weaver and M.E. Stevenson, The University of Alabama, Tuscaloosa
General Characteristics of Solid Materials
Engineering materials can be conveniently grouped into five broad classes: metals, ceramics and glasses,
intermetallic compounds, polymers, and composite materials. Metals, ceramics and glasses, polymers, and
composites represent the most widely utilized classes of engineering materials, whereas intermetallic
compounds (i.e., intermetallics), which are actually subcategories of metals and ceramics, are an emerging class
of monolithic materials. The general features of five major classes of materials are summarized in Fig. 1 and are
described in the following sections. Though this article deals with the properties of nonmetallic materials, a
brief discussion of the general characteristics of metallic materials is included where pertinent.
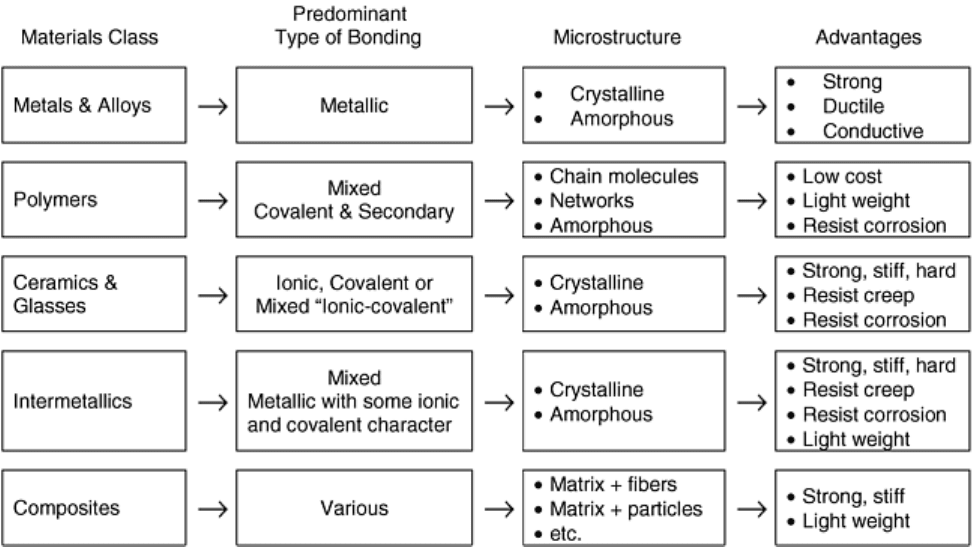
Fig. 1 General characteristics of major classes of engineering materials. Adapted from Ref 3
Metals
Metals represent the majority of the pure elements and form the basis for the majority of the structural
materials. The mechanical behavior of metals depends on a combination of microstructural and macrostructural
features, which ultimately depend upon bonding, chemical composition, and mode of manufacture. Metals are
held together by metallic bonds. Metallic bonds arise because on an atomic scale, the outer electron shells in
metals are less than half full. As a result, each atom donates its available outer shell (i.e., valence) electrons to
an electron cloud that is collectively shared by all of the atoms in the solid. This is referred to as metallic
bonding and is responsible for the high elastic moduli and the high thermal and electrical conductivity exhibited
by metals. Many metals also exhibit a limited solid solubility for other atoms (i.e., one metal can dissolve into
another). Consequently, engineers can often vary their properties by varying composition. In terms of atomic
arrangements, metals also have large coordination numbers (CNs), typically 8 to 12, which account for their
relatively high densities. Metals, by their nature, tend to be ductile in comparison to other engineering materials
and exhibit a high tolerance for stress concentrations. As such, many metals can deform locally to redistribute
load. Structurally, metals are generally crystalline, though amorphous structures (i.e., metallic glasses) are
possible using special processing techniques. Further information concerning structure-property relationships in
metals is provided in the article “Introduction to the Mechanical Behavior of Metals” in this volume.
Ceramics and Glasses
Ceramics and glasses include a broad range of inorganic materials containing nonmetallic and metallic
elements. Like metals, these materials can be formed directly from the melt or via powder processing
techniques (e.g., sintering or hot isostatic pressing) and their mechanical properties depend on structural (i.e.,
microstructural and macrostructural) features and chemical composition. They differ from metals in that strong
ionic, covalent, or intermediate bonds, which often result in higher hardness, stiffness, and melting
temperatures compared with metals, hold them together.
Ionic bonding occurs in compounds containing electropositive (i.e., metals, atoms on the left side of the
periodic table) and electronegative (i.e., nonmetals, atoms on the right side of the periodic table) elements. This
type of bonding involves the transfer of electrons whereby electropositive elements readily donate their valence
electrons to the electronegative elements, allowing the establishment of stable outer shell configurations in each
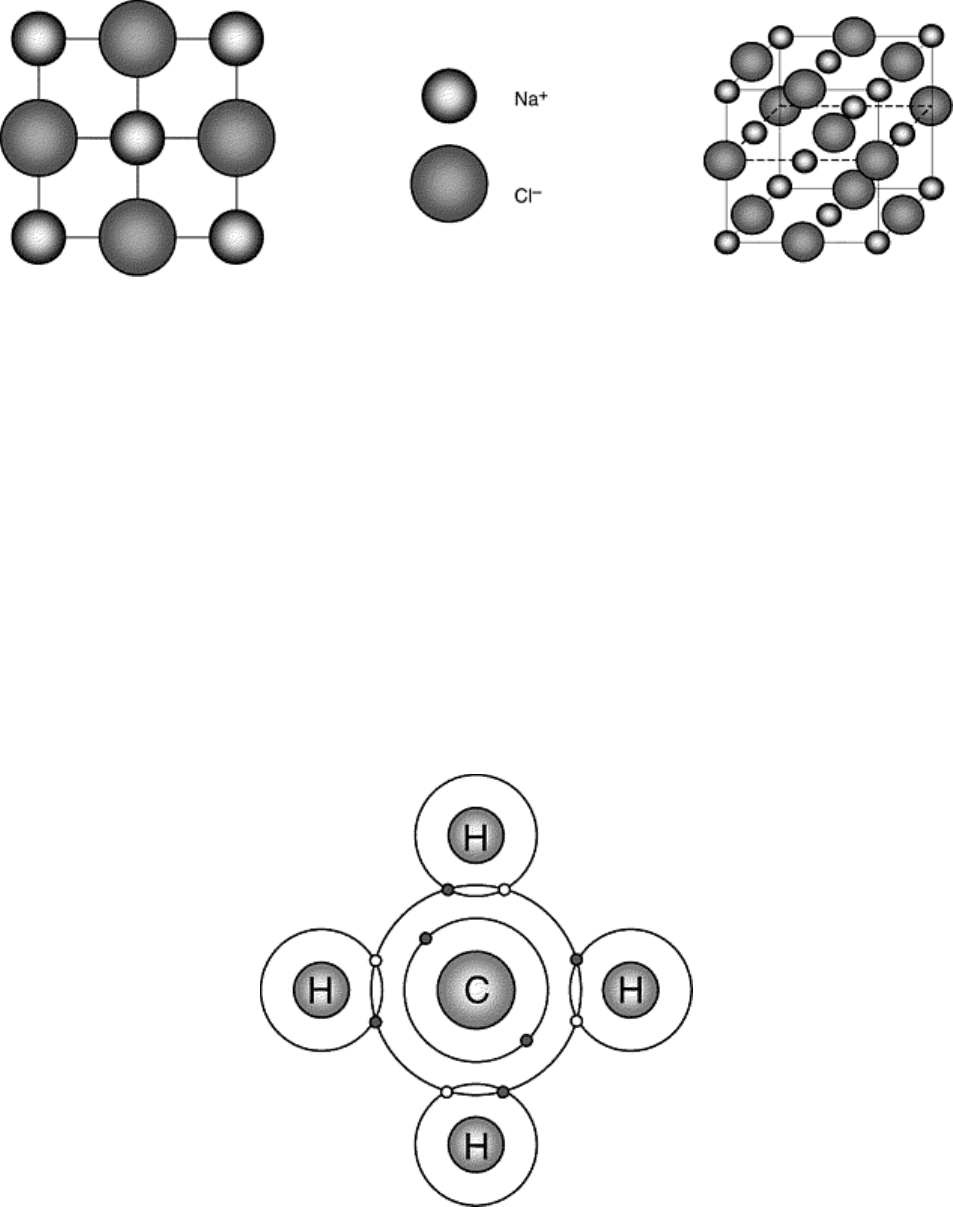
element. Figure 2 depicts ordinary table salt, NaCl, which is a perfect example of an ionically bonded solid. In
ionic solids, the coordination number (CN), which is defined as the number of cation/anion (i.e., positive
ion/negative ion) nearest neighbors, are typically lower than those in metals, which accounts for their slightly
lower densities compared with metals. Ionic solids are typically hard and brittle, and electrically and thermally
insulative (i.e., with lower electrical and thermal conductivity than metals). The insulative properties are a
direct result of the electron configurations within the ionic bond. Ionic solids usually form only in
stoichiometric proportions (e.g., NaCl and Al
2
O
3
), which cause them to have little tolerance for alloying.
Fig. 2 Schematic representation of ionically bonded NaCl. Note that this structure consists of Na
+
and Cl
-
ions sitting on interpenetrating fcc Bravais lattices.
Covalent bonding occurs in compounds containing electronegative elements. Covalent bonding involves the
sharing of valence electrons with specific neighboring atoms. This is schematically illustrated for methane
(CH
4
) in Fig. 3. For a covalent bond to occur between C and H, for example, each atom must contribute at least
one electron to the bond. These electrons are shared by both atoms, resulting in a strong directional bond
between atoms. The number of covalent bonds that form depends on the number of valence atoms that are
available in each atom. In methane, carbon has four valence electrons, while each hydrogen atom has only one.
Thus, each hydrogen atom can acquire one valence electron to fill its outer orbital shell. Similarly, each carbon
atom can accommodate four valence electrons to fill its outer shell. This type of bonding makes covalent solids
strong, brittle, and highly insulative because electrons are incapable of detaching themselves from their parent
and moving freely through the solid. Covalent solids also have lower CNs due to this localized electron sharing
resulting in lower densities. For example, diamond, which is an elemental covalent compound, has a CN of 4
(Fig. 4). Like ionic solids, covalent solids tend to exhibit very narrow composition ranges and exhibit little
tolerance for alloying additions. Examples of covalent molecules, elements, and compounds include H
2
O,
HNO
3
, H
2
, diamond, silicon, GaAs, and SiC.
Fig. 3 Schematic representation of covalent bonding in methane (CH
4
)
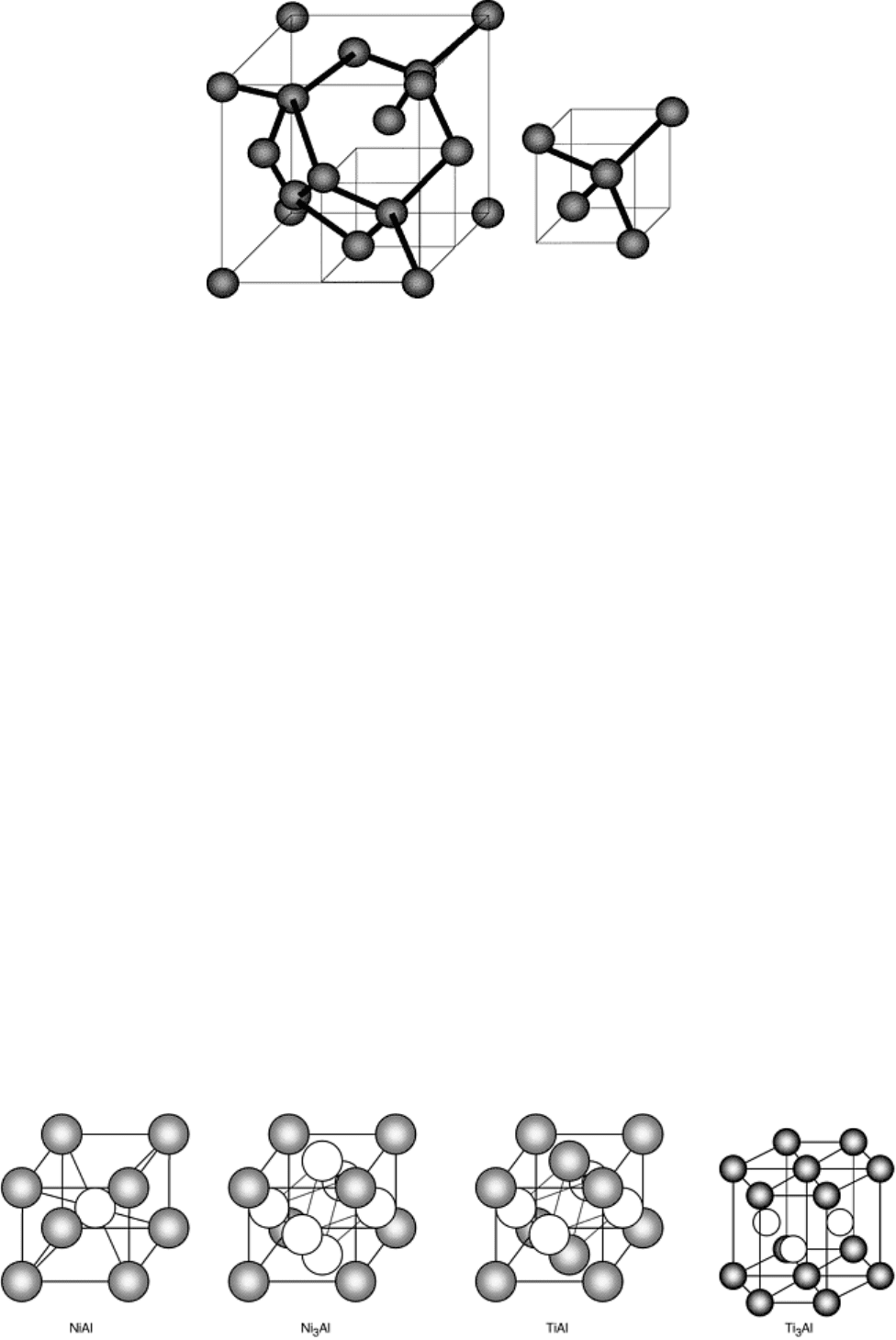
Fig. 4 An example of perfect covalent bonding in diamond
In general, most ceramic compounds exhibit a mixture of ionic and covalent bonding, the degree of which
depends on the positions of the constituent elements on the periodic table. For elements exhibiting a greater
difference in electronegativity, bonding tends to be more ionic, while for elements with smaller differences,
bonding tends to be more covalent. Solids that exhibit mixed bonding, which are termed polar covalent solids,
often exhibit high melting points and elastic moduli. Silica (SiO
2
) is a good example of a polar covalent solid.
Ceramics and glasses have higher elastic moduli than most metals and exhibit extremely high strengths when
deformed in compression. The presence of strong ionic and covalent bonds allows these materials to retain their
strength to high temperature and makes them extremely resistant to corrosion. However, these same bonds
render ceramics and glasses brittle at ambient temperatures, resulting in little tolerance for stress concentrations
(e.g., holes, cracks, and flaws) and usually in catastrophic failure during tensile or shear loading.
Intermetallic Compounds
In some cases, intermetallic compounds can form within alloys. These materials are composed of two or more
metallic or metalloid constituents and exhibit crystal structures that are distinctly different from its constituents.
Unlike solid solution alloys, these mixtures form stoichiometric compounds (e.g., NiAl, Ni
3
Al, TiAl, and
Ti
3
Al), and their bonding is typically a combination of metallic, ionic, and/or covalent types. In terms of
mechanical and physical properties, intermetallics occupy a position between metals and ceramics. As in the
case of ionic and covalent solids, extremely strong bonds exist between unlike constituents, which imparts
intermetallics with lower CNs and densities than metals, highly directional properties, higher stiffness and
strength, and good resistance to temperatures or chemical attack. These materials, which have intrinsically high
strengths and elastic moduli, are often used in precipitate form to strengthen commercial alloys (e.g., Ni
3
Al in
Ni-base superalloys), and their low densities and high microstructural stability makes them attractive for use in
high-temperature structural applications such as turbine blades, exhaust nozzles, and automotive valves.
Examples of some typical intermetallic compounds are illustrated in Fig. 5. Of the more than 25,000 known
intermetallic compounds, recent emphasis has focused on the development of NiAl, FeAl, Ni
3
Al, TiAl, and
MoSi
2
base alloys for use as monolithic alloys in structural applications (Ref 4). As in the cases of ceramics and
glasses, the same bonds that impart intermetallics with high strengths render most of them with low ductility
and fracture toughness at ambient temperatures.
Fig. 5 Some simple intermetallic crystal structures
Polymers
Polymers are long chain molecules (macromolecules) consisting of a series of small repeating molecular units
(monomers). Most common polymers have carbon (organic material) backbones, though polymers with
inorganic backbones (e.g., silicates and silicones) are possible. Polymeric materials exhibit strong covalent
bonds within each chain; however, individual chains are frequently linked via secondary bonds (i.e., van der
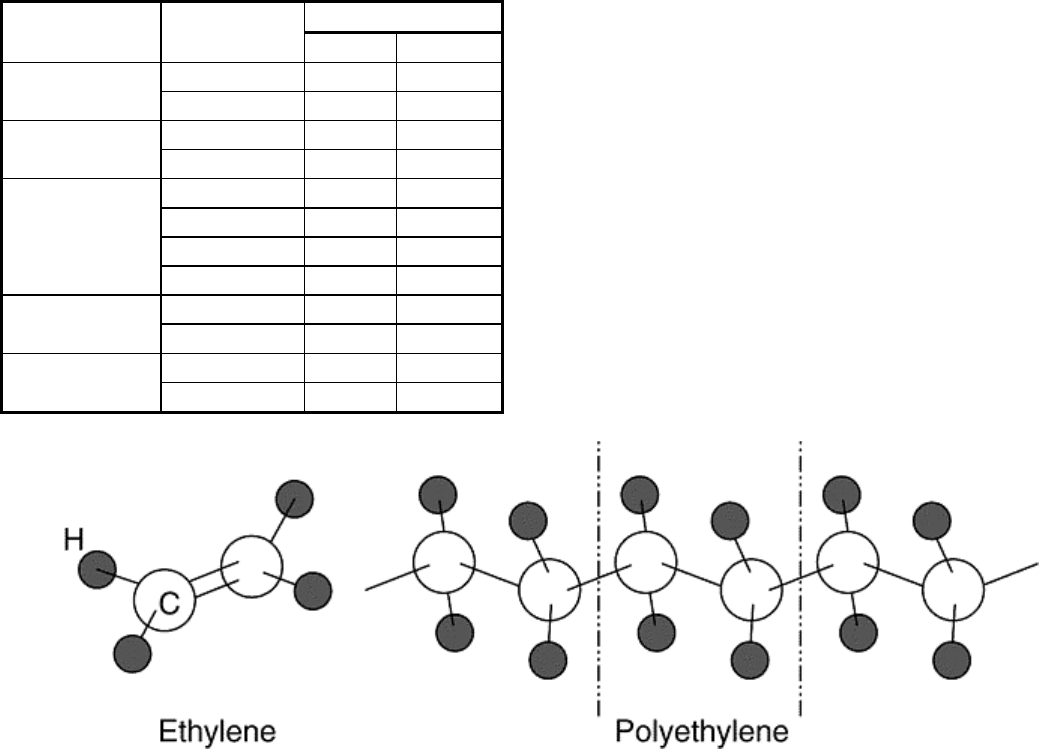
Waals, hydrogen, and so on) though cross-linking via primary bonds is possible. The polymer polyethylene, for
example, forms when the double bond between carbon atoms in the ethylene molecule (C
2
H
4
) is replaced by a
single bond to each of the adjacent carbon atoms, resulting in a long chain molecule (Fig. 6). In polymeric
materials, secondary bonds arise from atomic or molecular dipoles that form when positively charged and
negatively charged regions of an atom or molecule separate. Bonding results from coulombic attraction
between the positive and negative regions of adjacent dipoles as illustrated schematically in Fig. 7. These types
of interactions occur between induced dipoles, between induced dipoles and polar molecules, and between polar
molecules. The secondary bonds holding adjacent macromolecules together in Fig. 8 are a direct result of the
formation of molecular dipoles along the length of the polymer chain. Secondary bonds are much weaker than
primary bonds as indicated in Table 1, which accounts for the low melting temperatures, low stiffness, and low
strength exhibited by many polymers. Table 1 Bond energies for various materials
Bond energy
Bond type Material
kJ/mol
kcal/mol
NaCl 640
153
Ionic
MgO 1000
239
Si 450
108
Covalent
C (diamond)
713
170
Hg 68
16
Al 324
77
Fe 406
97
Metallic
W 849
203
Ar 7.8
1.8
van der Waals
Cl
2
31
7.4
NH
3
35
8.4
Hydrogen
H
2
O 51 12.2
Source: Ref 5
Fig. 6 Schematic representation of ethylene and polyethylene
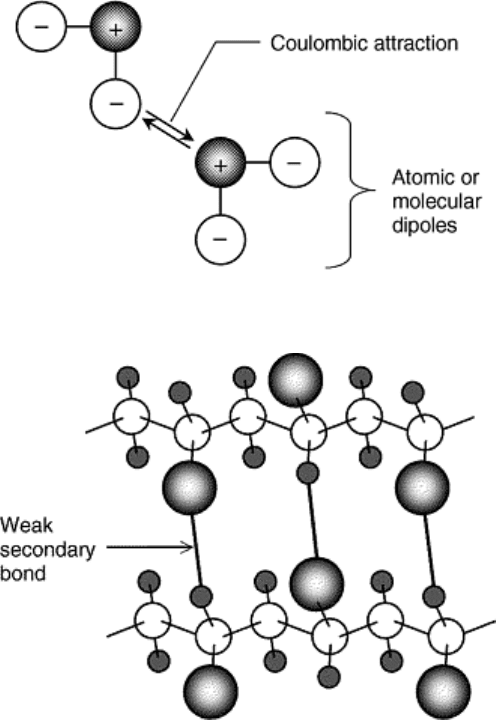
Fig. 7 Schematic representation of secondary bonding between two molecular dipoles
Fig. 8 Schematic representation of secondary bonding between two polymer chains
In comparison to metals, intermetallics, and ceramics and glasses, polymers have very low CNs, which is part
of the cause for their low densities; however, they also consist primarily of light atoms such as C and H, which
tends to result in lower density. The localized nature of electrons in polymers renders them good electrical
insulators and poor thermal conductors.
There are three categories of polymers: thermoplastics, thermosetting plastics, and elastomers. Thermoplastics
have linear chain configurations where chains are joined by weak secondary bonds as described above. These
materials often melt upon heating but return to their original solid condition when cooled. In thermosetting
polymers, covalent cross-links or strong hydrogen bonds occur between polymer chains resulting in three-
dimensional networks of cross-linked molecular chains. Phenol formaldehyde, or Bakelite, is a good example.
Thermosettings change chemically during processing and will not melt upon reheating. Instead, they will
remain strong until they break down chemically via charring or burning. Elastomers differ from thermoplastics
and thermosetting polymers in that they are capable of rubbery behavior and are capable of very large amounts
of recoverable deformation (often in excess of 200%). Structurally, these materials consist of networks of
heavily coiled and heavily cross-linked polymer chains, which impacts higher strengths than thermoplastics by
inhibiting the sliding of polymer chains past each other. Elastomers are typified by natural rubber and by a
series of synthetic polymers exhibiting similar mechanical behavior (e.g., polyisoprene).
Composites
Composites are relatively macroscopic arrangements of phases or materials designed to take advantage of the
most desirable aspects of each. As a result, the strengths and/or physical properties of composites are usually an
average of the strengths and/or properties of the individual constituents/phases. Most composites are composed
of a compliant, damage-tolerant matrix and a strong reinforcing phase/constituent, usually filaments, fibers, or
whiskers, that are too brittle for use in a monolithic form. In composites with brittle matrices (e.g., ceramic
matrix composites), the reinforcing constituent may toughen the material more than strengthen it.

References cited in this section
3. N.E. Dowling, Mechanical Behavior of Materials: Engineering Methods for Deformation, Fracture,
and Fatigue, 2nd ed., Prentice Hall, 1999, p 24
4. G. Sauthoff, Intermetallics, VCH Publishers, New York, 1995
5. W.D. Callister, Materials Science and Engineering—An Introduction, 4th ed., John Wiley & Sons,
1997, p 21
Introduction to the Mechanical Behavior of Nonmetallic Materials
M.L. Weaver and M.E. Stevenson, The University of Alabama, Tuscaloosa
Structures of Materials
Depending upon the application, engineering materials can be pure elements such as silicon, compounds such
as aluminum oxide (Al
2
O
3
) or gamma titanium aluminide (γ-TiAl), or combinations of different molecules
(polymers) or materials (composites). All materials are composed of a three-dimensional arrangement of atoms,
the general details of which are described subsequently.
Inorganic Crystalline Solids
The basic building blocks of crystalline solids are unit cells, which represent the smallest repeating unit within a
crystal. When stacked together, these repeating unit cells form a space lattice, which is a repeating three-
dimensional array of atoms. Due to geometrical considerations, atoms can only have one of 14 possible
arrangements, known as Bravais lattices (Fig. 9). Most metals and metallic alloys crystallize with face-centered
cubic (fcc), hexagonal close-packed (hcp), or body-centered cubic (bcc) crystal structures. However, the
structures in nonmetallic solids tend to be more complicated.
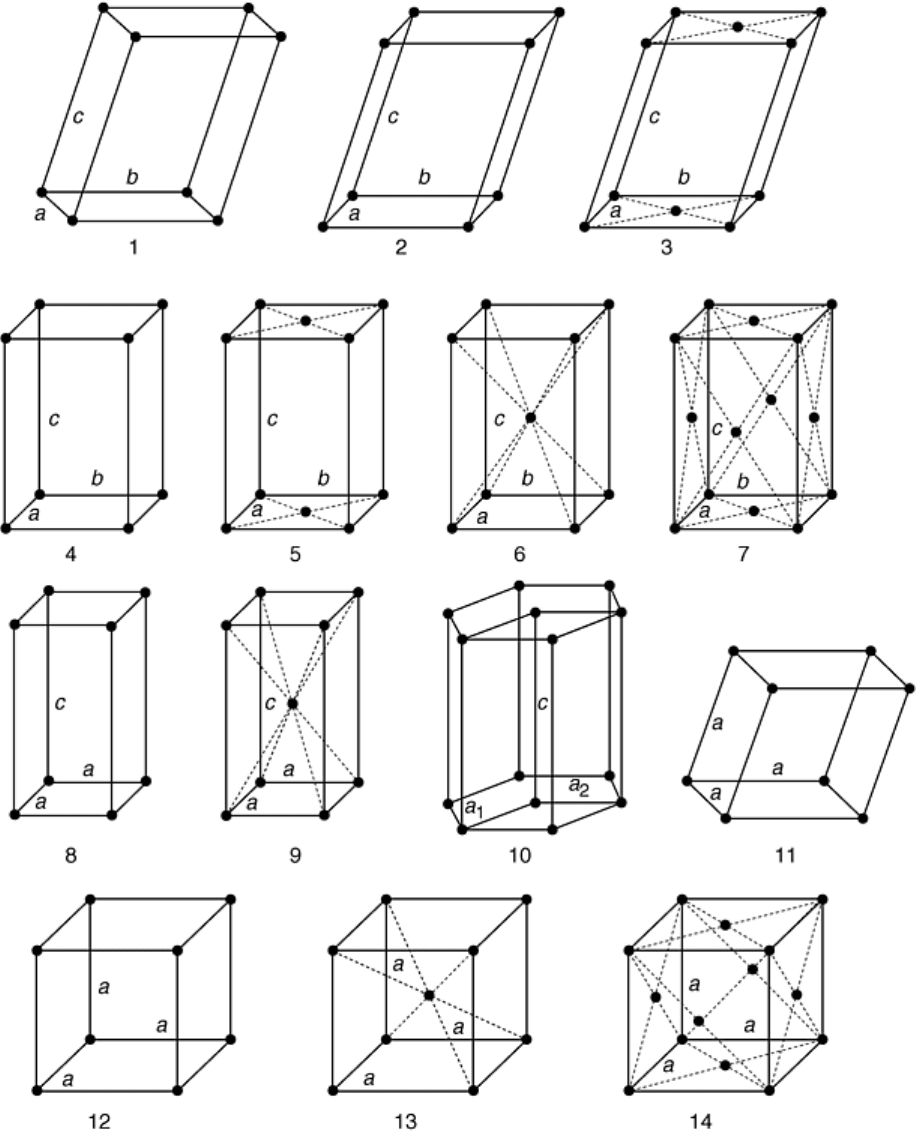
Fig. 9 The 14 Bravais lattices illustrated by a unit cell of each: 1, triclinic, primitive; 2, monoclinic,
primitive; 3, monoclinic, base centered; 4, orthorhombic, primitive; 5, orthorhombic, base centered; 6,
orthorhombic, body centered; 7, orthorhombic, face centered; 8, tetragonal, primitive; 9, tetragonal,
body centered; 10, hexagonal, primitive; 11, rhombohedral, primitive; 12, cubic, primitive; 13, cubic,
body centered; 14, cubic, face centered. Source: Ref 6
Crystalline ceramics and intermetallics have crystal structures consisting of multiple interpenetrating Bravais
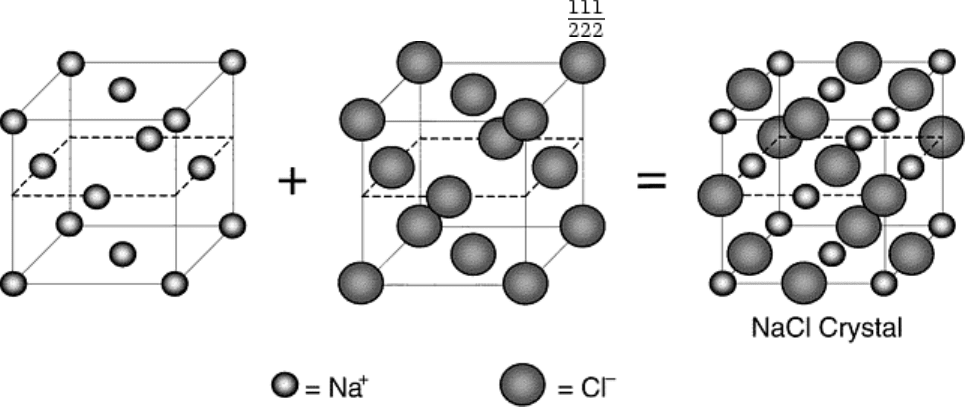
lattices, each of which is occupied by a specific atomic constituent. For example, common table salt (NaCl)
consists of two fcc (cubic F) Bravais lattices, slightly offset and overlaid on top of each other. One Bravais
lattice contains Cl
-
ions and is centered at origin, 0 0 0, while the second Bravais lattice contains Na
+
ions and is
centered at 0 ½0 (Fig. 10). The structure of the intermetallic compound β-NiAl, which is often called an ordered
bcc structure, actually consists of two simple cubic (primitive) Bravais lattices, one containing Ni atoms
centered at 0 0 0 and the second containing Al atoms centered at .
Fig. 10 Schematic illustration of the construction of NaCl from two interpenetrating fcc Bravais lattices
Inorganic Noncrystalline Solids
Not all solids are crystalline. Unlike their crystalline counterparts, noncrystalline materials do not display long-
range order. Instead, these solids exhibit some local order (i.e., short-range order) where atomic or molecular
subunits repeat over short distances. This group of materials, often collectively referred to as glasses, includes
many high molecular weight polymers, some polar-covalent ceramics, and some metallic alloys. Amorphous
structures arise because the mobility of atoms within these materials is restricted such that low energy
configurations (crystalline) cannot be reached.
To fully describe the nature of glassy materials, it is useful to consider the structure and properties of
commercial inorganic glasses. When cooling from the liquid state, materials may solidify in two different ways.
If the cooling rate is sufficiently slow, the liquid may freeze in the form of a crystalline solid. If the cooling rate
is extremely high, the liquid may pass through the freezing range without crystallizing so that it becomes a
supercooled liquid, which transforms to glass at lower temperatures. The critical cooling rate required for glass
formation in common inorganic glass is very low (≤10
-1
K/s), which means that it is very easy to form inorganic
glasses with these compositions. In metallic alloys, glass formation is more difficult and requires cooling rates
in excess of 10
5
K/s (Ref 7).
The supercooled liquid transforms to glass at the glass transition point, T
g
in Fig. 11(a). At this point, the
temperature dependence of the specific volume of the liquid changes. In glassy materials, there is little or no
change in volume upon cooling below the melting point, T
mp
. In contrast, crystallization is accompanied by a
sharp decrease in volume below T
mp
. At temperatures below T
g
, the slopes of both the glass and crystallized
solid curves are the same; however, the volume of glass is greater than the crystalline solid at all temperatures
where both forms can exist. The volume difference and the glass transition temperature depend on the cooling
rate, as illustrated in Fig. 11(b). The volume difference is related to the more open structure in the glass. The
difference is usually very small, in the range of a few percent for silicate glasses. In metallic glasses, it is
usually less than one percent (Ref 7).
