Baca A.G., Ashby C.I.H. Fabrication of GaAs Devices
Подождите немного. Документ загружается.

Cleaning and passivation of GaAs and related alloys
Transient conditions while the plasmas are first striking can pro-
duce a different film composition and/or density near the GaAs
interface. These are manifest in a higher refractive index for thin
films. For PECVD films, the index decreases with increasing film
thickness up to a thickness near 150 nm, and then remains rel-
atively constant. Similar behaviour is seen in ECR films. It is
especially important to keep this change in refractive index in
mind when growing AR coatings for optical applications. Since
the transients may be highly tool-dependent and can even vary
significantly on the same tool, it can be helpful to grow test films
to the calculated thickness, measure effective refractive index at
that thickness and then fine-tune the thickness right before device
fabrication to get optimum AR performance.
The ion bombardment energy can have a significant effect on
the leakage current for HBTs. Surface damage can increase current
conduction between the emitter-base and base-collector regions.
These damage-related current channels are more important at high
base-emitter voltages and high-injection levels. Ion damage will
also degrade FET performance.
The films that are produced from the same nominal source gases
and plasma conditions may actually be quite different when depos-
ited in different ECR tools. Plasma tools can be constructed with
different materials and with different designs. Since the plasma
interacts with the chamber walls in a way that changes the actual
plasma composition, it is possible to deposit a film that is NOT
what you might expect based on the composition of the source
gases. A good example is the deposition of SiN
x
and SiO
x
N
y
using ECR plasmas with SiH
4
and N
2
as the source gases. In one
ECR tool with minimal wall contributions, this forms partially
hydrogenated SiN
x
. In another tool, oxygen might be extracted
from the quartz window anodized Al parts, through which the
microwave radiation is launched into the plasma chamber, or from
the alumina ceramic liner of the chamber. Even a little oxygen
in the plasma will be incorporated at a significant level to give a
partially hydrogenated oxynitride. The source gases for SiO
2
are
SiH
4
and N
2
O. No appreciable nitrogen incorporation is observed
because of the strongly favoured formation of Si-O versus Si-N
bonds (Si-O = 124.7 kcal/mol versus Si-N = 105 kcal/mol).
In virtually all cases, some amount of hydrogen is incorporated
in the dielectric. We have deposited ECR films of nominally SiN
x
and SiO
x
N
y
with as little as 7% hydrogen and PECVD “SiN
x
”
films that were as much as 25% hydrogen. There tends to be more
H retained in PECVD films grown at lower wafer temperatures.
The presence of excessive H can be a problem for post-deposition
processing. For example, films with a high H content can
“bubble” due to hydrogen gas release and fail when the wafer
112
Cleaning and passivation of GaAs and related alloys
is subjected to rapid thermal annealing (RTA) to form good metal
contacts.
It is possible to grow films that are Si-rich. These display higher
refractive indices (n > 2.0) and lower resistivity. In PECVD,
increasing the silane fraction increases the Si content of the film. In
ECR, deposition at lower ECR powers will increase Si content for
a fixed gas composition; for example, decreasing the microwave
power from 400 to 160 W increases the index from 1.8 to 2.2.
Dielectric films are often characterised by measuring their
refractive index and assuming that the desired composition has
been attained when the “proper” refractive index is achieved.
Unfortunately, this is often an erroneous assumption. Refractive
index is a measurement of an optical property, not a composition.
It is affected by elemental composition, lattice structure, porosity,
etc. Addition of some Ar to the plasma will increase the refractive
index for a fixed set of reactants and plasma conditions because it
servestodensify the film. Addition of Ar also causes plasmas to run
in a more stable fashionandthereforeincreasesrun-to-run reprodu-
cibility. Films with the same composition but greater porosity will
have lower refractive indices. Films that contain Si-rich regions
will have higher refractive indices. For example, while the refract-
ive index of polycrystalline Si
3
N
4
is 2.0, it is possible to grow
ECR-deposited films with approximately the same index that are
actually Si
2.8
N
4
H
z
films with 7% H and SiO
x
N
y
H
z
with 60% Si,
20% N, 10% H, 7% O and 3% C. One PECVD “silicon nitride”
with index 2.0 was actually 35% Si, 40% N and 25% H. Although
it is a poor choice as the main diagnostic for composition, it can
be used very effectively as a measurement of the reproducibility
of a process since it combines the effects of composition and film
density. Hopefully, a specific process will produce uniform values
of these parameters when it is operating reproducibly.
Lower index films tend to etch faster than higher index ones,
due either to differences in composition or to the lower index films
being less dense. The effect is more pronounced in wet etchants
than dry, with a 5- to 20-fold increase over that for true Si
3
N
4
. Dry
etch rates are generally less than two-fold faster for the PECVD
films. Higher H-content films tend to etch faster. Having the same
refractive index does not mean there will be the same wet etch
rate. A factor of two spread in rate with 48% HF was obtained for
several PECVD films all with a refractive index of 2.0. This all
shows how important it is to test the actual etch rate of the films
deposited in your own hardware before attempting to etch them
during the fabrication of a valuable device.
Film stress is another important variable. ECR films tend to
be lower stress than PECVD films, but variations in this can
occur. Since high stress can lead to shifts in electronic properties
113
Cleaning and passivation of GaAs and related alloys
within a chip, e.g. offset voltages, resistance of thin-film transist-
ors, pinch-off voltages, hot electron degradation and mechanical
failure, it is generally desirable to use low-stress films.
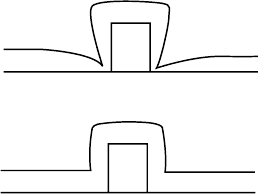
Conformality of the dielectric can be an issue especially when
the dielectric is deposited on a mesa, which can be either photores-
ist or semiconductor. With PECVD depositions on a mesa, a profile
with indentations near the mesa base (FIGURE 3.20(a)) often
forms. This non-uniform film thickness can be used to advantage
in some contact formation schemes. On the other hand, uniform
film thickness may be desired on all surfaces. While conformal
deposition is possible with PECVD, the room-temperature ECR
processes tend to automatically deposit in a more uniform fashion
(FIGURE 3.20(b)).
(a)
(b)
FIGURE 3.20 (a) Deposit with
non-uniform thickness with
PECVD. (b) Uniform thickness,
conformal deposit with ECR.
Pinholes can be a serious problem in dielectric deposition. These
can be caused by small particles on the wafer surface. These
particles may have been on the surface of the wafer as loaded in
the tool, or they may result from the deposition of particles within
the reactor. It is especially important to keep the deposition sys-
tem walls free of deposits that can flake and shed particles onto the
wafer surface. Excessively rapid deposition of the film increases
the likelihood of pinhole formation.
The use of silane as the Si source raises both safety and repeat-
ability concerns if there is even a tiny leak in the silane gas line.
Due to the pyrophoric nature of SiH
4
, fires and explosions are a
possibility with major leaks. Even a very small leak can lead to the
deposition of “sand”, i.e. tiny SiO
2
particles, in the line that can
gradually clog mass flow controllers and make the plasma com-
position vary slowly over time, leading to irreproducibility in film
composition.
3.4 CONCLUSION
The most important characteristic to remember when dealing with
cleaning and passivation issues is that materials properties at the
atomic level are what really matter. Composition and bonding
configurations at the monolayer level can totally dominate the
properties that affect device operation. Thinking “molecularly”
and careful attention to process details at that level will contribute
to achieving reproducible and reliable device results.
REFERENCES
[1] M.-G. Kang, H.-H. Park [Vacuum (UK) vol.67 (2002) p.91–100]
[2] K.R. Zavadil, C.I.H. Ashby, A.J. Howard, B.E. Hammons [J. Vac. Sci.
Technol. A (USA) vol.12 (1994) p.1045–9]
114
Cleaning and passivation of GaAs and related alloys
[3] P.R. Lefebvre, L. Lai, E.A. Irene [J. Vac. Sci. Technol. B (USA) vol.16
(1998) p.996–1001]
[4] C.J. Sandroff, F.S. Turco-Sandroff, L.T. Florez, J.P. Harbison [J. Appl.
Phys. (USA) vol.70 (1991) p.3632–5]
[5] S.I. Yi, P. Kruse, H.M. Hale, A.C. Kummel [J. Chem. Phys. (USA) vol.114
(2001) p.3215–23]
[6] M. Passlack, M. Hong, J.P. Mannaerts [Appl. Phys. Lett. (USA) vol.68
(1996) p.1099–101]
[7] H. Hasegawa [Jpn. J. Appl. Phys. (Japan) vol.38 (1999) p.1098–102]
[8] S.M. Sze [Semiconductor Devices: Physics and Technology (John Wiley
and Sons, New York, 1985)]
[9] D.E. Aspnes, A.A. Studna [Phys. Rev. B (USA) vol.27 (1983) p.985–1009]
[10] T. Ohno, K. Shiraishi [Phys. Rev. B (USA) vol.42 (1990) p.11194–7]
[11] J.-W. Seo, T. Koker, S. Agarwala, I. Adesida [Appl. Phys. Lett. (USA)
vol.60 (1992) p.1114–6]
[12] D. Wu, L. Liu, J. Marcano, Y. Darici, N. Paul, S. Mergui [Mater. Sci.
Eng. B (Switzerland) vol.46 (1997) p.61–4]
[13] C.I.H. Ashby, K.R. Zavadil, A.J. Howard, B.E. Hammons [Appl. Phys.
Lett. (USA) vol.64 (1994) p.2388–90]
[14] C.I.H. Ashby, K.R. Zavadil, A.G. Baca, P.-C. Chang, B.E. Hammons,
M.J. Hafich [Appl. Phys. Lett. (USA) vol.76 (2000) p.327–9]
[15] M.R. Ravi, A. DasGupta, N. DasGupta [IEEE Trans. Electron Devices
(USA) vol.50 (2003) p.532–4]
[16] S.M. Sze [Physics of Semiconductor Devices, 2nd Ed. (John Wiley and
Sons, New York, 1981)]
[17] M. Terman [Solid-State Electron. (UK) vol.5 (1962) p.285–99]
[18] K. Remashan, K.N. Bhat [Semicond. Sci. Technol. (UK) vol.17 (2002)
p.243–8]
[19] D. Shoji, T. Miura, M. Niwano, N. Miyamoto [Appl. Surf. Sci.
(Netherlands) vol.130–132 (1998) p.441–6]
[20] S. Hohenecker, T.U. Kampen, T. Werninghaur, D.R.T. Zahn, W. Braun
[Appl. Surf. Sci. (Netherlands) vol.142 (1999) p.28–32]
[21] S. Anantathanasarn, H. Hasegawa [Appl. Surf. Sci. (Netherlands) vol.216
(2003) p.275–82]
115
Chapter 4
Wet etching and photolithography of GaAs
and related alloys
Chapter scope p.117
Mechanism of wet etch
processes p.118
Rates and profiles p.118
Diffusion control p.121
Reaction-rate control p.123
Aging of etching solution p.124
Practical wet etching p.125
Photoresist issues p.127
Basic wet etches p.132
Acidic wet etches p.133
Metal etches p.135
Compositional selectivity p.136
GaAs versus AlGaAs selectivity p.137
Selectivity versus other materials p.139
Effects of doping type p.141
Electrolytic effects in wet
etching p.142
Effects of defects and damage p.144
Conclusion p.144
References p.144
4.1 CHAPTER SCOPE
Since most III–V devices require the exposure of buried layers
for metal contact formation and/or the formation of mesa struc-
tures for electrical isolation from adjacent devices on the same
wafer, etching will always be a critical part of their fabrication.
“Wet” (liquid-phase) etches are the simplest to implement since
they require only simple benchtop glassware (or plasticware if
HF is involved). There are, however, both advantages and dis-
advantages to using wet etching processes. The key issues to be
considered in selecting a particular etch process for any application
are rate, uniformity, selectivity, critical dimension control, feature
profile and surface damage that can degrade electronic properties
and, therefore, device performance.
The most favourable characteristic of wet etches is their ability
to cause virtually no surface electronic damage. When superior
surface electronic properties are most important, a wet process
will clearly be preferable to a dry process, which often degrades
surface electronic properties by ion bombardment.
The least desirable wet etch characteristic for many applications
is the unavoidable tendency to undercut etch masks, making pre-
cise dimensional control more difficult. This is observed whether
there are nearly equal vertical and lateral etch rates (isotropic
etching) or etch rates that depend on the specific exposed crys-
tal planes of the semiconductor (crystallographic etching). Precise
control of critical dimensions is made more difficult by the fre-
quently strong dependence of wet etch rates on temperature,
solution agitation and crystallographic alignment of the mask.
These aspects of wet etching, which will be discussed in some
detail in this chapter, present both difficulties and opportunities
for extra control.
A more detailed comparison between wet and dry (gas-phase)
etch processes is found in Chapter 5. In general, if feature profile
and tight control of dimensions are less importantthan surfaceelec-
tronic quality, a wet etch may be the process of choice. If vertical or
117
Wet etching and photolithography of GaAs and related alloys
controlled-angle profiles and small dimensions are needed, a dry
etch that achieves the best compromise between feature profile and
damage may be preferred. In this chapter, the issues of importance
in selecting and using a wet etch process will be discussed.
Advantages of wet etching
1) No surface electronic damage
2) Many chemistry choices
3) Chemical selectivity
4) Crystallographic etching
5) Inexpensive, simple equipment
Disadvantages of wet etching
1) Limited dimensional control
2) Vertical profiles hard to get
3) Solution waste disposal
When a pattern is to be etched, some method of protecting
or masking portions of the surface from the etchants must be
provided. Photolithography is the standard way of providing etch
masks for pattern delineation. Many issues related to photolitho-
graphy are independent of whether a wet or dry etch process is to be
used. Therefore, we will address many common photoresist issues
in this chapter. Some mask issues that are of importance only for
dry etching will be addressed at appropriate points in Chapter 5.
4.2 MECHANISM OF WET ETCH PROCESSES
The underlying chemical mechanism of most wet etching of GaAs
is the oxidation of the surface to form Ga and As oxides, fol-
lowed by the dissolution of these oxides by chemical attack with
acids or bases. Many GaAs etches call for the addition of hydro-
gen peroxide (H
2
O
2
) to serve as an oxidising agent to promote
the formation of the surface oxides. This is especially needed
for n-type material. Removal of electrons from the surface by an
oxidising agent like H
2
O
2
leaves an electron-deficient, “hole-rich”
surface that reacts readily to form the oxide. Some other chemicals
that have been used as the oxidising agent for etching GaAs are
HNO
3
,K
2
Cr
2
O
7
, CrO
3
,I
2
and Br
2
. An alternative way to create
a hole-rich surface in n-type material is to shine light on the sur-
face. This can produce photochemical etching of n-type material.
When ample quantities of chemical oxidising agent are present in
solution, this is usually not a major effect. However, with dilute
solutions, especially in bright light, photochemical etching may
be an unintentional consequence if samples are left in solution for
long periods of time. Under special conditions, local galvanic cells
may arise that can etch GaAs without light or a specific oxidising
agent.
Steps in wet etching
1) Diffusion of reactants to surface.
2) Formation of oxide on surface.
3) Acid or base attack on oxide and
oxide dissolution.
4) Diffusion of products from surface.
Overall rate controlled by step 2)
Profiles controlled by step 3).
4.3 RATES AND PROFILES
The most important consideration in selecting a wet etch is the
profile of the etched features. Since this is controlled primarily by
whether the etching chemistry is reaction-rate limited or diffusion
controlled, a brief discussion of reaction mechanisms is warranted.
A simplified representation of the chemistry typically involved
in GaAs etching is given by the following equations. To initiate
118
Wet etching and photolithography of GaAs and related alloys
the etching process, an oxide is formed on the surface of the GaAs
by reaction with an oxidising agent, typically H
2
O
2
.
GaAs + (oxidiser) → GaO
x
/AsO
y
(4.1)
The rate can be calculated from
rate = k
ox
[oxidiser]
m
{GaAs} exp(−E
∗
ox
/kT) (4.2)
Here, [oxidiser] represents the concentration of oxidiser, m is
related to the number of oxidiser molecules involved in oxidising
one Ga and one As, {GaAs} is the effective surface “concentra-
tion” of exposed Ga and As atoms, k
ox
is the reaction prefactor,
E
∗
ox
is the activation energy for the oxidation reaction, k is the
Boltzmann factor and T is temperature in Kelvin. While this reac-
tion may affect the overall rate, with rates being faster with higher
oxidiser concentration, it is the second step, the dissolution of the
oxide, that generally controls profiles.
The surface oxide is then attacked by either an acid or a base
and is dissolved to give Ga
3+
and AsO
2−
4
ions in solution.
GaO
x
/AsO
y
+ (acid or base) → Ga
3+
+ AsO
2−
4
(4.3)
It is this second step in the total etching process that is usually key
in determining whether the etching will be reaction-rate limited or
diffusion controlled.
rate = k
diss
[acid or base]
n
{oxide} exp(−E
∗
diss
/kT) (4.4)
Here, [acid or base] represents the concentration of acid or base,
n is related to the number of H
+
or OH
−
involved up to the slow-
est step in the dissolution reaction, {oxide} represents the effective
concentration of the surface oxide species that is slowest at dis-
solving, k
diss
is the reaction prefactor and E
∗
diss
is the activation
energy for the dissolution reaction.
Factors affecting diffusion versus
reaction-rate control
Reactant concentration
Solution mixing
Solution viscosity
Temperature
Assuming an adequate supply of oxidiser, whether the rate of
the dissolution process will be limited by the supply of acid or base
(diffusion controlled) or by the activation energy of the dissolution
reaction (reaction-rate limited) will be determined by the relative
magnitudes of their respective terms. Any reaction has the poten-
tial to be either reaction-rate limited or diffusion controlled. It is
merely a question of which term dominates under the conditions
that are being employed to etch GaAs. If an activation energy for an
etching process is less than 5 kcal/mol, it will probably be diffusion
119
Wet etching and photolithography of GaAs and related alloys
controlled, while a value greater than 10 kcal/mol probably means
reaction-rate control.
Rates can depend on:
Doping type and level
Crystallographic facet
Heterojunctions
pn junctions
Nearby exposed metal
It is easy to see from these equations that changing concen-
tration, solution mixing or temperature might shift the etching
reaction from one dominant mode to the other. In fact, some
common etching solutions perform well rather close to the switch-
over point. In general, low concentrations of reactants favour
reaction-rate control of etching while high concentrations favour
diffusion control. Higher viscosities also favour diffusion control.
Something as simple as diluting a solution to slow an etch rate can
switch the dominant mode from diffusion to reaction-rate control.
Consequently, caution must be used in altering “standard” recipes.
Unintentional switching from one type of control to the other can
produce very different results from those expected and destroy
your devices.
Rate dependence on doping
p
+
> p > n > n
+
n > SI
The activation energy for the initial oxidation of the surface
(E
∗
ox
) can vary appreciably depending on the crystallographic face
that is exposed and on the doping type of the material. Con-
sequently, it will always be important to determine the etch rate
for a particular wafer structure rather than assuming that a lit-
erature value will apply in any specific case. The presence of
heterojunctions and pn junctions in a particular device structure
can profoundly affect the energetics of the oxidation and, there-
fore, the etch rate. Consequently, it can affect the profile, with
rates generally being p
+
> p > n > n
+
and n > SI. This pro-
duces greater undercut for p-type material than n-type and a
sloped profile across the depletion region near the pn junction.
Doping effects are discussed in greater detail in Section 4.6 and
heterojunction issues will be discussed with selective etching in
Section 4.5.
Care must also be taken in aligning the mask with respect to the
crystallographic axes of the wafer since there is a difference in the
activation energy for oxidation of different crystal faces. This can
produce totally different etch profiles for devices that are aligned
differently with respect to the crystal axes. Crystallographic
dependences will be discussed in Section 4.3.2.
Excessively fast rates will cause poor surface quality. Oxida-
tion rate is generally controlled by the strength of the oxidiser so
increasing the H
2
O
2
fraction increases the rate; however surfaces
tend to roughen with etchants that are >15% H
2
O
2
. Dissolution of
the oxide is controlled by solution pH; surface roughness increases
at pH > 7.5. At higher pHs, there is less efficient oxide removal so
the thickening oxide can crack and float away in small segments.
This exposes the underlying surface for further oxidation. This
cracking and flaking away is inherently a non-uniform process
and roughening is the result.
120
Wet etching and photolithography of GaAs and related alloys
4.3.1 Diffusion control
The rate of diffusion-controlled reactions can be limited by the
diffusion of reactants to the surface or of products away from
the surface. For relatively narrow features, this rate limitation by
diffusion can lead to decreases in the reaction rate as features are
etched deeper and diffusion times into and out of the deepening
trench increase. The exponential term (exp[−E
∗
/k
B
T]) is of less
importance so these rates are relatively insensitive to temperature.
However, they are highly sensitive to the degree of agitation so
controlled stirring or rotation is important to achieve consistent
and predictable results.
Slow rotation (<100 rpm) permits very uniform thinning of a
wafer. Fast rotation in a centrifuge can decrease lateral under-
cutting. In practical terms, “rotation” often consists of manually
swirling the sample holder in solution, which may not be very
reproducible. Stirring at a reproducible rate with a magnetic
stirrer or an over-head propeller design can produce more predict-
able results. Automated equipment is available for higher volume
applications.
The classic profile of a diffusion-controlled reaction is shown in
FIGURE 4.1. Lateral undercutting proceeds at a rate comparable to
the vertical etch rate in the field region away from the mask. How-
ever, a shallow trench or downward bulge forms near the edge of
the mask and slopes back away from the edge. This profile is well
understood and has been quantitatively modelled [1] assuming no
closely adjacent additional mask edges. The degree of trenching
can be quite dependent on the areal ratio of exposed and masked
areas. The classic example of a diffusion-controlled etchant for
GaAs is the HCl/H
2
O
2
/H
2
O system. Under “ideal” diffusion con-
ditions, i.e. with no convection, the ratio of the bulge depth to
the lateral undercut beneath the mask is about 1.33 and the pro-
file would remain constant with time (FIGURE 4.1(a)). However,
some convection is always present during etching due to temper-
ature and/or concentration gradients generated near the surface
by the etching reaction itself. For short etch times, the bulge-to-
undercut ratio is essentially 1.33, but as the etch proceeds in time,
the mask-edge region appears more and more like the field region
more remote from the mask edge and the downwardbulge becomes
less prominent with time (FIGURE 4.1(b)).
The potential problem of trenching should be considered before
using these etches. For example, a trench-prone etch would be
totally unsuitable for gate-recess etching. For acid/peroxide ratios
of 1 HCl : y H
2
O
2
:1 H
2
O, negligible trenching is obtained for
y ≤ 10 and up to 10–20% deeper etching near mask edges is
obtained than at the centre between two masked areas for y = 40
121
