Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.


s24.2 The Flux Expressions for Heat and Mass
767
with
V
In a,), pressure diffusion (term with Vp), and forced diffusion (term with
g).
The d,
are so defined that Z,d,
=
0.
The entropy production in Eq. 24.1-6, which is a sum of products of fluxes and
forces, is the starting point for the nonequilibrium thermodynamics development. Ac-
cording to the "linearity postulate" each of the fluxes in Eq. 24.1-6 (q'"',
j,,
7,
and
-
G,/M,)
can be written as a linear function of all the forces (VT,
d,,
Vv,
and r,). How-
ever, because of "Curie's postulate," each of the
j,
must depend linearly on all of the
d, as well as on VT, and
q(h)
must depend linearly on VT as well as on all the d,, but
neither
j,
nor
q(h)
can depend on Vv or r,. Similarly the stress tensor
.r
will depend on
the tensor
Vv,
and also on the scalar driving forces r, multiplied by the unit tensor.
Since the "coupling" between
.r
and the chemical reactions has not been studied, we
omit any further consideration of it. In the next section we discuss the coupling among
all the vector forces and vector fluxes and the consequences of applying the "Onsager
reciprocal relations."
524.2
THE FLUX EXPRESSIONS FOR HEAT
AND
MASS
We now employ the "linearity postulate" to obtain for the vector fluxes
In these equations the quantities a,,,, a,,, a,,, and
asp
are the "phenomenological coeffi-
cients" (that is, the transport properties). Because the
j,
and d, are not all independent, it
must be required that a,,
+
2,a,,
=
0,
where the sums are over all
y
(except
y
=
P)
from
1
to
N.
Now according to the Onsager reciprocal relations, a,,
=
a,,
and
nap
=
a,,
for all
values of
cr
and /3 from
1
to
N.
Next we relate the phenomenological coefficients to the transport coefficients. First
we relabel a,, and ao, as
D:,
the multicomponent thermal diffusion coefficients. These have
the property that
2,~:
=
0.
Then we define the multicomponent Fick difisivities,'
ID,,,
by
D,,
=
-cRTaap/papp. These diffusivities are symmetric (Elap
=
D,,)
and obey the rela-
tions C,U,D,~
=
0.
Then Eq. 24.2-2 becomes
,
for the multicomponent mass fluxes. These are the generalized Fick equations. When the
second form of
Eq.
24.1-8 is substituted into
Eq.
24.2-3 we see that there are four contri-
butions to the mass-flux vector
j,:
the concentration diffusion term (containing the activ-
ity gradient), the pressure diffusion term (containing the pressure gradient), the forced
diffusion term (containing the external forces), and the thermal diffusion term (propor-
tional to the temperature gradient).
C.
F.
Curtiss,
J.
Chem. Pkys.,
49,2917-2919 (1968); see also
D.
W.
Condiff,
1.
Chem. Pkys.,
51,
42094212 (1969), and C.
F.
Curtiss
and
R.
B.
Bird,
Ind.
Eng. Chem. Research,
39,2515-2522 (1999); errata
41,1791 (2001). The
Dap
used here are the negatives
of
the
Curtiss
D,~,
which, in turn, are different from
the
DaB
used
by
J.
0.
Hirschfelder, C.
F.
Curtiss, and
R.
B.
Bird,
Molecular
Theo
y
of Gases
and
Liquids,
Wiley, New York (1954), second corrected printing (1964, Chapter 11.

768
Chapter
24
Other Mechanisms for Mass Transport
Equation 24.2-3 can be turned "wrong-side out"',' and solved for the driving forces d,:
These are the generalized Maxwell-Stefan equations, a special case of which was given in
Eq. 17.9-1. The
Bop
are called the mulficomponent Maxwell-Stefan diffusivities, and they
have been proven to be ~ymrnetric;~ their relation to the Dap will be discussed presently.
When the expression for d, in Eq. 24.2-4 is substituted into Eq. 24.2-1, we get
The thermal conductivity of a mixture is defined to be the coefficient of proportionality
between the heat-flux vector and the temperature gradient when there are no mass
fluxes in the system. Thus, the quantity in brackets is, by general agreement, the thermal
conductivity
k
times the absolute temperature
T.
If we combine this result with the defin-
ition in
Eq.
24.1-7, w get for the final expression for the heat flux:3
We see that the heat flux vector
q
consists of three terms: the heat conduction term (con-
taining the thermal conductivity), the heat diffusion term (containing the partial molar
enthalpies and the mass fluxes), and finally the Dufour term (containing the thermal dif-
fusion coefficients and the mass fluxes). The heat diffusion term, already encountered in
Eq. 19.3-3, is generally important in diffusing systems. The Dufour term is usually small
and can usually be neglected.
Equations 24.2-3,4, and
6
are the principal results of nonequilibrium thermodynam-
ics. We now have the mass- and heat-flux vectors expressed in terms of the transport
properties and the fluxes.
Next we discuss the relation between the matrix of Fick diffusivities
Dep
and that of
the Maxwell-Stefan diffusivities
Bag.
Both matrices are symmetric and of order
N
X
N,
and both have
~N(N
-
1) independent elements. The
Bap
are obtained thus:3
in which
(Ba)pY
=
-
DPy
+
Day-that is, the
p
y-component of a matrix called
B,,
which is
of order (N
-
1)
x
(N
-
1)-and adj
B,
is the matrix adjoint to
B,.
For binary and ternary
H.
J.
Merk,
Appl.
Sci.
Res.,
A8,73-99 (1959);
E.
Helfand,
J.
Chem.
Phys.,
33,319-322 (1960).
Hendrik
Jacobus
Merk
(1920-1988) performed the inversion of the mass-flux expressions when he was a graduate
student in engineering physics at the Technical University of Delft; from 1953 to 1987 he was a professor
at the same institution.
C.
F.
Curtiss
and
R.
B.
Bird,
Ind.
Eng.
Chm.
Research,
38,2515-2522 (1999); errata,
40,1791
(2001).
s24.2 The Flux Expressions for Heat and Mass
769
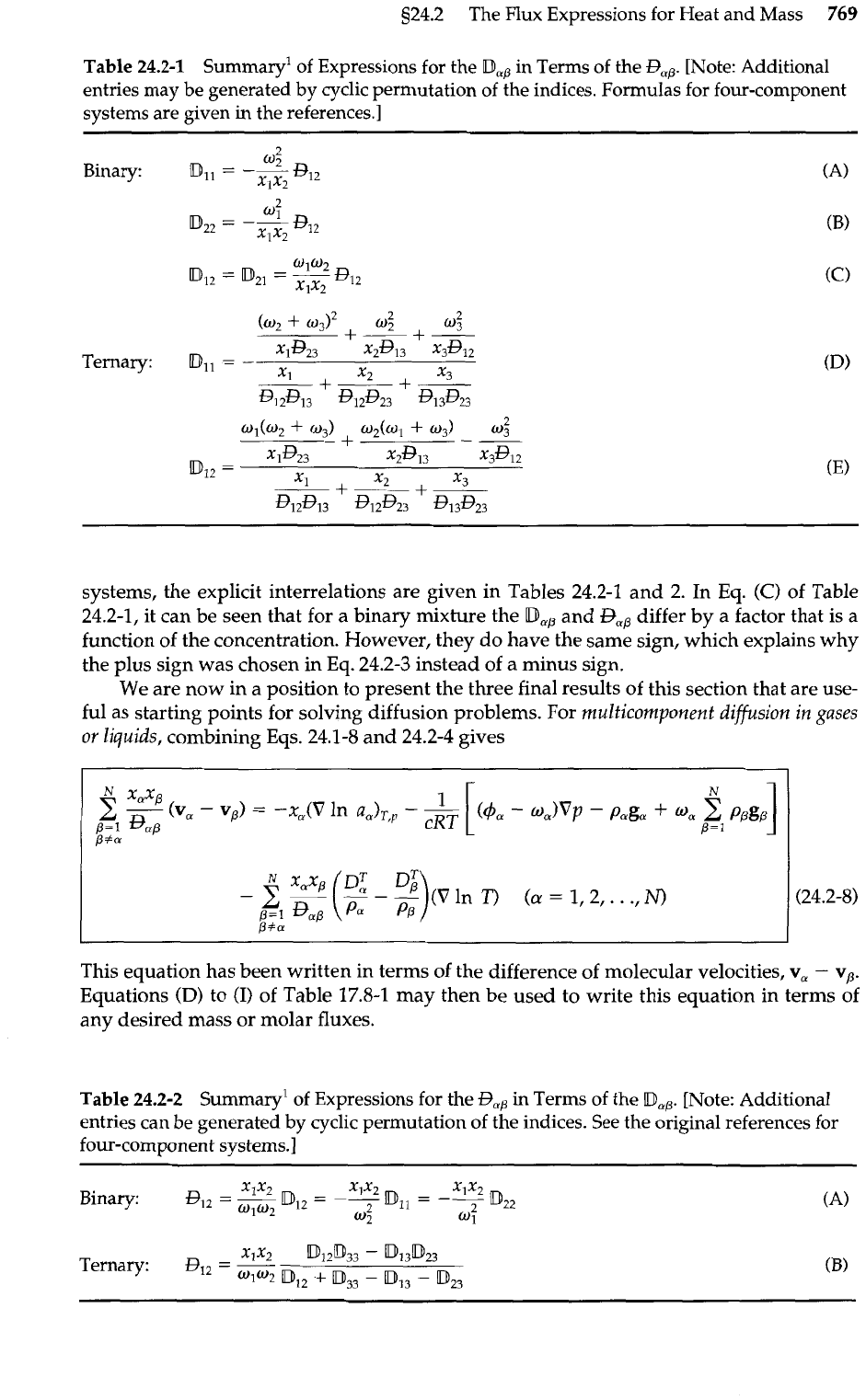
Table
24.2-1 Summary1 of Expressions for the
Dmp
in Terms of the
Rp.
[Note: Additional
entries may be generated
by
cyclic permutation of the indices. Formulas for four-component
systems are given
in
the references.]
Binary:
Ternary:
systems, the explicit interrelations are given in Tables 24.2-1 and 2. In Eq.
(C)
of Table
24.2-1, it can be seen that for a binary mixture the
Dap
and
Bap
differ by a factor that is a
function of the concentration. However, they do have the same sign, which explains why
the plus sign was chosen in
Eq.
24.2-3 instead of a minus sign.
We are now in a position to present the three final results of this section that are use-
ful as starting points for solving diffusion problems. For
multicomponent
diffusion in gases
OY
liquids,
combining Eqs. 24.1-8 and 24.2-4 gives
This equation has been written in terms of the difference of molecular velocities,
v,
-
v,+
Equations
(D)
to (I) of Table
17.8-1
may then be used to write this equation in terms of
any desired mass
or
molar fluxes.
Table
24.2-2 Summary' of Expressions for the
R1,
in
Terms of the
map.
[Note: Additional
entries can be generated
by
cyclic permutation of the indices. See the original references for
four-component systems.]
Xlx2
D12D33
-
D13D23
Ternary:
QI2
=
-
W1°2
DI2
+
IDs3
-
DI3
-
[Di23

770
Chapter 24 Other Mechanisms for Mass Transport
If one wishes to designate one species
y
as being special (for example, the solvent),
then Eq. 24.2-8 can be rewritten thus (see Problem 24C.1):
Note that in
Eq.
24.2-8 there are
N(N
-
1)/2 symmetric diffusivities, Boa, and that the
Go,
do not appear and are hence not defined. However, in
Eq.
24.2-9, there are
N(N
+
1)/2 symmetric diffusivities, but the
B,,
(N
of them) now appear, and therefore
we have to supply an auxiliary relation Z,(x,/Bffp)
=
0,
in which the sum is over all
a.
Equation 24.2-9, with the auxiliary relation, is equivalent to Eq. 24.2-8, and both of these
generalized Maxwell-Stefan equations are equivalent to the generalized Fick equations
of Eq. 24.2-3, together with its auxiliary relation.
For
multicomponent diffusion in gases at low density,
the activity may be replaced by the
mole fraction, and furthermore, to a very good approximation, the
Bop
may be replaced
by 5?hap. These are the
binary
diffusivities for all pairs of species in the mixture. Since the
Bffp vary only slightly with concentration, whereas the
DUp
are highly concentration-
dependent, it is preferable to use the Maxwell-Stefan form (Eq. 24.2-4) rather than the
Fick form (Eq. 24.2-3).
For
binary diffusion in gases or liquids,
Eq.
(C)
of Table 24.2-1 and
Eq.
17B.3-1 may be
used to simplify
Eq.
24.2-8 as follows:
In this equation we have introduced the
thermal difusion ratio,
defined by
k,
=
-
=
+
(Di/&B)(~A~B/~A~B). Other quantities encountered are the
thermal diffusion factor
a,
and the
Soret coefficient
a,,
defined by
k,
=
cu,
x,x,
=
a,
xAxBT. For gases
a,
is almost inde-
pendent of composition, and
a,
is the quantity preferred for liquids. When
k,
is positive,
species
A
moves toward the colder region, and when it is negative, species
A
moves toward
the warmer region. Some sample values of
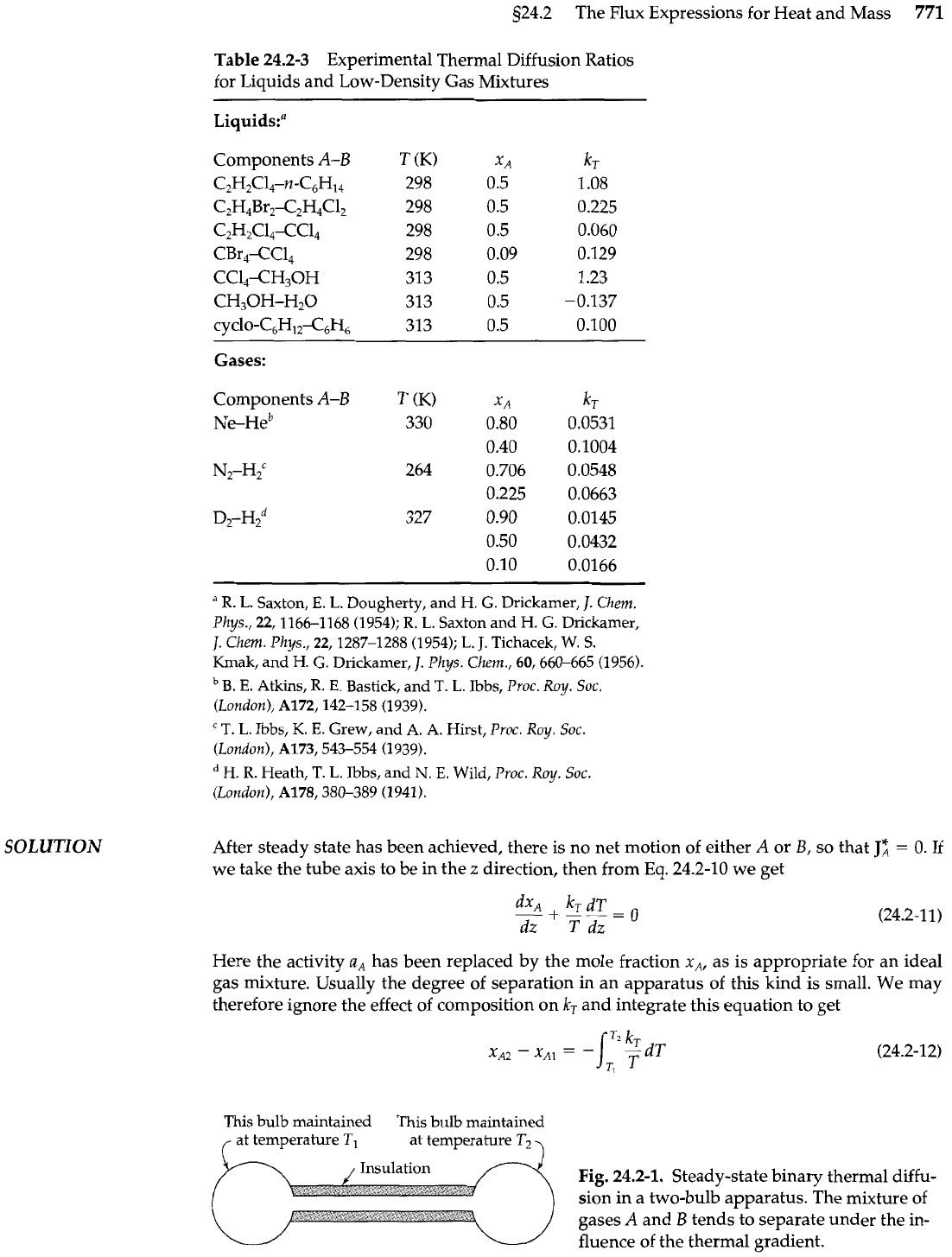
k,
for gases and liquids are given in Table 24.2-3.
For binary mixtures of dilute gases, it is found by experiment that the species with
the larger molecular weight usually goes to the colder region. If the molecular weights
are about equal, then usually the species with the larger diameter moves to the colder re-
gion. In some instances there is a change
in
the sign of the thermal diffusion ratio as the
temperature is lowered."
In the remainder of the chapter, we explore some of the consequences of the mass-
flux expressions in Eqs. 24.2-8,9, and
10.
EXAMPLE
24.2-1
Thermal
Diffusion
and
the
Clusius-Dickel
-
-
In this example we discuss the diffusion of species under the influence of a temperature gra-
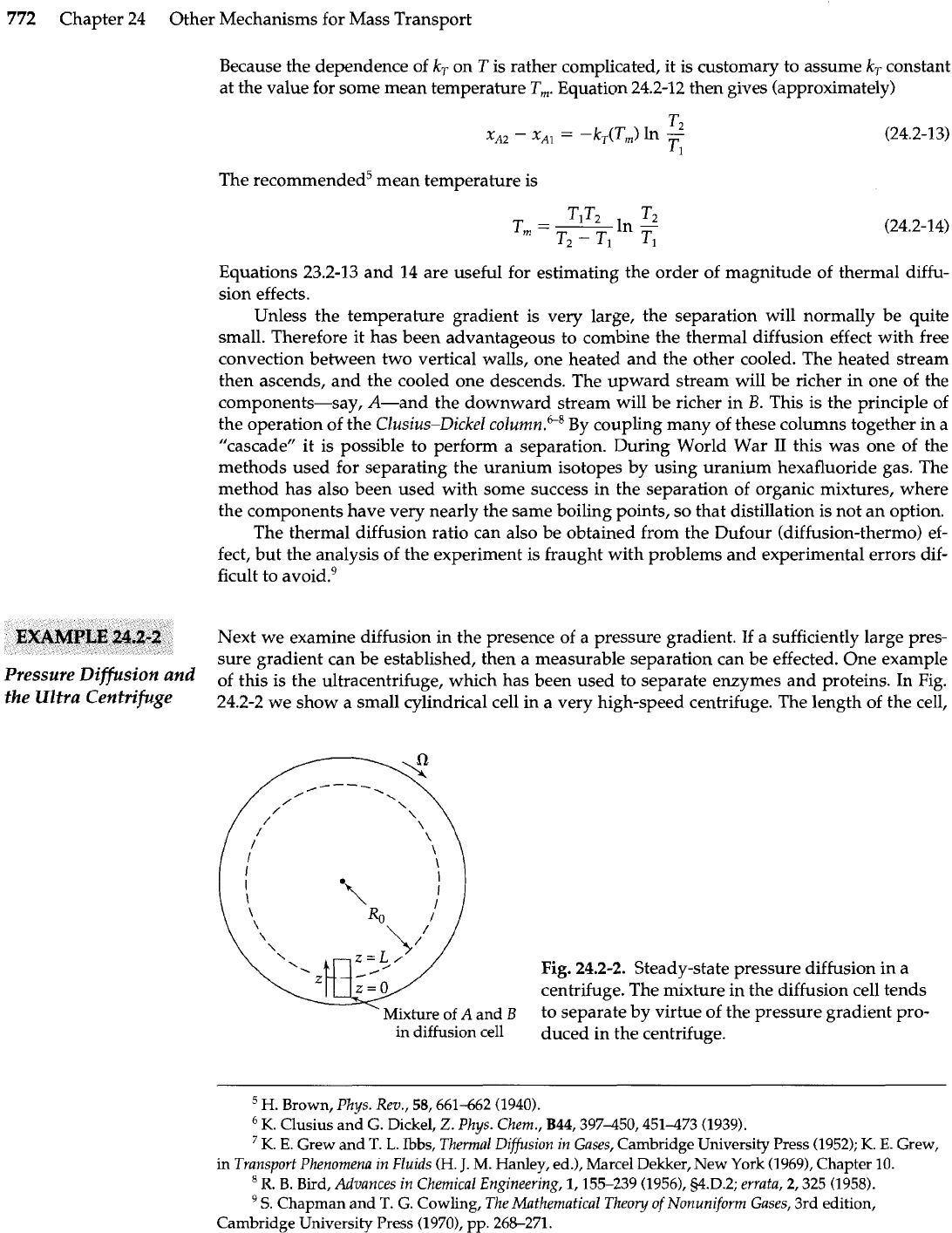
dient. To illustrate the phenomenon we consider the system shown in Fig.
24.2-1,
two bulbs
joined together by an insulated tube of small diameter and filled with
a
mixture of ideal gases
A
and
B.
The bulbs are maintained at constant temperatures
TI
and
T,,
respectively, and the
Column
diameter of the insulated tube
is
small enough to eliminate convection currents substantially.
Ultimately the system arrives at a steady state, with gas
A
enriched at one end of the tube and
depleted at the other. Obtain an expression for
xA2
-
x,,,
the difference of the mole fractions
at the two ends of the tube.
%.
Chapman and
T.
G.
Cowling,
The
Mathemnticnl
Theory
of
Non-Uniform
Gases,
3rd
edition,
Cambridge
University
Press
(1970),
p.
274.
s24.2 The Flux Expressions for Heat and Mass
771
SOLUTION
Table
24.2-3 Experimental Thermal Diffusion Ratios
for Liquids and Low-Density Gas Mixtures
Liquids:"
Components A-B
C2H,C14-n-C6H14
C2H,Br2-C2H4C12
C2H2C14-CC14
CBr,-CCl,
CC14-CH30H
CH30H-H20
CYC~O-C~H~~<~H~
Gases:
Components A-B
T
(K)
X
A
~e-~e~
330
0.80
0.40
N2-H2C 264 0.706
0.225
D,-H~~
327
0.90
0.50
0.10
a
R.
L.
Saxton,
E.
L.
Dougherty, and
H.
G.
Drickamer,
J.
Chem.
Phys., 22,1166-1168 (1954);
R.
L.
Saxton and
H.
G.
Drickamer,
J.
Chem. Phys., 22,1287-1288 (1954);
L.
J.
Tichacek, W.
S.
Kmak, and
H.
G.
Drickamer,
J.
Phys. Chem., 60,660-665 (1956).
B.
E.
Atkins,
R.
E.
Bastick, and
T.
L.
Ibbs,
PYOC. Roy. SOC.
(London), A172,142-158 (1939).
'
T.
L.
Ibbs,
K.
E.
Grew, and
A.
A. Hirst,
Proc. Roy. Soc.
(London), A173,543-554 (1939).
H.
R.
Heath, T.
L.
Ibbs, and
N.
E.
Wild,
Proc. Roy. SOC.
(London), A178,380-389 (1941).
After steady state has been achieved, there is no net motion of either A or
B,
so that
Jz
=
0.
If
we take the tube axis to be in the
z
direction, then from Eq. 24.2-10 we get
Here the activity
aA
has been replaced by the mole fraction xA, as is appropriate for an ideal
gas mixture. Usually the degree of separation in an apparatus of this kind is small.
We
may
therefore ignore the effect of composition on
kT
and integrate this equation to get
This bulb maintained
This bulb maintained
at temperature
TI
at temperature
T2
7
Fig.
24.2-1. Steady-state binary thermal diffu-
sion in a two-bulb apparatus. The mixture of
gases A and
B
tends to separate under the in-
fluence of the thermal gradient.
772
Chapter 24 Other Mechanisms for Mass Transport
Because the dependence of
k,
on
T
is rather complicated, it is customary to assume
k,
constant
at the value for some mean temperature
T,.
Equation
24.2-12
then gives (approximately)
The recommended5 mean temperature is
Equations 23.2-13 and 14 are useful for estimating the order of magnitude of thermal diffu-
sion effects.
Unless the temperature gradient is very large, the separation will normally be quite
small. Therefore it has been advantageous to combine the thermal diffusion effect with free
convection between two vertical walls, one heated and the other cooled. The heated stream
then ascends, and the cooled one descends. The upward stream will be richer in one of the
components-say, A-and the downward stream will be richer in
B.
This is the principle of
the operation of the
Clusius-Dickel c~lurnn.~~
By coupling many of these columns together in a
"cascade" it is possible to perform
a
separation. During World War I1 this was one of the
methods used for separating the uranium isotopes by using uranium hexafluoride gas. The
method has also been used with some success in the separation of organic mixtures, where
the components have very nearly the same boiling points, so that distillation is not an option.
The thermal diffusion ratio can also be obtained from the Dufour (diffusion-thermo) ef-
fect, but the analysis of the experiment is fraught with problems and experimental errors dif-
ficult to avoid.9
Next we examine diffusion in the presence of a pressure gradient.
If
a sufficiently large pres-
sure gradient can be established, then a measurable separation can be effected. One example
Pressure
Diffusion
and
of this is the ultracentrifuge, which has been used to separate enzymes and proteins. In Fig.
the Ultra Centqge
24.2-2 we show a small cylindrical cell in a very high-speed centrifuge. The length of the cell,
and
B
Fig.
24.2-2.
Steady-state pressure diffusion in a
centrifuge. The mixture in the diffusion cell tends
to separate by virtue of the pressure gradient pro-
in diffusion cell
duced in the centrifuge.
H.
Brown,
Phys.
Rev.,
58,661-662 (1940).
K.
Clusius and
G.
Dickel,
Z.
Phys. Chem.,
B44,397450,451473 (1939).
K.
E.
Grew
and
T.
L.
Ibbs,
Thermal Difision in Gases,
Cambridge University Press (1952);
K.
E.
Grew,
in
Transport Phenomena in Fluids
(H.
J.
M.
Hanley, ed.), Marcel Dekker, New
York
(1969), Chapter 10.
R.
B. Bird,
Advances in Chemical Engineering,
1,155-239 (1956),
9.D.2;
errata,
2,325 (1958).
S.
Chapman and
T.
G.
Cowling,
The Mathematical Theory of Nonuniform Gases,
3rd edition,
Cambridge University Press (19701,
pp.
268-271.

s24.2 The Flux Expressions for Heat and Mass
773
L,
is short with respect to the radius of rotation R,, and the solution density may be consid-
ered a function of composition only. Determine the distribution of the two components at
steady state in terms of their partial molar volumes and the pressure gradient. The latter is
obtained from the equation of motion as
For simplicity, we assume that the partial molar volumes and the activity coefficients are con-
stant over the range of conditions existing in the cell.
At steady state
j,
=
0,
and the relevant terms in Eq. 24.2-10 give for species
A
Inserting the appropriate expression for the pressure gradient and then multiplying by
(V5/xA)dz, we get for species
A
Then we write a similar equation for species
B,
which is
Subtracting Eq. 24.2-18 from Eq. 24.2-17 we get
We now integrate this equation from
z
=
0
to some arbitrary value of
z,
taking account of the
fact that the mole fractions of
A
and
B
at
z
=
0
are
XAO
and xBU, respectively. This gives
If
g,
is treated as constant over the range of integration, then we get
Then we take the exponential of both sides to find
This describes the steady-state concentration distribution for
a
binary system in a constant
centrifugal force field. Note that, since this result contains no transport coefficients at all, the
same result can be obtained by an equilibrium thermodynamics analysis." However, if one
wishes to analyze the time-dependent behavior of a centrifugation, then the diffusivity for the
mixing
A-B
will appear in the result, and the problem cannot be solved by equilibrium
thermodynamics.
lo
E.
A.
Guggenheim,
Thermodynamics,
North-Holland, Amsterdam
(1950),
pp.
356-360.
774
Chapter 24 Other Mechanisms for Mass Transport
524.3
CONCENTRATION DIFFUSION
AND
DRIVING
FORCES
In Chapter 17 we wrote Fick's first law by stating that the mass (or molar) flux is propor-
tional to the gradient of the mass (or mole) fraction, as summarized in Table 17.8-2.
On the other hand, in
Eq.
24.2-10 it appears that the thermodynamics of irreversible
processes dictates using the activity gradient as the driving force for concentration diffu-
sion. In this section we show that either the activity gradient or the mass (or mole) frac-
tion gradient driving force may be used, but that each choice requires a different
diffusivity. These two diffusivities are related, and we illustrate this for a binary mixture.
When we drop the pressure-, thermal-, and forced-diffusion terms from Eq. 24.2-10,
we get
This may be rewritten by making use of the fact that the activity coefficient is a function
of xA to obtain
dln
UA
J;
=
-cgAB(-)
d
In
XA
T,P
vxA
The activity may be written as the product of the activity coefficient and the mole frac-
tion (a,
=
yAxA)
SO
that
If the mixture is "ideal," then the activity coefficient is equal to unity, Eq. 24.3-3 becomes
the same as
Eq.
(B)
of Table 17.8-2, and
BA,
=
9,,.
If the mixture is "nonideal," one can express the binary diffusivity
9AB
as
then Eq. 24.3-2 and 3 become
which is one of the forms of Fick's law (see Eq.
(B)
of Table 17.8-2). In order to measure
B,,,
one has to have measurements of the activity as a function of concentration, and for
this reason
BAB
has not been popular.
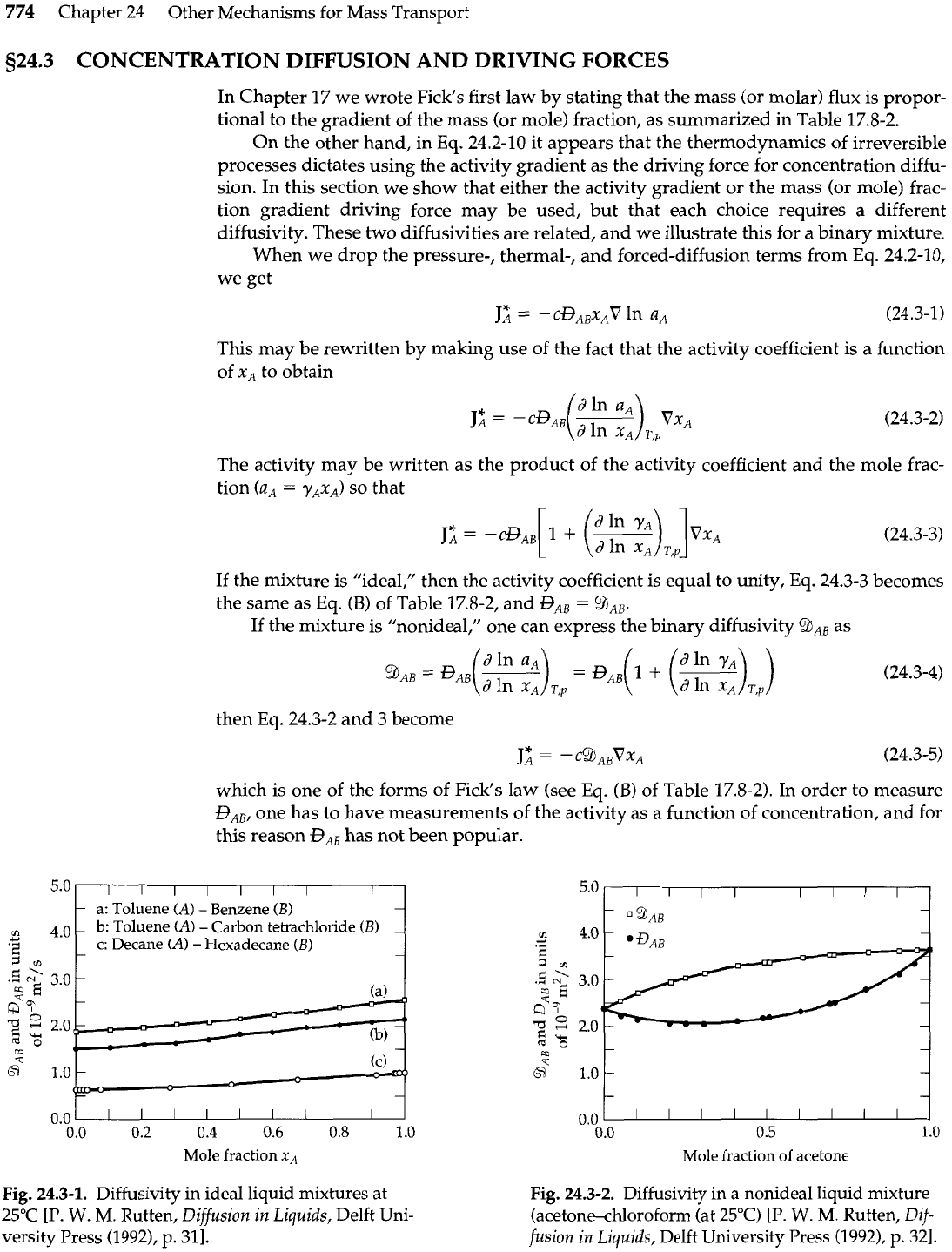
a: Toluene (A)
-
Benzene
(B)
cn
b:
Toluene (A)
-
Carbon tetrachloride
(B)
Y
.
+
G
c: Decane
(A)
-
Hexadecane
(B)
1
rn
0.0
1
I
I
I
I
I
I I
I
I
0.0
0.2
0.4
0.6
0.8
1
.O
Mole fraction
xA
Fig.
24.3-1.
Diffusivity in ideal liquid mixtures at
25°C
[P.
W.
M.
Rutten, Diffusion in Liquids, Delft Uni-
versity Press (1992), p.
311.
Mole fraction of acetone
Fig.
24.3-2.
Diffusivity in a nonideal liquid mixture
(acetone-chloroform (at 25°C) [P.
W.
M.
Rutten,
Dif-
firsion in Liquids, Delft University Press (1992), p. 321.
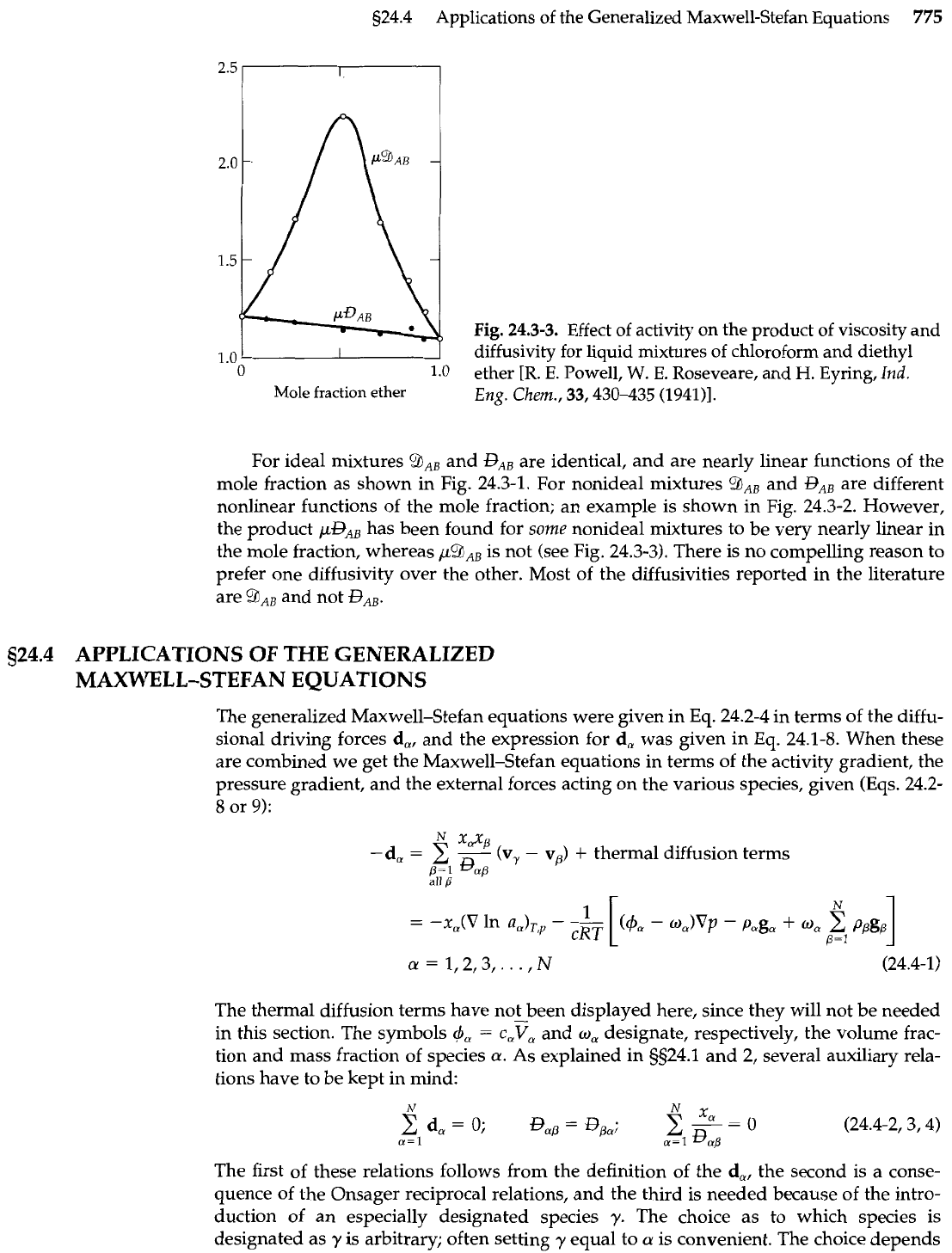
s24.4 Applications of the Generalized Maxwell-Stefan Equations
775
Fig.
24.3-3.
Effect of activity on the product of viscosity and
1
.0
diffusivity for liquid mixtures of chloroform and diethyl
0
1.0
ether
[R.
E.
Powell,
W.
E.
Roseveare, and
H.
Eyring,
Ind.
Mole
fraction
ether
Eng.
Chem.,
33,430435 (194111.
For ideal mixtures
?hAB
and
GAB
are identical, and are nearly linear functions of the
mole fraction as shown in Fig. 24.3-1. For nonideal mixtures
9AB
and
RB
are different
nonlinear functions of the mole fraction; an example is shown in Fig. 24.3-2. However,
the product
pGAB
has been found for some nonideal mixtures to be very nearly linear in
the mole fraction, whereas
/&JAB
is not (see Fig. 24.3-3). There is no compelling reason to
prefer one diffusivity over the other. Most of the diffusivities reported in the literature
are and not
BAB.
924.4
APPLICATIONS OF
THE
GENERALIZED
MAXWELL-STEFAN EQUATIONS
The generalized Maxwell-Stefan equations were given in
Eq.
24.2-4 in terms of the diffu-
sional driving forces d,, and the expression for d, was given in
Eq.
24.1-8. When these
are combined we get the Maxwell-Stefan equations in terms of the activity gradient, the
pressure gradient, and the external forces acting on the various species, given (Eqs. 24.2-
8 or
9):
X,Xfi
-
d,
=
-
(v,
-
vp)
+
thermal diffusion terms
n=1
goo
The thermal diffusion terms have not been displayed here, since they will not be needed
in this section. The symbols
m,,
=
cNl/,
and
w,
designate, respectively, the volume frac-
tion and mass fraction of species
a.
As explained in SS24.1 and 2, several auxiliary rela-
tions have to be kept in mind:
The first of these relations follows from the definition of the d,, the second is a conse-
quence of the Onsager reciprocal relations, and the third is needed because of the intro-
duction of
an
especially designated species
y.
The choice as to which species is
designated as
y
is arbitrary; often setting
y
equal to
a
is convenient. The choice depends

776
Chapter 24 Other Mechanisms for Mass Transport
on the nature of the system under study, and this point will be illustrated in the exam-
ples that follow.
In all previous chapters, the only external force that has been considered has been
the gravitational force. In this section we set the external force per unit mass
g,
equal to a
sum of forces
Here
g
is the gravitational acceleration,
z,
is the elementary charge on species
a
(for ex-
ample, -1 for the chloride ion C1-),
F
=
96485
abs.-coulombs/g-equivalent
is the Fara-
day constant,
4
is the electrostatic potential, and the subscript
m
on the Kronecker delta
S,,
refers to any mechanically restrained matrix, such as
a
permselective membrane.
In sum, for solving multicomponent diffusion problems in isothermal systems, we
now have
N
mass-flux equations (of which only
N
-
1
are independent), the species
equations of continuity, and the equation of motion. This set of equations has proven to
be useful for solving wide classes of mass transfer problems, and we discuss some of
these in the following examples.
Of course, in order to solve multicomponent diffusion problems one needs the
Maxwell-Stefan diffusivities
Bmp
that occur in Eq. 24.4-1. Very few measurements have
been made of these quantities, which require the simultaneous measurement of the ac-
tivity as a function of concentration. Among the few examples of such measurements are
those made by ~utten.'
EXAMPLE
24.4-1
Centrifugation
of
Proteins
Protein molecules are large enough that they can be concentrated by centrifugation against
the dispersive tendencies of Brownian motion, and this process has proven useful for molecu-
lar weight determination as well as for small-scale preparative separations. Show how the
be-
havior of protein molecules in a centrifugal field can be predicted, and the kind of
information that can be obtained from their behavior in a centrifuge tube (see Fig. 24.4-1).
As
we shall see in Example 24.4-3, we may treat the protein and its attendant counter-ions as a
single large electrically neutral molecule. Choose the protein as species
y
and begin with the
mass-flux equation for it. The small ionic species needed for protein stability play no signifi-
cant role in this development and can be ignored.
SOLUTION
We consider here a pseudobinary system of a single globular protein
P
in a solvent
W,
which
is primarily water, and initially we restrict the discussion to a dilute solution rotating in a
tube perpendicular to the axis of rotation (Fig. 24.,4-la) at a constant angular velocity
R.
For
such a system
x,
=
1
and the solute flow field with respect to stationary axes will be that
of
rigid-body rotation-namely,
v,
=
6,Ru.
f
Initial protein
band
2
4-
2
y..
0
.II
2
Fig.
24.4-1.
Ultracentrifugation of proteins, with two possi-
I
ble orientations of the centrifuge tube.
Ph.
W.
M.
Rutten,
Diffusion
in
Liquids,
Delft University Press, Delft, The Netherlands
(1992).
