Durst F. Fluid Mechanics: An Introduction to the Theory of Fluid Flows
Подождите немного. Документ загружается.

54 3 Physical Basics
Fig. 3.3 Definition of the pressure in
a fluid P (x
i
,t) by momentum exchange
x
1
x
2
x
3
F
j
Fig. 3.4 Fluctuation while determining
the pressure in a fluid
10
−12
to 10
−14
m
2
and therefore the mean numbers of molecules impinging
on such an area are sufficient to have the force effect of the molecules per
unit area. This, however, corresponds to a local definition of the pressure is
permissible, P (x
i
,t) for a fluid.
Similarly to the above continuum mechanics quantities ρ(x
i
,t)andP (x
i
,t),
there are other local field variables such as temperature, internal energy, and
enthalpy of a fluid, etc., for which the above considerations can be repeated.
Analogously to the above treatments for the density and pressure, it becomes
apparent, that molecular properties define continuum properties. This again
shows that it is possible for fluid mechanics considerations to neglect the
complex molecular nature of fluids. It is sufficient to introduce continuum
mechanics quantities into fluid mechanics considerations that correspond to
mean values of corresponding molecular properties. Fluid mechanics consid-
erations can therefore be carried out on the basis of continuum mechanics
properties of fluids.
However, there are some important domains in fluid mechanics where con-
tinuum considerations are not appropriate, e.g. the investigation of flows in
highly diluted gas systems. No clear continuum mechanics quantities can be
defined there for the density and pressure with which fluid mechanics pro-
cesses can be resolved. The required spatial resolution of the fluid mechanics

3.3 Transport Processes in Newtonian Fluids 55
considerations does not provide, due to the dilution, sufficient numbers of
molecules for the necessary establishment of the mean values of the consid-
ered continuum properties. Hence there are insufficient molecules available in
the considered δV for the introduction of the continuum mechanics quanti-
ties. When treating such fluid flows, priority has to be given to the molecular
theory rather than continuum mechanics considerations. In the present in-
troduction to fluid mechanics, the domain of flows of highly diluted gases is
not dealt with, so that all required considerations can take place in the ter-
minology of continuum mechanics. For continuum mechanics considerations,
molecular effects, e.g. within the conservation laws for mass, momentum and
energy, are presented in integral form, i.e. the molecular structure of the con-
sidered fluids is not neglected but taken into consideration in the form of
integral quantities.
3.3 Transport Processes in Newtonian Fluids
3.3.1 General Considerations
When treating fluid motions including the transport of heat and momentum
as well as mass transport, molecular transport processes occur that cannot
be neglected and that, hence, have to be taken into account in the general
transport equations. A physically correct treatment is necessary that orients
itself on the general representations of these transport processes and this is
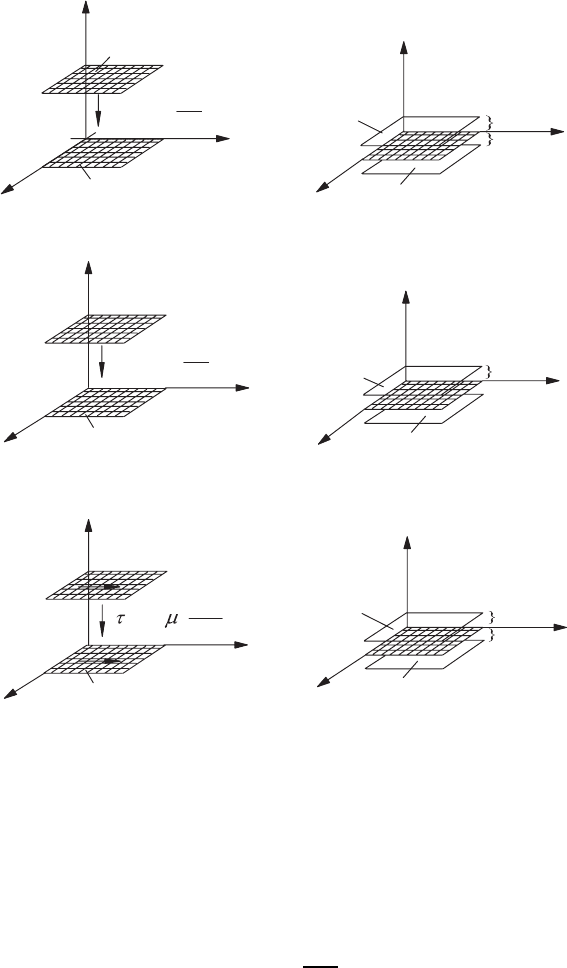
indicated below. For explanation we refer to Figs. 3.5a–c. These figures show
planes that lie parallel to the x
1
−x
3
plane of a Cartesian coordinate system.
In each of these planes the temperature T = constant (a), the concentration
c = constant (b) or the velocity (U
j
) = constant (c). There distributions in
space are such that, when taking into account an increase in the quantities in
the x
2
direction = x
i
direction, a positive gradient in each of these quantities
exists. It is these gradients that result in the molecular transports of heat,
mass and momentum.
In Fig. 3.5, the heat transport occurring, as a consequence of the molecular
motion, is given by the Fourier law of heat conduction and the mass transport
occurring analogously given by the Fick’s law of diffusion. The Fourier law
of heat conduction reads:
˙q
i
= −λ
∂T
∂x
i
, (3.5)
where λ = coefficient of heat conduction, and Fick’s law of diffusion reads:
˙m
i
= −D
∂c
∂x
i
, (3.6)
where D = mass diffusion coefficient.
56 3 Physical Basics
x
2
=x
i
T
A
>
x
1
x
3
q
i
=
λ
•
∂
T
∂
x
i
T
B
x
2
x
1
x
3
l
l
T
(
x
i
- l
)
T
(
x
i
+l
)
x
2
x
3
x
1
c
(
x
i
+l
)
c
(
x
i
-l
)
l
l
}
l
l
x
2
x
1
x
3
U
j
(
x
i
- l
)
U
j
(
x
i
+ l
)
x
2
= x
i
c
A
> c
B
m =
i
D
•
∂
c
∂
x
i
−
−
x
1
x
3
x
2
= x
i
x
3
x
1
= x
j
c
B
(
U
j
)
A
> (
U
j
)
B
i
j
=
−
∂
∂
x
i
U
j
(
U
j
)
B
a) Explanations of heat transport
b) Explanations of transport of chemical species
c) Explanations of momentum transport
T
B
Fig. 3.5 Analogy of the transport processes dependent on molecules for (a)heat
transport, (b) mass transport, and (c) momentum transport
In an analogous way, the molecule-dependent momentum transport also
has to be described by the Newtonian law, which in the presence of only one
velocity component U
j
can be stated as follows (see Bird et al. [3.1]):
τ
ij
= −µ
∂U
j
∂x
i
, (3.7)
where µ = dynamic viscosity.
In ˙q
i
,˙m
i
,andτ
ij
in the above three equations, the direction i indicates
the “molecular transport direction”, and j indicates the components of the

3.3 Transport Processes in Newtonian Fluids 57
Fig. 3.6 Exchange of mass and
momentum. Illustrative explana-
tion of τ
ij
as momentum transport
Exchange of mass and momentum
velocity vector for which momentum transport considerations are carried out.
It will be shown in Chap. 5 that the complete equation for τ
ij
, in the presence
of a Newtonian medium, can be represented as follows:
τ
ij
= −µ
∂U
j
∂x
i
+
∂U
i
∂x
j
+
2
3
µδ
ij
∂U
k
∂x
k
, (3.8
1
)
where τ
ij
represents the momentum transport per unit area and unit time and
therefore represents a “stress”, i.e. force per unit area. It is therefore often
designated “shear stress” and the sign before the viscosity coefficient µ is
chosen positive. This has to be taken into account when comparing treatments
of momentum in this book with corresponding treatments in other books.
The existing differences in the viewpoints are considered in following two
annotations.
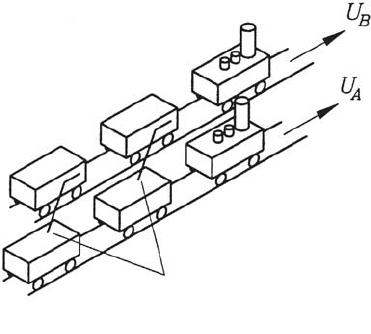
Annotation 1: The illustrative example in Fig. 3.6 shows how the viscosity-
dependent momentum transport, introduced in continuum mechanics, is
caused by motion of molecules. Two passenger trains may run next to one
another at different speeds. In each of the trains, persons are assumed to
travel carrying sacks along with them. These sacks are being thrown by the
passengers in one train to the passengers in the other train, so that a mo-
mentum transfer takes place; it should be noted that the masses m
A
and m
B
of the trains do not change. Because the persons in the faster train catch
the sacks that are being thrown to them from the slower train, the faster
train is slowed down. In an analogous way, the slower train is accelerated.
Momentum transfer (of the momentum in the direction of travel) takes place
by a momentum transport perpendicular to the direction of travel. This idea
transfers to the molecule-dependent momentum transport in fluids, is in ac-
cordance with the molecule-dependent transport processes of heat and mass
that were stated above.
1
τ
ij
as molecule-dependent momentum transport, as introduced here, is to be dif-
ferentiated, in principle, from the shear stress that is introduced in some books;
see Sect. 3.3.3.
58 3 Physical Basics
Fig. 3.7 Interaction of friction.
Illustrative representation of the
τ
ij
term as a friction term
Friction acting on side
walls of wagons of trains
Annotation 2: In continuum mechanics, the viscosity-dependent interaction
between fluid layers is generally postulated as “friction forces” between lay-
ers. This would, in the above-described interaction between trains (Fig. 3.7),
running alongside each another, correspond to passengers in each of the trains
exerting a friction force of the other train with bars, by scratching along the
other train’s wall. This idea does not correspond to the concept of molecular-
dependent transport processes between fluid layers of different speeds.
If one carries out physically correct considerations regarding the molecular-
dependent momentum transport τ
ij
, derivations have to be carried out as
presented in Sect. 3.3.3. In addition, considerations are presented below con-
cerning the existence of pressure and the occurrence of heat exchange and
mass diffusion in gases in order to show the connection between molecular
and continuum-mechanics quantities and transport processes.
3.3.2 Pressure in Gases
From the molecular theory point of view, the gaseous state of aggregation of a
fluid is characterized by a random motion of the atoms and/or molecules. The
properties that materials assume in this state of aggregation are described
fairly well by the laws of an ideal gas. All the laws for ideal gases result
from derivations that are based on mechanical laws for moving spheres. They
interact by ideal elastic collisions and in the same way the moving molecules
also interact with walls, e.g. with container walls. Between these collisions,
the molecules move freely and in straight lines. In other words, no forces
act between the molecules, except when their collisions take place. Likewise,
container walls neither attract nor repel the molecules and the interactions
of the walls with the moving molecules are limited to the moment of the
collision. The most important properties of an ideal gas can be stated as
follows:

3.3 Transport Processes in Newtonian Fluids 59
(a) The volume of the atoms and/or molecules is extremely small compared
with the distances between them, so that the molecules can be regarded
as material points.
(b) The molecules exert, except at the moment of their collisions, neither
attractive nor repulsive forces on each other.
(c) For the collisions between two molecules or a molecule and a wall, the
laws of perfect elastic collisions hold (collisions of two molecules take
place exclusively).
When one takes into account the characteristic properties of an ideal gas
listed in points (a)–(c), the derivations indicated below can be formulated
to obtain the pressure. This fluid property represents a characteristic con-
tinuum mechanics quantity of the gas, but it can be derived by taking
well-known molecular-theoretical considerations into account. The deriva-
tions in the theory only consider the known basic laws of mechanics and
the molecule properties indicated above in (a)–(c).
In order to derive the “pressure effect” of the molecules on an area, the
derivations are carried out by considering a control volume consisting of
acubewithanedgelengtha as shown in Fig. 3.8. Regarding this control
volume, the area standing perpendicular to the axis x
1
is hatched. All con-
siderations are made for this area. For other areas of the control volume, the
derivations have to be carried out in an analogous way, so that the consider-
ations for the hatched area in Fig. 3.8 can be considered as generally valid,
e.g. see Ref. [3.2].
In the control volume shown in Fig. 3.8, N molecules are present. With
the introduction of n molecules per m
3
(molecular density), this number N
is given by:
N = na
3
. (3.9)
From n molecules per unit volume, n
α
molecules with a velocity component
(u
1
)
α
may move in the direction of the axis x
1
and interact with the hatched
area in Fig. 3.8. In a time ∆t all molecules will hit the wall area that are at
Fig. 3.8 Control volume for derivations
of pressure from the molecular interaction
with walls
a
a
a
60 3 Physical Basics
adistanceof(u
1
)
α
∆t from it
z
α
= n
α
a
2
(u
1
)
α
∆t. (3.10)
Each of the z
α
molecules exerts a momentum on the wall that is formulated
by the law of ideal elastic collision:
∆(i
1
)
α
= −m∆(u
1
)
α
=2m(u
1
)
α
(3.11)
For the total momentum transferred by z
α
molecules to the wall, we can
write:
∆(J
1
)
α
= z
α
∆(i
1
)
α
= n
α
a
2
(u
1
)
i
∆t[2m(u
1
)
α
] (3.12)
∆(J
1
)
α
=2ma
2
∆tn
α
(u
2
1
)
α
. (3.13)
The wall experiences a force (K
1
)
α
:
(K
1
)
α
=
∆(J
1
)
i
∆t
=2ma
2
n
α
(u
2
1
)
α
(3.14)
and the following pressure (P
1
)
α
results:
(P
1
)
α
=
(K
1
)
α
a
2
=2mn
α
(u
2
1
)
α
. (3.15)
The total pressure which is exerted on the hatched area in Fig. 3.8 summarizes
the pressure contributions (P
1
)
α
of all differing velocities (u
1
)
α
.Ifonewants
to calculate the total pressure, one has to sum up over all these contributions.
Then one obtains from the above equation:
P
1
=
n
x
α=1
(P
1
)
α
=2m
n
x
α=1
n
α
(u
2
1
)
α
. (3.16)
The summation occurring in the above relation can be substituted by the
following definition of the mean value of the velocity squared,
u
2
1
:
n
x
α=1
n
α
(u
2
1
)
α
= n
x
(u
2
1
), (3.17)
If n
x
is the total number of molecules per unit volume moving in the positive
direction x
1
, i.e.
n
x
=
1
6
n, (3.18)
where n
x
represents the mean number of molecules present in a unit volume;
u
2
1
represents the square of the “effective value” of the molecular velocity,
which, according to the above derivations, can be defined as follows:
(u
2
1
)=
1
n
α
n
x
α=1
n
α
(u
2
1
)
α
=
6
n
n
x
α=1
n
α
(u
2
1
)
α
. (3.19)
3.3 Transport Processes in Newtonian Fluids 61
The thermodynamic pressure P in a free fluid flow is defined generally as the
mean value of the sum of the pressures in all three directions:
P =
1
3
(P
1
+ P
2
+ P
3
)=
2m
3
⎡
⎣
N
α
α=1
n
α
!
u
2
1
"
α
+
N
β
β=1
n
β
!
u
2
2
"
β
+
N
γ
γ=1
n
γ
!
u
2
3
"
γ
⎤
⎦
=
2m
3
n
x
u
2
1
+ n
y
u
2
2
+ n
z
u
2
3
=
2m
3
1
6
n
3
u
2
=
1
3
mn
u
2
=
1
3
ρ
u
2
The pressure with which the hatched area is associated is therefore:
P =
1
3
mn
(u
2
). (3.20)
As m is the mass of a single molecule and n the mean number of molecules
per unit volume, the expression mn corresponds to the density ρ in the
terminology of continuum mechanics:
P =
1
3
mn
(u
2
)=
1
3
ρ
(u
2
) (3.21)
P =
1
3
ρ
(u
2
). (3.22)
This relationship contains the continuum property ρ and also the mean
molecular velocity squared. The squared mean velocity can be eliminated
by another quantity of continuum mechanics, namely the temperature T of
an ideal gas (see H¨ofling [3.3]).
The mean kinetic energy of a molecule can be written according to the
equipartition law of statistical physics:
e
k
=
1
2
m
(u
2
)=
3
2
kT, (3.23)
where k =1.380658 ×10
−23
JK
−1
represents the Boltzmann constant. From
(3.22) and (3.23) the following expression results:
P =
1
3
ρ(3
k
m
T )=ρ
k
m
T. (3.24)
Further,
k =
L
=
universal gas constant
Loschmidts’s number
. (3.25)
Then,
P =
T
Lm
ρ, (3.26)

62 3 Physical Basics
where M = Lm is the mass per kmol of an ideal gas, so that υ = M/ρ can
be written:
Pυ = T, (3.27)
where υ represents the gas volume per kmol and is the general ideal gas
constant (see to Bosnjakovic [3.4]).
Strictly, the above derivations can only be stated for a monatomic gas,
with the assumption of ideal gas properties. However, the above law can be
transferred to polyatomic gases with “ideal gas properties” if the additional
degrees of freedom present in polyatomic gases and the corresponding con-
stituents of the internal energy of a gas are taken into account. Generally,
the energy content of a gas can be stated as follows:
e
gas
=
α
2
kT, (3.28)
where α indicates the degrees of freedom of the molecular motion:
α = 3 with monatomic gases,
α = 5 with a diatomic gases,
α = 6 with triatomic and polyatomic gases.
The above derivations have shown that the properties of an ideal gas, that
are known from continuum mechanics, can be derived from molecular theory
considerations. This means that the laws of continuum mechanics, at least for
the pressure derived here, with the introduction of density and temperature,
are consistent with the corresponding considerations of the mechanical theory
of molecular motion.
3.3.3 Molecular-Dependent Momentum Transport
In Sect. 3.3.1, transport processes that are caused by the thermal motion of
the molecules were considered in an introductory way. Attention was drawn to
the analogy between momentum heat and mass transport and it was pointed
outthattheτ
ij
terms used in fluid mechanics are not considered to be caused
by friction, i.e. physically they represent no friction-caused “shear stress”
but represent molecular-caused momentum transport terms occurring per
unit area and time; the index i represents the considered molecular trans-
port direction and j the direction of the considered momentum. In order to
give an introduction into physically correct considerations of the molecular-
dependent momentum transport terms, the following derivations for an ideal
gas are made, where only an x
1
momentum transport in the direction x
2
is
considered, i.e. the term τ
21
.
For the derivations below, a velocity distribution has to be used that cor-
responds to the equilibrium distribution (Maxwell distribution), in which
3.3 Transport Processes in Newtonian Fluids 63
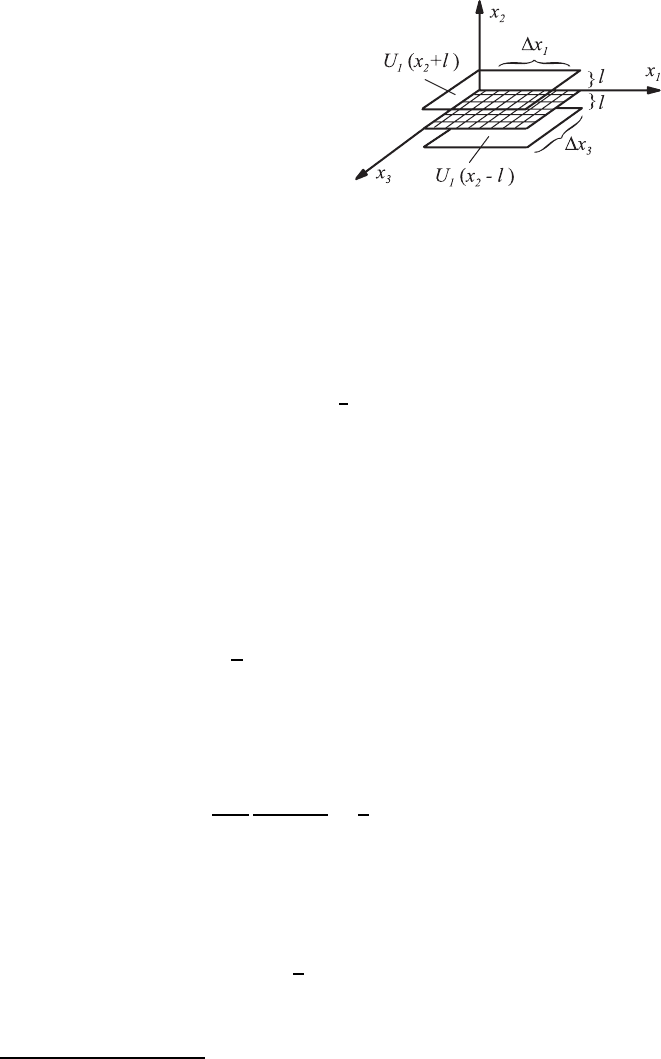
Fig. 3.9 Molecular motion and shear
stress: consideration of transport in the x
2
direction of U
1
momentum
˙m
i
=0.
2
The following simple model for a mean velocity distribution is used.
One-sixth of all molecules at a time move with a velocity (−¯u, 0, 0), (¯u, 0, 0),
(0, −¯u, 0), (0, ¯u, 0), (0, 0, −¯u), (0, 0, ¯u), with the directions of motion being
perpendicular to the axis of the coordinates. When one assumes a molecular
concentration per unit volume of n, i.e. n molecules per unit volume, one-
thirdofthemonanaveragemovewithavelocity¯u in the direction x
2
and of
these again half, i.e. each n/6 molecules per unit volume, move in a negative
and positive x
2
direction. On average,
1
6
n¯u molecules per unit time and unit
area move through the area of the plane x
2
= constant, which is indicated in
Fig. 3.9.
The molecules which traverse the plane x
2
= constant in the positive x
2
direction have, on average, collided the last time at a distance l with molecules
below the plane, where l represents the mean free path of the molecular
motion. The molecules coming from below thus possess, on average, the mean
velocity which the flowing medium has in the plane (x
2
−l). Consequently, the
molecular transport in the positive direction x
2
posseses an x
1
momentum,
which can be stated as follows:
∆J
1
=
1
6
n¯u[mU
1
(x
2
− l)]∆t ∆x
1
∆x
3
. (3.29)
This is connected with an “effective force” per unit time and unit area, i.e.
a force acting on the hatched area of Fig. 3.9, arising as a consequence of an
x
1
momentum that is transported by molecular motion in the positive x
2
direction:
τ
+
21
=
∆J
1
∆t
1
∆x
1
∆x
3
=
1
6
mn¯uU
1
(x
2
− l). (3.30)
In an analogous way, the molecular motion through the plane x
2
= constant
in the negative x
2
direction can be stated to carryout a x
1
momentum trans-
port per unit time and unit area. For the latter the resultant stress can be
calculated as:
τ
−
21
= −
1
6
nm¯uU
1
(x
2
+ l). (3.31)
Hence the entire momentum exchange per unit area and unit time which the
hatched plane x
2
= constant experiences can be expressed as:
2
The derivations are only valid if no self diffusion of mass is present in a flow.
