Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.

To summarize, there is much to be learned regarding the toxicity of nanostructures.
The data thus far are contradictory in many respects, which may depend on the
processing parameters of the nanostructures used within particular in vitro/in vivo
tests. In an attempt to define the risks in a more tangible and coordinated way, there
has been a recent attempt at developing a risk-based classification system for nanoma-
terials.
[56]
However, research in this area must continue to expand in order for us to
truly understand the risks that may accompany this exciting “nanofrontier”.
[57]
In addition to these toxicological-based questions, a number of ethical consi dera-
tions must also be addressed
[58]
:
(i) Equity issues – will nanotechnology be utilized to solve third-world problems, or
will it primarily be used to increase the prowess of industrially advanced countries?
(ii) Priv acy issues – imagine a world where you have invisible sensors/micro-
phones – need we say more...
(iii) Security – how will our country and others defend itself against invisible
nanoweaponry?
(iv) Hum an–machine interactions – there are many religious and philosophical
issues associated with embedded nanodevices within the human body.
6.2. WHAT IS “NANOTECHNOLOGY”?
Although there is much current excitement and hype
[59]
about nanomaterials, there
is really nothing new about nanoscience. In fact, the earliest civilizations used
nanoscale materials for a variety of applications. For example, the Mayans used a
magnesium aluminum silicate clay called palygorskite, which contained nanosized
channels that were filled with water. The Mesopotamian civilizations used colored
glass for decorative applications that contained embedded metallic nanoparticles.
Physics Nobel Laureate Richard Feynman gave the first lecture regarding the
applications for nanoscale materials. His talk, entitled “There’s Plenty of Room at
the Bottom,” was delivered on 29 December 1959 at the annual American Physical
Society meeting on the campus of Caltech. Appendix B contains a transcript of his
entire talk, which contains references to a future world that was never before imagined.
Feynman pointed out that designing materials atom-by-atom is a real possibility, as it
would not violate any physical laws. He also predicted such sci-fi accomplishments as
writing 24 volumes of the Encyclopedia Britannica on the head of a pin, and even more
amazingly, the complete reproduction of every book ever produced to fit within a small
handheld pamphlet of less than 40 pages! To put these prophetic statements into
context, at the time he delivered this speech, computers such as UNIVAC 1 filled an
entire room (Figure 6.6) and carried a price tag of over $1 million.
The first use of the term “nanotechnology” was by Norio Taniguchi in 1974 at the
International Conference on Precision Engineering (ICPE). His definition referred to
“production technology to get extra high accuracy and ultra fine dimensions, i.e., the
preciseness and fineness on the order of 1 nm (nanometer), 10
9
m in length.”
[60]
Although many definitions for nanotechnology have been suggested, NASA
recently suggested the most thorough description:
468 6 Nanomaterials
The creation of functional materials, devices and systems through control of
matter on the nanometer length scale (1–100 nm), and exploitation of novel
phenomena and properties (physical, chemical, biological) at that length scale.
[61]
Although Feynman and Drexler certainly popularized nanotechnology, their
influence did not directly lead to the design of nanoscale materials. Rapid progress
in nanotechnology could only take place after the arrival of sophisticated instrumen-
tation, capable of viewing and manipulating materials on the nanoscale. In the
1980s, scanning probe microscopy (SPM) was developed which allowed scientists
to fulfill Feynman’s vision of pushing individual atoms around a surface (Figure 6.7).
This technique was co-invented by Calvin Quate and Hemantha Kumar Wickra-
masinghe. Interestingly, when Quate and Binnig first submitted their work to the
peer-reviewed journal Physical Review Letters, it was rejected due to such far-
fetched claims as being able to measure forces on individual atoms. However,
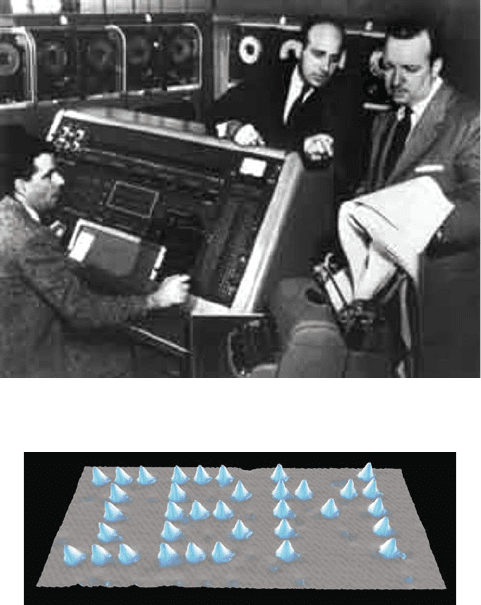
Figure 6.6. Photo of the room-sized UNIVAC 1 computer system that was introduced in the late 1950s.
Figure 6.7. Scanning tunneling microscope image of the placement of individual Xe atoms on a Ni(110)
surface – no surprise, by researchers at IBM. Reproduced with permission from Eigler, D. M.; Schweizer,
E. K. Nature 1990, 344, 524. Copyright 1990 Macmillan Publishers Ltd.
6.2. What is “Nanotechnology”? 469

these results were eventually publishe d, directly influencing the future of molecular
nanotechnology. The 1986 Nobel Prize in Physics was awarded to Gerd Binnig and
Heinrich Rohrer to honor their design of the scanning tunneling microscope (STM).
They shared the Prize with Ernst Ruska, the inventor of the first electron microscope,
another essential tool for the modern nanomaterials scientist. In fact, the resolution
of modern electron microscopes are now high enough to provide images of
individual atoms, and are often fitted with detectors that are capable of determining
the chemical composition and/or oxidation state of the surface atoms. Chapter 7 will
describe these and other instruments that are commonly used for materials-related
research and development.
6.3. NANOSCALE BUILDING BLO CKS AND APPLIC ATIONS
The first question almost everyone new to the nanoregime asks is “why are nanoma-
terials so special?” The leading advantage of this size regime is the relatively large
surface area/volume ratio exhibited by nanomaterials; that is, with decreasing size, an
increasingly larger percentage of (coordinatively unsaturated) atoms reside at its
surface (Figure 6.8 and Table 6.3). Accordingly, this translates to a very high surface
reactivity with the surrounding surface, ideal for catalysis or sensor applications.
Further, since biological systems feature the systematic organization of nanoscale
materials (e.g., proteins are 1–20 nm in size, the diameter of DNA is ca. 2.5 nm),
being able to fabricate materials in this size regime holds promise for artificial
Figure 6.8. Comparison of the surface area/volume ratio of macroscopic particles (marbles) and
nanoscopic aluminum oxide particles. Since nanoparticules contain a proportionately large number of
surface atoms, there are a significantly greater number of adsorption/reaction sites that are available to
interact with the surrounding environment. Further, whereas bending of a bulk metal occurs via
movement of grains in the >100 nm size regime, metallic nanostructures will have extreme hardness,
with significantly different malleability/ductility relative to the bulk material.
470 6 Nanomaterials

components within cells (that have ca. 10,000–20,000 nm diameters) to diagnose/fight
diseases, illnesses, viruses, and other superficial weaknesses (e.g., artificial muscles).
Another key benefit for nanomaterials is the ability of varying their funda-
mental properties (e.g., magnetization,
[62]
optical properties (color), melting point
(Figure 6.9), hardness, etc.), relative to bulk materials without a change in chemical
composition. Bulk propert ies such as decreased melting point, enhanced solubility,
and high hardness are related to an increase of surface energy generated by the high
density of surface atoms. In contrast, the size-tunable electronic properties are due
to quantum-confinement effects, as discussed in later in this chapter.
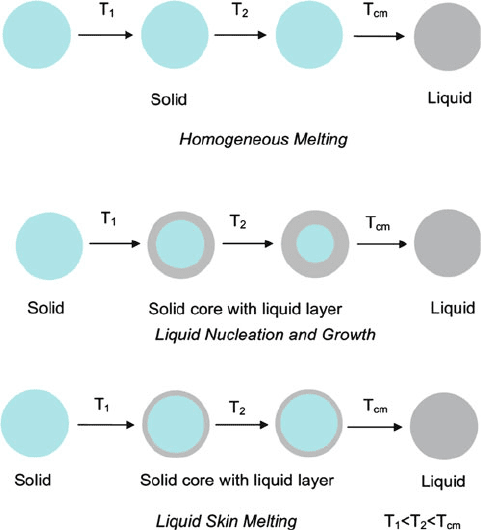
There are three hypotheses that describe the depressed melting point of nanos-
tructures relat ive to their bulk counterparts (Figure 6.10).
[63]
The first, known as
homogeneous melting (HM) assumes that no surface melting occurs. In contrast,
liquid skin melting (LSM) assumes that a liquid layer is formed around a solid core at
a relatively low temperature. The nanoparticle is thought to remain in that state until
it is completely transformed into a liquid at its melting point. The third process is
referred to as liquid nucleation and growth (LNG), which is a surface-melting
phenomenon wherein a liquid layer is formed at lower temperatures and grows
with increasing temperatures. Considering these three hypotheses, the general
expression for the nanostructural melting temperature, T
m,n
, may be expressed as
[63]
:
T
c;n
T
c;b
¼ 1
2Vðg
sv
g
lv
Þa
DH
f
ðD 2dÞ
;ð1Þ
Table 6.3. Comparison of the % Surface Atoms for Gold Nanoparticles
a
Particulate radius (nm) Total # of atoms % Surface atoms
0.65 79 76
0.71 116 67
0.81 140 69
0.87 201 64
0.98 225 62
1.1 309 52
1.2 459 51
1.4 807 43
1.5 976 40
1.6 1,289 37
2.0 2,406 31
2.2 2,951 30
2.4 4,033 27
2.6 4,794 26
2.8 6,266 23
a
Data taken from Hostetler, M. J.; Wingate, J. E.; Zhong, C. -J.; Harris, J. E.;
Vachet, R. W.; Clark, M. R.; Londono, J. D.; Green, S. J.; Stokes, J. J.;
Wignall, G. D.; Glish, G. L.; Porter, M. D.; Evans, N. D.; Murray, R. W.
Langmuir 1998, 14, 17. Au nanoparticles were stabilized by dodecanethiol,
and sizes were controlled by varying the sodium borohydride reduction
temperature and rate of addition, and the molar ratio of dodecanethiol:
HAuCl
4
xH
2
O.
6.3. Nanoscale Building Blocks and Applications 471
where: T
c,n
and T
c,b
¼ melting points of nanostructures and bulk, respectively;
g
sv
and g
lv
¼ surface energies of the solid-vapor and liquid-vapor interfaces
of the material, respectively;
DH
f
¼ bulk latent heat of fusion
D ¼ average d iameter of the nanostructures
a ¼ 2 for LNG; a ¼ 3 for HM and LSM
d > 0 for LSM and < 0 for either HM or LNG
Since we live in a macroscopic world, the next generation of materials will be of
similar physical dimensions as today’s consumer products. That is, we have shrunk
down the size of cell phones and computers to almost their useful limits – any further,
and one would inconveniently need to use a sharp stylus to dial a phone number!
However, as articulated in Chapter 4, although the size of electronic devices will
remain somewhat constant, the speed and computational ability of these d evices must
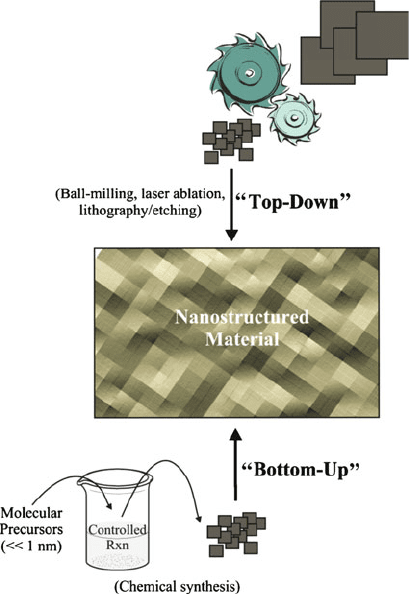
continue to increase. This translates to materials that are built from the ground up, one
nanoscale building block at a time. However, it is synthetically too expensive (and
not industrially scalable) to arrange such small units into their desired positions by
hand. Consequently, materials chemists are largely focused on “bottom-up” techni-
ques that afford the self-assembly of nanoscale species. As we saw in Chapter 4,
parallel efforts in “top-down” processing are being developed by materials engineers
to yield nanoscale building blocks and devices through advanced lithographic,
ablation, and etching techniques (Figure 6.11). In this respect, one can consider a
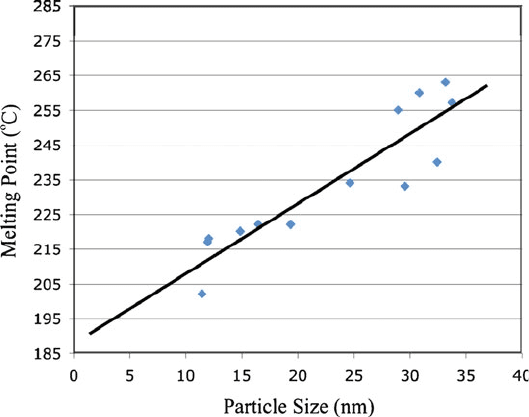
Figure 6.9. Decrease in the melting point of gold nanoparticles with decreasing diameter. It should be
noted that the melting point of bulk gold is 1,064
C! Adapted with permission from Unruh, K. M. et al.
“Melting Behavior in Granular Metal Thin Films,” Materials Research Society Symposium Proceedings,
vol. 195. Materials Research Society, Apr 16–20, 1990. Copyright 1990 Materials Research Society.
472 6 Nanomaterials
nanoscale object as being “mesomolecular” or “mesoatomic” – an aggregate of
smaller molecular/atomic subunits.
There are three primary types of nanoscale building blocks that may be used for
further device fabrication and applications:
(i) 0-D (e.g., nanoparticles, nanoclusters, nanocrystals, quantum dots)
(ii) 1-D (e.g., nanotubes, nanofibers, nanowires, nanor ibbons)
(iii) 2-D (e.g., thin films (see Chapter 4 ), graphene/graphene oxide sheets)
The direct incorporation of these nanoarchitectures in existing materials to
improve their properties is often referred to as incremental nanotechnology. How-
ever, as we will see later in this chapter, the self-assembly of nanosized building
blocks into 3-D architectures may yield entirely new devices and functionalitie s –
referred to as evolutionary nanotechnology.
6.3.1. Zero-Dimensional Nanomaterials
Analogous to the period in this sentence, a “zero-dim ensional” structure is the
simplest building block that may be used for nanomaterials design. These materials
have diameters <100nm, and are denoted by nanoparticles, nanoclusters, or
Figure 6.10. Illustration of nanoparticle melting hypotheses. Reproduced with permission from Nanda,
K. K. Pramana - J. Physics, 2009, 72, 617. Copyright 2009 Indian Academy of Sciences.
6.3. Nanoscale Building Blocks and Applications 473
nanocrystals, which are used synonymously in the literature. However, in order to
continue our rapid nanoscience developments, the active participants must share a
common language. Since there has been no broad adoption of such terminology,
[64]
a goal of this chapter is to provide explicit definitions and examples (Figure 6.12),
in order to avoid the current nomenclature ambiguities.
The term nanoparticle is generally used to encompass all 0-D nanosized building
blocks (regardless of size and morphology), or those that are amorphous and possess
a relatively irregular shape. Herein, we will define nanoparticles as amorphous or
semicrystalline 0-D nanostructures with dimens ions larger than 10 nm, and a
relatively large (15%) size dispersion. For amorphous/semicrystalline nanostruc-
tures smaller in size (i.e., 1–10 nm), with a narrow size distribution, the term
nanocluster is more appropriate. This distinction is a simpl e extension of the term
“cluster,” which is typically used in inorganic/organometallic chemistry to indicate
small molecular cages of fixed sizes (Figure 6.12).
Analogous to bulk materials, the agglomeration of noncrystalline nanostructural
subunits should best be termed a nanopowder (Figure 6.12). Though the gross
Figure 6.11. Schematic comparison of the “top-down” and “bottom-up” approach to nanomaterials synthesis.
474 6 Nanomaterials
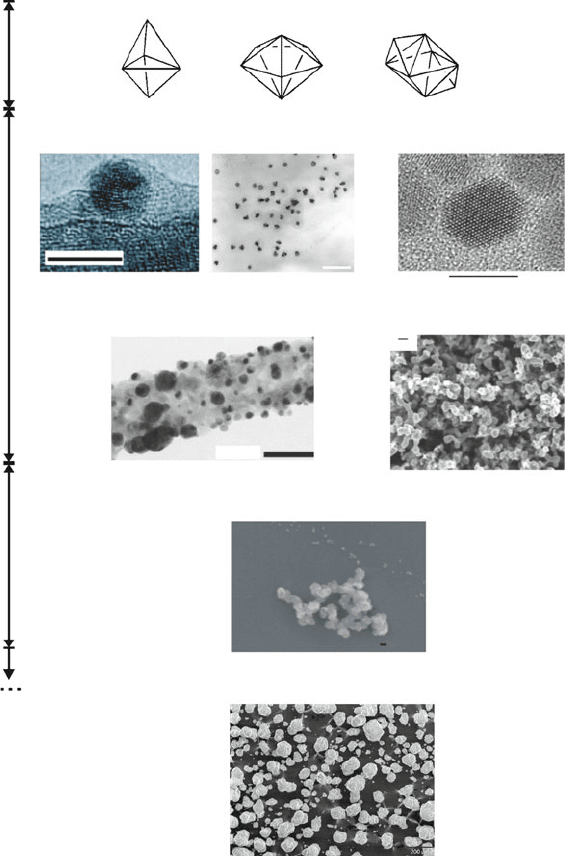
Clusters
Nanoclusters
Nanoparticles Nanopowder
Sub-Micron Particles
Bulk Powder
Nanocrystals
a
b
c
<< 1 nm1 - 100 nm100 nm - 1000 nm
1 - 100 µm: “microstructures”
> 100 µm: “particulates”
50 nm
5 nm
500 nm
5 nm
100 nm
100 nm
Figure 6.12. 0-D nanostructure nomenclature. Shown are the well-defined cage sizes of molecular
clusters (Os
5
(CO)
16
), (Os
6
(CO)
18
), [Os
8
(CO)
22
]
2
.
[65]
Comparatively, nanoclusters should be used to
describe 0-D nanostructures of a homogeneous size distribution.
[66]
By contrast, nanoparticles exhibit a
greater range of sizes/shapes.
[67]
Nanocrystals are characterized by the presence of an ordered lattice array
of the constituent subunits, as illustrated by a single nanocrystal of CdSe.
[68]
In stark contrast to a
nanocrystal, an example of a nanopowder is shown that consists of microscopic grains, each comprised
of nanoscale amorphous units.
[69]
The size regime that is intermediate between the nano- and
microregimes is best referred to as submicron.
[70]
The bulk powder scale bar is 200 mm.
6.3. Nanoscale Building Blocks and Applications 475
dimensions of these particulates are typically much greater than 100 nm, they will
exhibit a large specific surface area (SSA), which may be easily determined by
particle-size analyzers (BET) that quantifies the surface adsorption of an inert gas
such as nitrogen. Using this parameter, one can then properly define a nanopowder
as having a specific surface area >60 m
2
/g, which corresponds to the SSA of solid
spheres of unit density with a diameter of exactly 100 nm.
It is also important here to note the difference between nanoparticles/nanoclusters
and traditional colloids, which date back to the early 1860s (Table 6.4). We are all
familiar with the term colloid, which is used to describe solid/liquid and solid/gas
suspensions such as milk, paints, butter, smoke, and smog. Although both types of
materials have sizes within the nanoregime, the leading difference is the control one
has over composition and morphology. As we will see shortly, in order to stabilize
metal nanostructures, a stabilizing agent is often used to prevent agglomeration into
a larger powder. This is also the case for colloids, which generally employ poly-
dispersed organic polymers and other ionic species that may adsorb to the colloid
surface. Such a variation in the nature of the encapsulating environment leads to a
large dispersity in overall morphology and properties of colloids. By contrast, in
order for nanomaterials to be used for “bottom-up” design, their synthesis and
resultant properties must be reproducible. This is easily accomplished through the
use of stabilizing agents with well-defined structures that do not react with/surface
deactivate the entrain ed nanostructures (e.g., dendrimers, polyoxoanions, etc.).
Thus far, we have defined nomenclature for amorphous 0-D nanostructures.
Analogous to bulk materials, any nanomaterial that is crystalline should be referred
to as a nanocrystal. This term should be reserved for those materials that are single-
crystalline; if a particle exhibits only regions of crystallinity, it is better termed a
nanoparticle or nanocluster depending on its dimensions. Transmission electron
microscopy, especially in tandem with electron diffraction is most useful in deter-
mining the crystallinity of any nanostructure (Figure 6.13).
A special case of nanocrystal that is comprised of a semiconductor is known as
a quantum dot (QD).
[73]
Typically, the dimensions of these n anostructures lie in
Table 6.4. Comparison of 0-D nanoarchitectures with traditional colloids
[71]
Nanoparticles/nanoclusters Colloids
Size: 1–100 nm (nanoclusters: 1–10 nm) Typically >10 nm
Homogeneous molecular composition 15% Size
dispersion (less polydispersity for nanoclusters
relative to nanoparticles)
Poorly defined compositions >15% Size dispersion
Reproducible synthesis (control over size, shape,
and composition)
Nonreproducible, uncontrollable morphology/
composition
Reducible physical properties and catalytic activity Nonreproducible properties (esp. irreproducible
catalytic activities
[72]
)
Soluble in polar/nonpolar organic solvents
(depending on stabilizing agent)
Typically only soluble in polar solvents
Contain clean surfaces Contain surface-adsorbed species such as —OH,
—X, —OH
2
, etc.
476 6 Nanomaterials
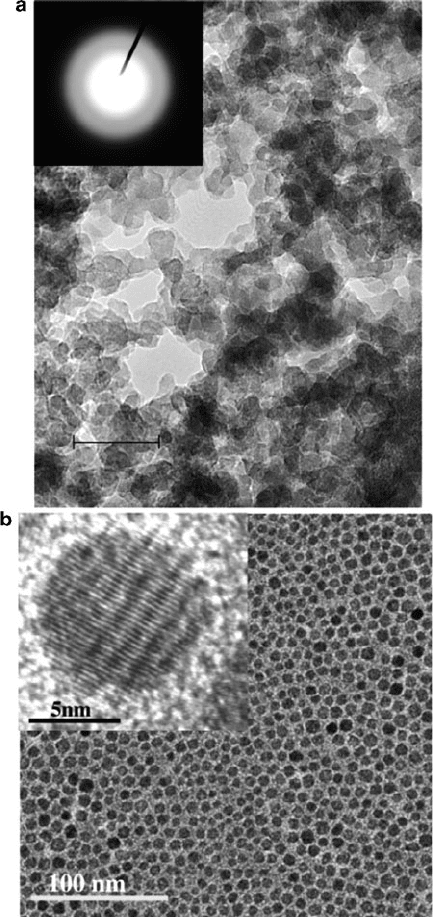
Figure 6.13. TEM images of amorphous nanoclusters (a), and nanocrystals, (b). The inset of (a) shows
selected area electron diffraction (SAED); the absence of a pattern indicates an amorphous structure. The
scale bar is 20 nm. The high-resolution TEM image inset in (b) shows lattice spacings of an individual
nanocrystal. Reproduced with permission from (a) Liu, S.; Fooken, U.; Burba, C. M.; Eastman, M. A.;
Wehmschulte, R. J. Chem. Mater. 2003, 15, 2803. (b) Tirosh, E.; Shemer, G.; Markovich, G. Chem.
Mater. 2006, 18, 465. Copyright 2003 & 2005 American Chemical Society.
6.3. Nanoscale Building Blocks and Applications 477
