Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


13.1 Introduction 405
Table 13.1 Groups, formula, structural description and specifi c minerals of the clay group.
Group Formula Description Mineral(s)
Clays Kaolinite Al
2
Si
2
O
5
(OH)
4
Silicate sheets (Si
2
O
5
)
bonded to aluminum
oxide/hydroxide layers
(Al
2
(OH)
4
)
Kaolinite
Dickite
Nacrite
Smectite (Ca, Na, H)(Al, Mg,
Fe, Zn)
2
(Si,
Al)
4
O
10
(OH)
2
– x H
2
O
S ilicate layers sandwiching
a g ibbsite [Al
2
(OH)
4
] (or
brucite [Mg
2
(OH)
4
]) layer in
between, in an s - g - s
stacking sequence. Variable
amounts of water
molecules lie between the
s - g - s sandwiches
Pyrophyllite
Talc
Vermiculite
Sauconite
Saponite
Nontronite
Montmorillonite
Illite (Mica) (K, H)Al
2
(Si,
Al)
4
O
10
(OH)
2
– x H
2
O
S ilicate layers sandwiching
a g ibbsite - like layer in
between, in an s - g - s
stacking sequence. The
variable amounts of water
molecules would lie
between the s - g - s
sandwiches as well as the
potassium ions
Biotite
Lepidolite
Muscovite
Paragonite
Phlogopite
Zinnwaldite
Chlorite X
4 – 6
Y
4
O
10
(OH, O)
8
a
S ilicate layers sandwiching
a b rucite or brucite - like
layer in between, in an
s - b - s stacking sequence
similar to the above groups.
There is an extra weakly
bonded brucite layer in
between the s - b - s
sandwiches. This gives the
structure an s - b - s b s - b - s b
sequence. The variable
amounts of water
molecules would lie
between the s - b - s
sandwiches and the brucite
layers
Amesite
Baileychlore clinochlore
Cookeite corundophilite
Daphnite
Delessite
Gonyerite
Nimite
Odinite
Orthochamosite penninite
Pannantite
Rhipidolite (prochlore)
Sudoite
Thuringite
etc.
a The X represents either aluminum, iron, lithium, magnesium, manganese, nickel, zinc or, rarely, chromium.
The Y represents either aluminum, silicon, boron, or iron but mostly aluminum and silicon.
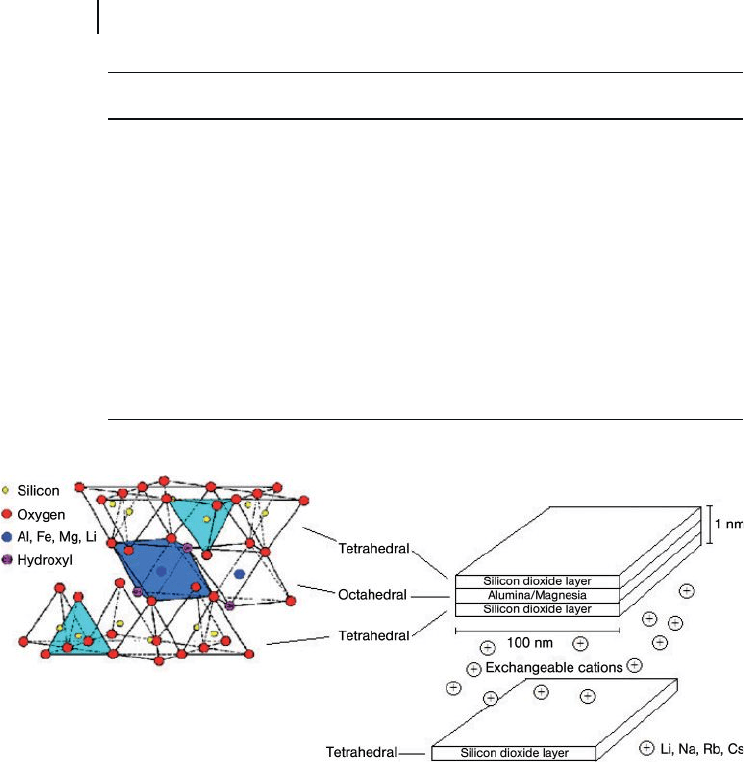
406 13 Nanocomposites Based on Phyllosilicates
Montmorillonite ( MMT ) a member of the smectite group, is commonly used in
polymer – clay nanomaterials. The layered structure of MMT (Figure 13.1 ) consists
of two silica tetrahedral (corner - shared) sheets fused to one (edge - shared) octahe-
dral sheet of alumina (aluminosilicate) or magnesia (magnesium silicate). The
lateral dimensions of these layers vary from 100 nm to a few microns, and about
1 nm in thickness, depending on the particular silicate. Due to an isomorphic
substitution of alumina into the silicate layers (Al
3+
for Si
4+
) or magnesium for
aluminum (Mg
2+
for Al
3+
), each unit cell has a negative charge of between 0.5 and
1.3. The layers are held together with a layer of charge - compensating cations, such
as Li
+
, Na
+
, K
+
, and Ca
2+
; the charge - compensating cations help the intercalation
and surface modifi cation of clays, which is required to disperse clays at the
Table 13.2 Charge/unit formula for different phyllosilicates minerals.
Mineral species Interlayer cations Charge/unit formula
Kaolinite - serpentine Kaolinite, Halloysite – 0.0
Pyrophillite - talc Antigorite, Chrysotile – 0.0
Smectite Montmorillonite,
Beidelite, Nontronite,
Saponite, Hectorite
Na, Ca 0.25 – 0.6
Vermiculite Dioctahedral - vermiculite Mg 0.6 – 0.9
Trioctahedral - Vermiculite
Mica Muscovite, Biotite,
Phlogopite
K 1.0
Figure 13.1 Structure of montmorillonite clay.

13.1 Introduction 407
Table 13.3 Structure and physical characterization of two organoclays.
Organic moiety CEC
(mEq kg
− 1
)
Moisture
content (wt%)
Loss on
ignition
Basal Spacing
(nm)
HDTM
Hexadecyltrimethylammoniumbromide
0.91 1.13 29.98 1.9
SMB
Stearyldimethylbenzylammoniumchloride
0.91 1.05 34.19 2.5
nanoscale into polymers. The cation - exchange capacity ( CEC ), which is indicative
of the intercalation capacity, is defi ned as the number of exchangeable interlayer
cations, and has units of mEq 100 g
− 1
. Typically, MMT has a CEC ranging from
approximately 75 to 115 mEq 100 g
− 1
[11] .
Other clays are saponite , a synthetic material that closely resembles MMT, and
hectrite , which is a magnesium silicate (CEC 55 mEq 100 g
− 1
) and its synthetic
equivalent, Laponite ® . Typically, sheets of Laponite are 25 – 30 nm wide, whereas
MMT has sheets that are approximately 200 nm in width.
The dispersion of a hydrophilic nanofi ller into a hydrophobic polymer matrix
requires a preliminary modifi cation of the clay to render it more organophilic.
Consequently, a treatment is often carried out with suitable chemicals to replace
the inorganic exchange ions in the galleries between the layers with alkylammo-
nium surfactants or other organic cations. Clays treated in this way are referred
to as “ organoclays ” . The number of onium ions that can be packed into the gal-
leries depends on the charge density of the clay and the CEC. In fact, at lower
charge densities the surfactants pack in monolayers while, as the charge increases,
bilayers and trilayers can be formed with different orientations of packed alkyl
chains. The intercalation of organophilic cations into the layers can be followed
using X - ray diffraction ( XRD ) analysis, with the distance between a plane in the
unit layer and the corresponding plane in the next unit layer being defi ned as the
“ basal plane spacing ” d
001
, often known as the “ interlayer distance ” . When a sur-
factant replaces sodium in the galleries, an increase in interlayer distance can be
observed. The organic moiety used for preparing organoclays depends on the host
polymer to be used as a matrix for the nanocomposites. Both, the structural and
physical characteristics of two common ammonium salts (hexadecyltrimethylam-
monium bromide and stearyldimethylbenzylammonium chloride) are reported in
Table 13.3 [7] .
408 13 Nanocomposites Based on Phyllosilicates
The aim of the ion exchange process is to enlarge the interlayer distance so as
to promote the accommodation of monomers or polymers, thus affording an
organophilic surface and improving wetting between the clays and polymers.
13.1.2
Morphology of Composites
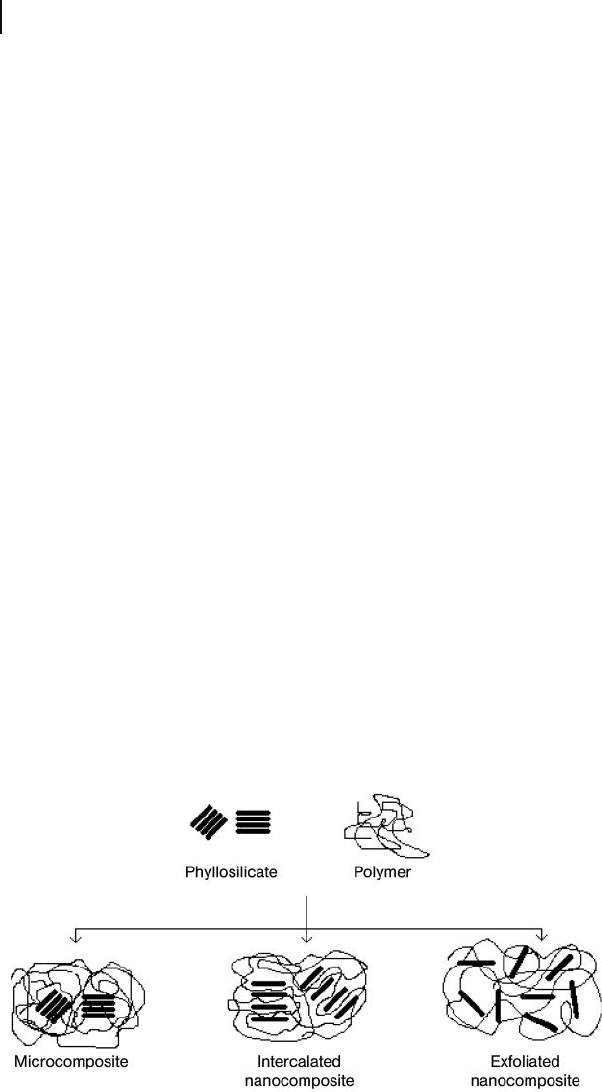
Polymer – clay composites based on layered silicates can be classifi ed into three
types, depending on the extent of separation of the silicate layers [12] , namely
conventional microcomposites, intercalated nanocomposites, and exfoliated nano-
composites (these are shown schematically in Figure 13.2 ). If the polymer does
not enter the galleries, the d
001
of the clay will remain unchanged, in agreement
with the achievement of a microcomposite. If an organic species enters the galler-
ies and causes an increase in d
001
, but the clay layers remain stacked, then the
composite is considered “ intercalated. ” However, if the clay layers are completely
pushed apart to create a disordered array, the composite is termed “ exfoliated. ”
Usually, a composite for which d
001
> 10 nm (a spacing that cannot be determined
by conventional XRD analysis) is designated as exfoliated. Recently, Sinha Ray
et al . [13] defi ned another type of nanocomposite within the set of “ intercalated
nanocomposites ” that they termed intercalated - and - fl occulated nanocomposites,
and which contained aggregates of intercalated silicate layers due to the hydroxy-
lated edge – edge interaction of the silicate layers. In fact, most intercalated tactoids
include both single stacks and several connected stacks of clay layers, such that
the distinction between fl occulation and intercalation will rest on an electron
microscopic analysis of the structure. Moreover, the “ fl occulation ” could be attrib-
uted to the long molecular chains that intercalate into two or more clay galleries
and play a bridging role. Electron microscopy studies have often shown that
most nanocomposites are both intercalated and exfoliated, but this cannot be
readily deduced from XRD measurements. This situation may occur because the
preparation method has not allowed suffi cient time for adsorption and penetration
Figure 13.2 Classifi cation of morphologies for polymer – clay composites.

13.1 Introduction 409
of the galleries to be completed, or because the dispersive mixing has been less
effective and that, if more time were available, the polymer – clay system might
develop either fully intercalated or exfoliated structures. The extent of clay disper-
sion in polymer is dependent on the intrinsic properties of the polymer and clay,
including the aspect ratio of the clay platelets, the volume fraction of the clay, the
nature/structure of polymer, the interactions between the polymer, clay and clay
modifi er, and the processing conditions used. Fully exfoliated polymer – clay nano-
composites are, in theory, only found for volume fractions of clay less than 3%,
as calculated by considering the small size of clay platelets [14] .
Three principal methods are available to determine the exfoliated fraction:
• Quantitative XRD, using a strong and independent refl ection from an internal
standard, can potentially be used to track the decrease in the ratio d
001
for the clay
to the standard peak as exfoliation proceeds.
• A method based on NMR, which was recently established [15] .
• Transmission electron microscopy ( TEM ) provides an indication, but is diffi cult
to use for precise measurement unless very thin specimens are prepared, a large
number of images are captured, and quantitative microscopy coupled with
stereology is used.
Recently, Luo et al . [16] developed a statistical TEM image analysis methodology
to evaluate the dispersion parameters D
0.1
, based on measurement of the free - path
spacing distance between the single clay sheets. Drummy et al . [17] reported a
morphological characterization of layered silicate nanocomposites by using both
electron tomography and small - angle X - ray scattering ( SAXS ). The latter method
has the advantage of providing complementary results with respect to the electron
tomography. In fact, it can be used to probe materials with subnanometer resolu-
tion while at the same time sampling a large number of nanoparticles, thus pro-
viding more than adequate statistics.
Rheological testing [18] might also be developed to measure the degree of exfolia-
tion, but at present this is only used for semi - quantitative analysis. Hence, the
morphological characterization of layered silicate nanocomposites requires the use
of different techniques in order to achieve a relevance not only in the three -
dimensional ( 3 - D ) representation of the nanometer - scale part of the sample, but
also regarding the average distribution of platelets in the systems.
The characterization of layered silicate polymer blends is complicated by the
need to study both the phase and fi ller distribution. In miscible systems, such as
poly(methyl methacrylate) ( PMMA )/ poly(ethylene oxide) ( PEO ) [19, 20] , the mor-
phological analysis can be carried out as in a general polymeric matrix. However,
in immiscible systems, such as poly(propylene) ( PP )/ poly(styrene) ( PS ), both XRD
patterns and TEM observations have shown that the silicate layers were either
intercalated or exfoliated, depending on their interactions with the polymer pair,
and were located at the interface between the two polymers [21] . In this case, the
compatibilizing action of an organically modifi ed layered silicate resulted in a
decrease in the interfacial tension and particle size, and in a remarkable increase

410 13 Nanocomposites Based on Phyllosilicates
in the mechanical properties of the modifi ed immiscible blends. A similar result
was obtained by Fang et al . [22] , who investigated the morphology of nanocom-
posites based on 80/20 and 20/80 (w/w) poly( ε - caprolactone) ( PCL )/PEO immis-
cible blends and organophilic layered silicates prepared by melt extrusion. From
the TEM analysis, it was observed that the exfoliated silicate platelets were located
preferentially at the interface between the two blend phases. However, when the
blend - based nanocomposites were prepared via a two - step process, in which the
silicates were fi rst premixed with the PEO component or with the PCL component,
the silicate layers migrated from the PEO phase or PCL phase to the interface. The
emulsifying capability of layered silicates in immiscible blends depends on the
structure and physical properties of the couple of polymer components. In fact, in
PP/ poly(amide) ( PA ) blends [23] , it was shown, using electron microscopy, that in
all cases the inorganic fi ller was enriched in the PA phase, and this resulted in a
phase coarsening in comparison with the unfi lled PP/PA blend. In contrast, in
blends of a poly(vinylidene fl uoride)/nylon - 6 (PVDF/PA6) 30 : 70 melt compounded
with various organoclays either directly or sequentially [24] , the nanocomposite
with the best mechanical properties was characterized by a good dispersion of
particles throughout the matrix (PA6) and at the PVDF/PA6 interface. The authors
ascribed this good result to a suppression of the coalescence of PVDF domains.
Moreover, the crystallization of the PVDF domains was suppressed, ultimately
creating a blend nanocomposite that was stiffer, stronger, and tougher than the
blend without nanoparticles.
In agreement with the latter results, organoclay strongly infl uences the dynamic
evolution of phase morphology, both in partially miscible and immiscible blends.
A partially miscible blend was examined by investigating the poly(vinyl methyl
ether) ( PVME )/ poly(styrene) ( PS ) [25] demixing in the presence of organically
modifi ed Laponite. The phase separation of these near - critical blends proceeds by
a spinodal decomposition, even with added nanoparticles. However, the presence
of nanoparticles slowed the phase - separation kinetics. In immiscible systems,
such as poly(ethylene ) ( PE )/PA6 blends, the presence of an organoclay, preferen-
tially distributed in the polyamide phase, effectively stabilized a co - continuous
phase morphology [26] which persisted for more than 500 s. The authors explained
this experimental evidence by suggesting that the co - continuous morphology
could evolve to a phase - separated morphology in the timescale of their experi-
ments, as the organoclay framework would radically slow down the melt state
dynamics of the percolating network formed during the melt processing.
The presence of a compatibilizer [e.g., ethylene – propylene random copolymers
(EPM), functionalized with maleic anhydride (EPM - g - MAH), or maleic anhydride -
functionalized PP (PP - g - MAH)] in PP/PA6 blends containing organoclay, improves
the phase morphology of the blends and promotes the formation of exfoliated
nanocomposites, with the nanoplatelets preferentially distributed in the polyamide
phase [27, 28] . In particular, atomic force microscopy ( AFM ) studies [29] carried
out after selective chemical or physical etching of PP - g - MAH compatibilized PA6/
PP/organoclay polished sample surface, showed that the organoclay was embed-
ded in the PA6 - g - PP phase of the PA6/PP blends compatibilized with PP - g - MAH.
The preferential location of the clay in the PA6 - g - PP phase was attributed to pos-

13.2 Polyolefi n-Based Nanocomposites 411
sible chemical interactions between the PA6 and the organic surfactant of the clay.
The use of a proper organoclay, modifi ed with the reactive (glycidoxypropyl)tri-
methoxy silane, in poly(lactic acid) ( PLA )/ poly(butylene succinate) ( PBS ) blends
[30] , allowed an exfoliated blend to be obtain contemporarily, and improved the
phase adhesion between the two polymers. The authors ascribed this result to the
formation of a grafted hybrid in the interphase region, based on the reaction of a
glycidyl unit with the terminal hydroxyl or carboxylic groups of the polyesters.
In general, the phase morphology development and the compatibilization of
nanofi lled blends requires more studies to be conducted, in order to create a
rational scheme concerning the effect of nanoscaled interactions, physical fea-
tures, and rheological properties on fi ller and phase distribution, with the aim of
modulating the fi nal properties of the composites.
13.1.3
Properties of Composites
The dispersion of phyllosilicates into polymer or blends at nanometer scale allows
improvements to be made to the properties of the polymer matrix [31] . In
particular:
• the barrier properties to gases are enhanced [32] , not only when an exfoliated
morphology is obtained [33] , but also in an intercalated morphology [34] . In fact,
the results of fi tting of experimental data with proper models showed that the
chain confi nement enhanced the barrier properties of the intercalated
nanocomposites [35] .
• Flame retardancy is improved, based on the dispersion of nanoclays, such that
char formation is favored on the burning surface [36, 37] .
• Stiffness is improved, with an increase in tensile modulus frequently being
observed in phyllosilicate – polymer nanocomposites [21] .
• Thermal stability, as degradation temperature, determined via thermogravimetric
analysis ( TGA ), shows an increase of at least 7 – 8 ° C when an exfoliated
nanocomposite is tested [38] .
Other properties of nanocomposites are currently the object of much research,
and in some cases – an example is that of thermal behavior [39, 40] – these inves-
tigations have begun to establish new properties that might be exploited such that
the nanocomposites can be used for a variety of industrial applications.
13.2
Polyolefi n - Based Nanocomposites
The polyolefi n s ( PO s) represent one of the most widely used classes of thermo-
plastic materials, as they offer a unique combination of high thermomechanical
properties and good environmental compatibility, at low cost.

412 13 Nanocomposites Based on Phyllosilicates
Currently, a huge effort is being devoted to extend the fi eld of application for
POs and to satisfy their market demand, the latter point being based on these
materials having specifi c and advanced properties, and being adaptable to the
environment and, ideally, recyclable. In particular, the preparation of PO nano-
composites by the addition of very small amounts of layered silicate loadings is a
very common approach that provides drastic improvements in the elasticity
modulus, strength and heat resistance of the materials, as well as a substantial
decrease in their gas permeability, when compared to either virgin POs or con-
ventional microcomposites [41 – 49] .
Attempts have been made to prepare either intercalated and exfoliated nano-
composites, through different routes, and extensive studies have been conducted
to promote an understanding of the fundamental aspects associated with the clay
dispersion, the resultant physical properties, and the inter - relationship between
both. Indeed, the properties of polymer – clay nanocomposites depend largely on
the synergistic effect of nanoscale structure and interactions occurring between
the clay and polymer.
The achievement of a desired level of clay dispersion into the polymer, and
interaction between the phases, involves numerous chemical and technological
implications. Generally, the chemical modifi cation of one or both system phases
is necessary. For this, the hydrophilic clay is often changed to hydrophobic either
by the cation exchange or grafting of alkoxysilanes, by exploiting the cation -
exchange capability in one case and the reactivity of silanol groups present on the
edges of the clay platelets in the other case. Typically, a compatibilizer – that is, a
functionalized PO or copolymer – is added to assist in this situation. At present, it
is not completely clear which conditions lead to exfoliated layers and which cause
only the intercalation of polymer chains into the gallery spacing of the stacked
silicate layers. However, what does appear well established is that the interface is
not only fundamental for the adhesion between the two phases and stabilization
of the attained morphology, but also plays a major role in the bulk properties, due
to its large extension [39] .
Clearly, the ability to predict and control the morphology of nanocomposites
would be of major interest, by virtue of the strict connection between structural
control and the fi nal properties of the nanocomposite [49, 50] . Consequently, the
main objective of this section is to review the concepts and methods relating to
the optimization of layered silicate dispersion in PO matrices by melt intercalation,
notably with regard to the use of a compatibilizer and identifi cation of the pre-
ferred process conditions.
13.2.1
Overview of the Preparation Methods
Two major synthetic strategies are generally followed for preparing PO/layered
silicates nanocomposites: (i) polymer intercalation (including melt intercalation
[51 – 56] and solution intercalation methods [57] ); and (ii) in situ intercalative
polymerization [58 – 62] .

13.2 Polyolefi n-Based Nanocomposites 413
The in situ intercalative polymerization method, which is based on the thermo-
mechanical forces generated during the process of olefi n polymerization inside
the interlayers of the silicate, and the energy released during the polymerization,
is reported to promote silicate layer dispersion and exfoliation in the nonpolar PO
matrix. However, a very poor interaction between the polar silicate layers and the
hydrophobic PO matrix makes the nanocomposite structure thermodynamically
instable. In fact, it has been demonstrated that melting processes of this type of
nanocomposite material cause agglomeration of the silicate layers and collapse of
the nanocomposite structure. Even the more recently developed polymerization
fi lling technique , which consists of attaching the polymerization catalyst to the
surface and into the interlayers of the silicate and polymerizing the olefi n in situ ,
although favoring exfoliated structures does not allow thermodynamically stable
nanocomposites to be prepared [63] . The method which should be adopted for
stabilizing the morphology is to copolymerize the olefi n with a functionalized
comonomer, thus improving the necessary interaction with the clay. However,
many challenges remain for the catalytic polymerization of polar monomers with
both traditional Ziegler – Natta and metallocene catalysts, due mainly to the catalyst
deactivation.
For solution intercalation , the enthalpy of dispersion of the silicate layers in solu-
tion must balance the entropy loss of the solvent molecules which intercalate into
the silicate layers [64] . Moreover, this method is poorly sustainable because it
requires a large amount of organic solvent to ensure a good clay dispersion.
Melt intercalation is the most commonly applied process for preparing PO
nanocomposites, because it is sustainable, versatile, and inexpensive. This meth-
odology is limited by the low interaction enthalpy between the nonpolar PO
chains and the polar silicate layers. In fact, when using polymer chain intercala-
tion between the silicate layers, an effective and stable morphology is achieved
only if the interaction enthalpy balances the entropy loss of the polymer chains
confi ned within the silicate interlayers. However, it has been recognized that
the entropy loss due to such polymer confi nement is only partially compensated
by the entropy gain associated with the increased conformational freedom of
the surfactant tails as the interlayer distance increases with polymer intercalation
[65, 66] , whereas favorable enthalpic interactions are critical for determining the
nanocomposite structure [67] . Hence, even if a good dispersion of the clay can
be achieved simply by applying strong shear forces during the process, the
structure will be unstable and reagglomeration of the particles may occur quite
rapidly if an effective interaction between the polymer and clay surface is not
ensured [44] .
It is somewhat evident that the PO hydrophobicity makes it almost impossible
to obtain intercalation without chemical modifi cation of the clay or polymer, or
both. In order to overcome the above - described thermodynamic obstacles, and to
promote the interaction between the hydrophobic PO and the polar silicate layers,
the general approach is to use either: (i) organophilic layered silicate s ( OLS s),
obtained via cation exchange; or (ii) polymer compatibilizers (i.e., functionalized
polyolefi ns).

414 13 Nanocomposites Based on Phyllosilicates
Whilst the dispersal of layered silicates in polar polymers is relatively straight-
forward, the achievement of comparable results with hydrophobic POs remains a
challenge. Favorable interactions at the inorganic/organic interface are necessary
in order to separate the clay monolayers and to successively grant cohesion forces
between the exfoliated layers and polymer chains, thus achieving a stable
nanostructure.
13.2.2
Organophilic Clay and Compatibilizer: Interactions with the Polyolefi n Matrix
Two main methods can be applied for clay modifi cation: (i) to replace the metallic
cations that were originally present on the clay surface with large organic cations;
and (ii) to graft alkoxysilanes onto the clay by exploiting the reactivity of the silanol
groups [64] .
The fi rst of these methods has been widely applied, using several types of cation
(mostly dialkyldimethyl - or alkyltrimethyl - ammonium cations). The introduction
of quaternary ammonium salts with long alkyl chains between the layers (as dis-
cussed above) allows the interlamellar space to be expanded, reduces the interac-
tion among the silicate sheets, and also facilitates the diffusion and accommodation
of the polymeric matrix. In contrast, the grafting of alkoxysilanes has been used
only rarely with layered silicates, due to the small number of reactive - edge silanols
present. Moreover, it has been shown recently, by using a combination of mor-
phological analysis and multinuclear solid - state nuclear magnetic resonance
( NMR ) experiments [68] , that the modifi cation of a synthetic Laponite with a com-
bination of organic ammonium cations and alkoxysilanes will result in a disor-
dered arrangement of the clay platelets (which favors interplatelet interactions).
This effect was due to self - condensation occurring among clay silanols at the
platelet edges, thus limiting dispersion into the polymer matrix.
Several reports have described the use of OLSs modifi ed with alkylammonium
surfactants of different nature and length for the preparation of PO/OLS nano-
composites [52, 69] . In particular, the effect of factors such as the length and
number of alkyl groups of the cationic surfactant was investigated. It became
apparent that the equilibrium structure of the polymer/OLS depended on the
nature of the polymer, on the charge density of the layered silicate, and also on
the chain length and structure of the cationic surfactant. Balazs et al . [70, 71]
showed that an increase in surfactant length improved the separation of the layers
by allowing the polymer to adopt more conformational degrees of freedom. Both,
exfoliated and intercalated PE/OLS nanocomposites were obtained when the
number of methylene units in the alkylammonium chain exceeded 16 [72] , whereas
it was reported that an excessive density of tethered chains would prevent the
formation of intercalated structures. The dispersion level was found to depend
strictly on the amount of OLS; in particular, it was shown that the morphology of
the PO/OLS systems could be shifted from disordered exfoliated to predominantly
intercalated by varying the OLS content from 6 to 36 wt% [73] . Finally, from a
thermodynamic point of view, OLSs modifi ed with alkylammonium salts were
