Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


342 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
reactions with polymers, involving the formation of new compounds, at
coverages of about one monolayer and higher. It has been argued that the
data for polyimide do not imply reactive metals like Cr and Ti to be
bonded directly to the polymer, but rather suggest a disruption of the poly-
imide and the formation of oxidic, nitridic, and carbidic compounds.
[28, 47]
Co was also found to react strongly with PMDA-ODA.
[68]
Ni appears to
be much less reactive, but more reactive than Cu.
[19, 58, 69]
The alkali met-
als K
[28]
and Cs
[29]
have been demonstrated to reduce PMDA-ODA by
transferring an electron to the PMDAunit of the polymer. Dianion forma-
tion was also observed at high alkali metal concentrations.
The high reactivity of Cr, Ti, Al, and Co in the high-coverage regime
does not necessarily imply that individual metal atoms are strongly bound
to polymer chains. Based on measurements of the near-edge x-ray absorp-
tion fine structure in the very early stages of interface formation,
Strunskus et al.
[28]
ruled out purely physical interactions between Cr and
polyimide and discussed p-complex formation
[17, 48]
as one possible inter-
action mechanism. On the other hand, the fact that an intermixing layer
was observed at the Al-polyimide interface in cross-sectional TEM
studies
[17, 19]
points to relatively weak interactions of this reactive metal at
very low coverages. Our XPS and TEM experiments carried out in the Cr-
polyimide system at very low Cr coverages also show short-range inter-
mixing at the interface. However, the absence of long-range diffusion
even after very slow deposition at elevated temperatures in conjunction
with the lack of any influence of the deposition rate on the extent of inter-
mixing (compare Sec. 7.4) clearly points to strong chemical interactions.
[70]
7.4 Diffusion in the Polymer Bulk
Surface spectroscopy experiments indeed confirmed a strong correla-
tion between reactivity and mobility of metal atoms. In these experiments,
the metal was deposited at room temperature, and the drop of the metal
intensity was measured after annealing. While substantial intensity drops
were observed for noble metals,
[50, 69, 71–73]
reflecting appreciable metal
mobility, interfaces of polymers with Cr and Ti proved to be thermally
stable.
[17, 60, 69]
Al showed features of some mobility,
[17, 65]
and the mobility
of Ni turned out to be somewhere between that of Al and Cu. Note that the
metal mobility reflected in a drop of the metal intensity in surface analyt-
ical techniques cannot necessarily be taken as evidence of metal diffusion
into the polymer bulk; it is often caused by metal clustering at the surface,
as discussed below.
The ability of K and Cs to reduce polymers proved to have drastic
consequences on the diffusion behavior. Since positive ions repel each
Ch_07.qxd 11/12/04 4:10 PM Page 342

other, their diffusion is not impeded by the formation of immobile clus-
ters. Moreover, positive ions are much smaller than the neutral atoms. As
a result, these ions were found to be highly mobile in polymers, and
almost uniform K and Cs distributions were observed by means of angular-
resolved XPS
[29]
and Fourier-transform infrared reflection-absorption
spectroscopy
[28]
in polyimide films of appreciable thickness. A similar
behavior was seen for In in films of the organic semiconductor perylenete-
tracarboxylic dianhydride (PTCDA).
[30]
Here even Al was seen to diffuse
into the organic film quite extensively, which was attributed to the rela-
tively low ionization energy. In contrast, Ti, Sn, Ag, and Au turned out to
form interfaces that display evidence of overlayer metallicity at coverages
as small as 5 to 10 nm.
The first direct evidence of noble metal diffusion and aggregation in
the polymer bulk has been provided by cross-sectional TEM studies of
interface formation between Cu and polyimide (PMDA-ODA). In these
studies, LeGoues et al.
[19]
observed marked clustering of Cu at consider-
able distances below the polyimide surface after metal deposition at ele-
vated temperatures (but still well below T
g
) and low deposition rates.
However, no clustering in the bulk was observed at high deposition rates
and even elevated temperatures. After room-temperature deposition and
subsequent annealing, no metal particles were detected either. Investigations
carried out by Kiene et al.
[22]
have essentially confirmed these early results
and have shown that other noble metal-polymer systems, for example,
Ag/PMDA-ODA polyimide
[21, 74, 75]
and Au/TMC polycarbonate,
[32, 76]
exhibit a similar behavior.
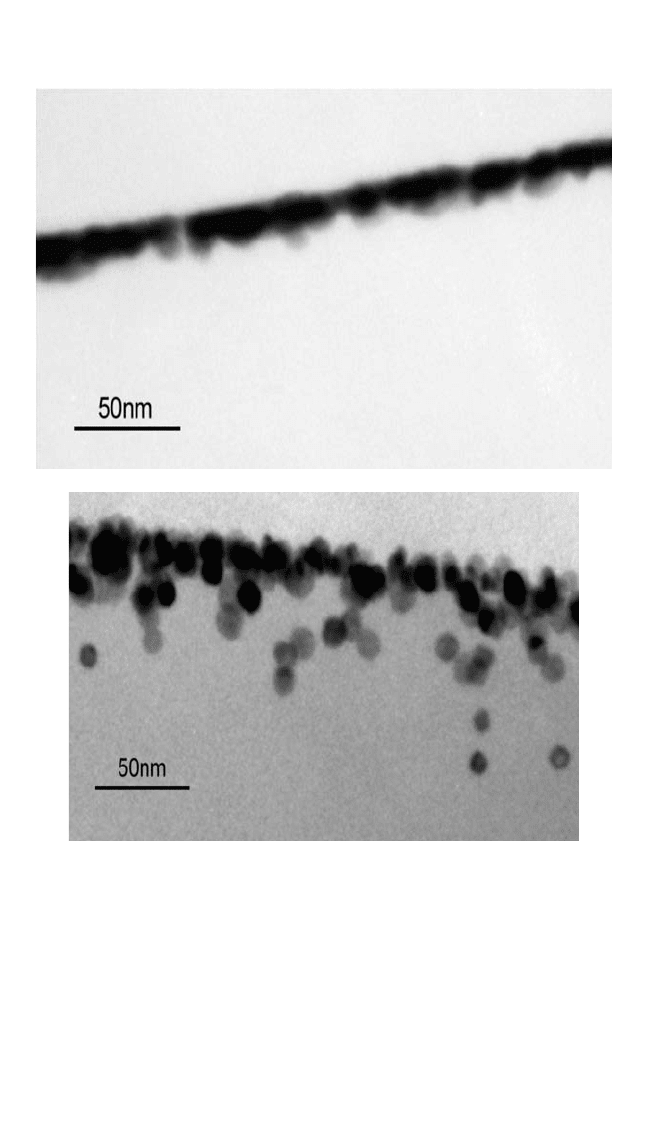
Figure 7.3 gives a striking example that demonstrates the crucial role
of the metallization conditions on the interfacial structure. While deposi-
tion of Cu at a very low rate at 350°C produces a rather spread-out inter-
face, implying pronounced Cu diffusion (Fig. 7.3, bottom), deposition at
room temperature and subsequent annealing at the same temperature
results in a sharp interface without cluster formation inside the polyimide
(Fig. 7.3, top).
[22]
Note that the metal films in Fig. 7.3 are still not contin-
uous, despite the relatively large nominal metal coverage of about
30 monolayers. The impression of a continuous film is a consequence of the
finite thickness of the samples of 40 to 100 nm, depending on the cutting
procedure. Upon tilting, isolated and connected clusters are clearly visible.
The results depicted in Fig. 7.3 and corresponding results obtained for
other noble metal systems
[9]
show that metal clustering at the surface
effectively impedes metal diffusion into the bulk and strongly suggests
that no significant metal diffusion into the polymers is expected from a
continuous film or an arrangement of large clusters. This conclusion is
also corroborated by the pioneering medium-energy ion scattering exper-
iments of Cu diffusion in polyimide by Tromp et al.
[18]
On the other hand,
METAL DIFFUSION IN POLYMERS, FAUPEL ET AL. 343
Ch_07.qxd 11/12/04 4:10 PM Page 343
344 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
the absence of clusters in cross-sectional TEM images does not allow dif-
fusion of metal atoms into polymers and the formation of small clusters
that are not detectable in TEM to be ruled out.
We have used a very sensitive radiotracer technique to study atomic
diffusion. Diffusion profiles of the radiotracer atoms in the polymer films
Figure 7.3 Cross-sectional TEM micrographs showing the striking differences in
the copper diffusion characteristics between Cu deposition at room temperature
(8 nm at 0.16 nm/min.) followed by subsequent annealing at 350°C for 30 min.
(top) and very slow evaporation of Cu (4 nm at 0.16 nm/min.) at 350°C (bottom).
Ch_07.qxd 11/12/04 4:10 PM Page 344

are measured by means of ion-beam microsectioning in a high-vacuum
chamber. A very high depth resolution of typically 3 to 4 nm can be
achieved by use of argon or krypton ions of energies as low as 100 to
200 eV
[77]
(as opposed to several keV in secondary ion mass spectrome-
try). Surface charging of the insulating polymer film is avoided by use of
an electron-emitting neutralizer filament. The sputtered-off material of
each section is collected on a catcher foil, which is advanced like a film
in a camera. After sputtering, the catcher foil is cut into pieces correspon-
ding to the individual sections, and the radioactivity of each section,
which is proportional to the tracer concentration, is counted. After depth
calibration, a penetration profile is obtained by plotting the radioactivity
of the individual sections against the penetration depth. Using a calibrated
source, absolute metal concentrations can be determined.
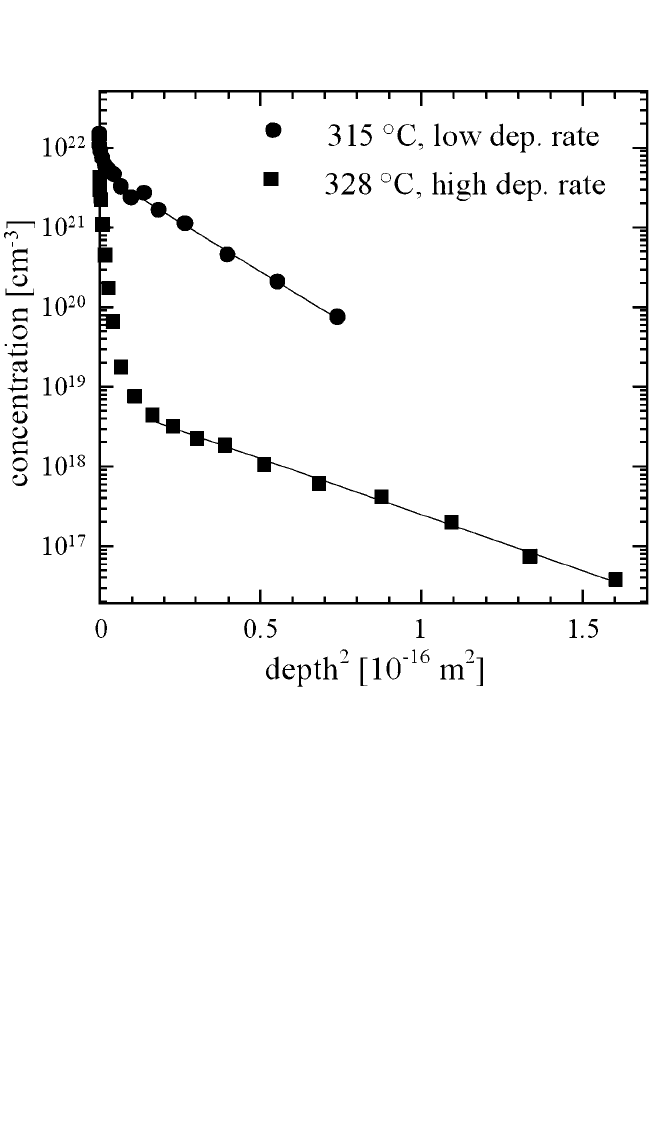
Representative penetration profiles are shown in Fig. 7.4. Here the
logarithm of the activity of
110m
Ag is plotted versus the square of the pen-
etration depth x. The total amount of metal is extremely small. On this
scale, ordinary diffusion of the tracer according to the thin-film solution
of Fick’s second law,
c(x) const. exp
, (5)
leads to a straight line of slope 1(4Dt). In Eq. (5), D is the tracer diffu-
sivity and t is the diffusion time. It is obvious from Fig. 7.4 that silver dif-
fuses deeply into polyimide during deposition at elevated temperatures.
However, only the upper profile, obtained after evaporation of silver onto
the hot polymer sample at an extremely low rate, is nearly Gaussian. At
high and moderate deposition rates, profiles often resemble the lower pro-
file in Fig. 7.4, where most of the metal deposit is located at or very close
to the polymer surface. The fraction of atoms that diffuse over large dis-
tances and contribute to the linear range is very small. (Note the logarith-
mic scale.) Metal diffusion into the bulk is only detectable because of the
high sensitivity of the radiotracer technique. Nevertheless, diffusion of
even trace amounts of Cu through the polymer into Si devices may cause
damage in ultra-large-scale integrated chips if it is not effectively blocked
by a barrier. Penetration profiles of a similar shape have been recorded in
various measurements for noble-metal tracers in polyimides
[9, 24, 78, 79]
and
polycarbonates.
[80, 81]
Only the ratio of metal in the near-surface region and
in the tail varied from case to case.
In view of the characteristic shape of the penetration profiles and the
well-known metal aggregation tendency, special attention was paid to
sputtering artifacts.
[9, 23, 80, 82]
For example, it was shown that the sputter
x
2
4Dt
METAL DIFFUSION IN POLYMERS, FAUPEL ET AL. 345
Ch_07.qxd 11/12/04 4:10 PM Page 345
346 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
rate of noble metals is even slightly higher than that of polycarbon-
ates.
[23, 82]
Hence retarded sputtering of metal clusters, which would mimic
metal diffusion, can be excluded. In all cases, a resolution function was
recorded by sputtering a very thin tracer layer on a polymer film without
diffusion annealing. Moreover, sputtering was performed not only in the
direction of diffusion but also in the opposite direction after removing the
polymer film from the substrate.
[23, 82]
Finally, at a given temperature, we
determined diffusivities from the Gaussian tails of the diffusion profiles
(see Fig. 7.5) and showed that the resulting diffusivities were independent
of the annealing time.
[75, 81]
Monte Carlo simulations have shown that the dominant role of the
deposition rate is a direct consequence of the interplay of atomic diffusion
Figure 7.4 Effect of the deposition rate on penetration profiles of
110m
Ag into
PMDA-ODA polyimide. The tracer was deposited continuously at a rate of about
0.1 ML/min. during annealing at 315°C and flash evaporated at the annealing tem-
perature of 328°C.
Ch_07.qxd 11/12/04 4:10 PM Page 346
and aggregation.
[9, 83, 84]
Such effects are not observed in ordinary diffu-
sion experiments. Simulated metal concentration profiles and simulated
TEM images were found to exhibit the characteristics observed in the
experiments if we incorporated the condition that stable clusters form
whenever metal atoms encounter each other on their diffusion path. This
condition is implied by our observation of a critical cluster size of i 1
(see Sec. 7.3).
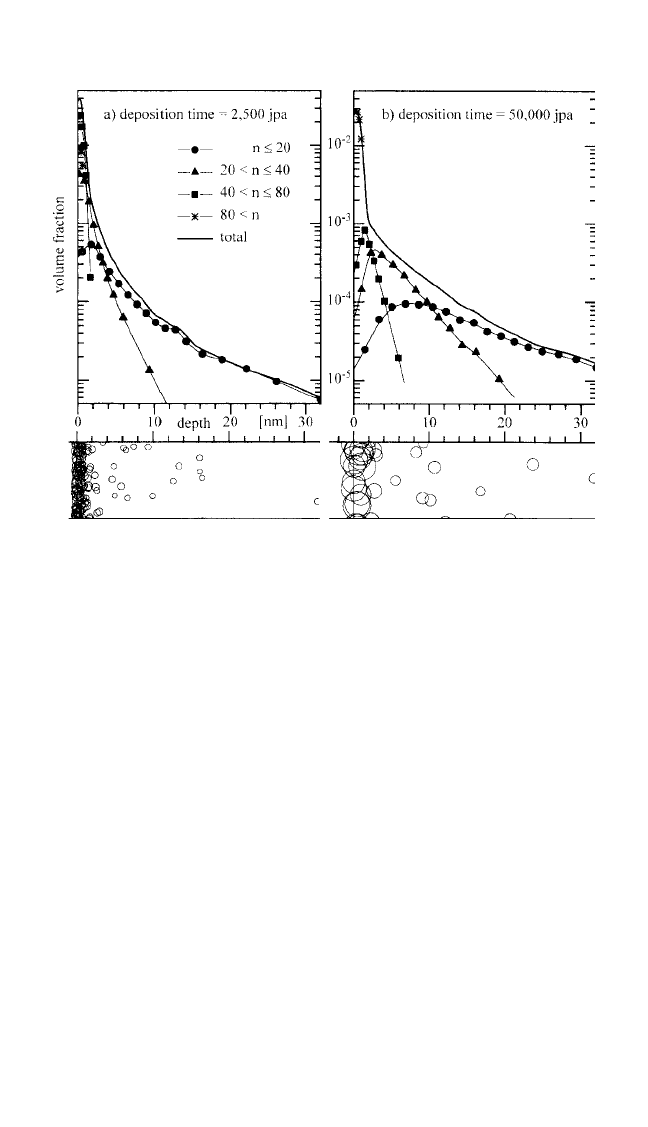
An example is given in Fig. 7.5. The Monte Carlo simulations also
reflect the experimental observation that the total metal concentration
found at large concentration depth decreases strongly with increasing dep-
osition rate due to the metal immobilization through clustering.
[9]
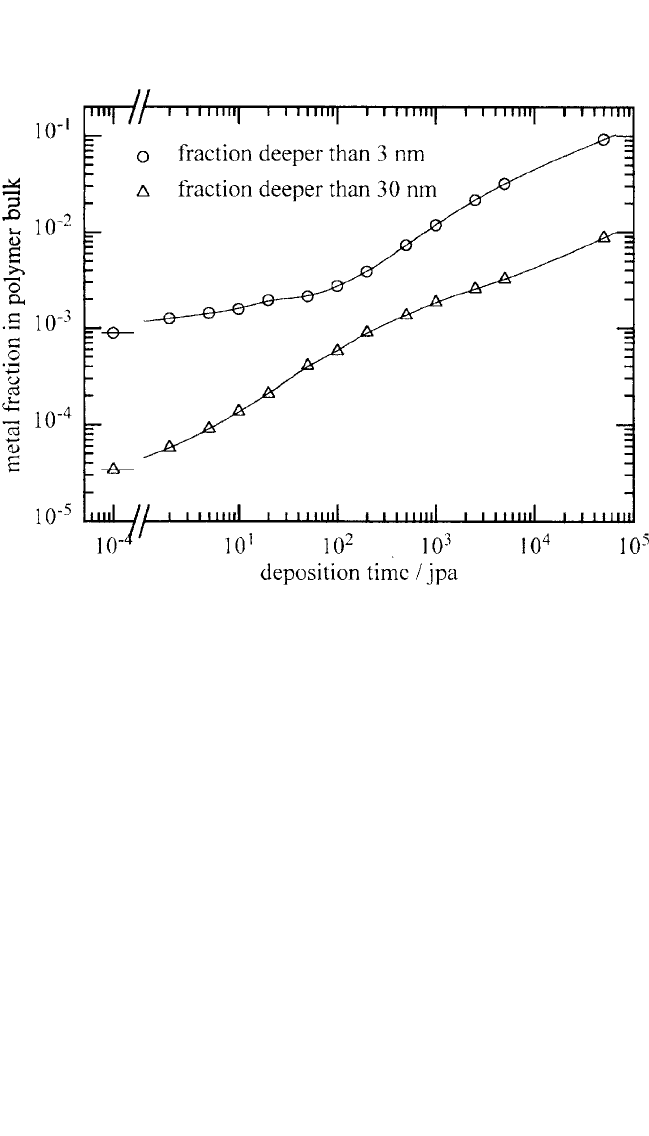
This is
illustrated in Fig. 7.6, where the fraction of metal atoms at depths of
3 and 30 nm is plotted against the deposition time (1rate). We can
METAL DIFFUSION IN POLYMERS, FAUPEL ET AL. 347
Figure 7.5 Simulated penetration profiles for the total metal concentration and for
metal clusters of different size ranges, indicated by the number n of atoms, after
deposition during (a) 2,500 and (b) 50,000 jumps per free atom (jpa) and subse-
quent annealing. The total annealing time of 5 10
5
jpa, the nominal metal cov-
erage of 0.2 monolayer, and the ratio of surface and bulk diffusivity of D
s
D 60
are equal for both runs. Cross-sectional views at the bottom show clusters that
have formed in a section of the simulated volume. The calibration of the deposi-
tion rates depends on the experimental diffusion temperature. For diffusion of
noble metals in polyimides and polycarbonates, a deposition rate of the order of 1
ML/min. typically corresponds to 100 jpa in the vicinity of the glass transition
temperature.
[9]
Ch_07.qxd 11/12/04 4:10 PM Page 347
348 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
conclude that at industrial metallization rates, only traces of noble metal
are to be expected in the polymer bulk. Because trace amounts of metals
can cause device failures in chip interconnects involving organic low-k
dielectrics, diffusion barriers must be used.
Based on the notion that metal diffusion into polymers is an interplay
between atomic diffusion and aggregation, suggested by the Monte Carlo
simulations and the experiments described in this chapter, the extended
linear tails in the low-concentration range of the radiotracer profiles (see
Fig. 7.4) were attributed to diffusion of isolated metal atoms. Arrhenius
plots of the tracer diffusivities for Cu and Ag noble metals in polyimides
(PMDA-ODA and BPDA-BDA) and polycarbonates, determined from
the linear tails of penetration profiles similar to those in Fig. 7.4, are
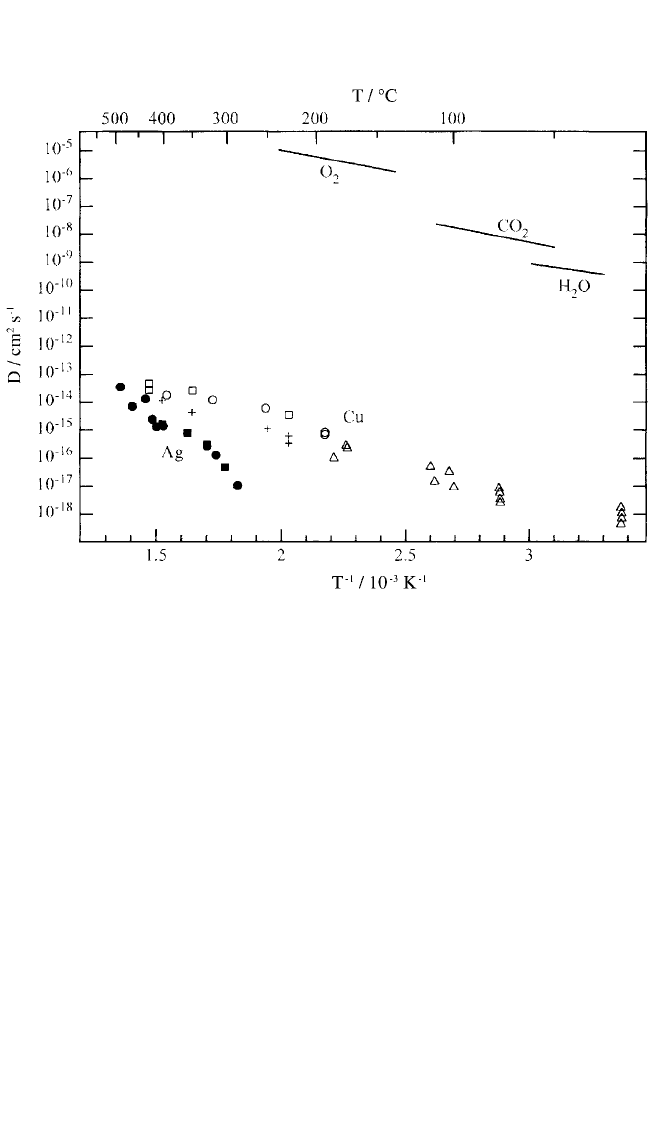
shown in Fig. 7.7. Diffusivities of O
2
, CO
2
, and H
2
O are also shown. Our
results from ion-beam depth profiling in conjunction with x-ray photo-
electron spectroscopy on Cu diffusion in SiLK
®
indicate that Cu diffusion
is more than an order of magnitude slower in SiLK
®
than in the polyimide
PMDA-ODA at 315°C.
[39]
This is in accord with the higher adsorption
Figure 7.6 Total metal concentration found in the computer simulations at pene-
tration depths 3 and 30 nm, as a function of the metal deposition time in units
of jumps per free atom.
Ch_07.qxd 11/12/04 4:10 PM Page 348
energy of Cu on SiLK
®
, discussed in Sec. 7.2, which indicates stronger
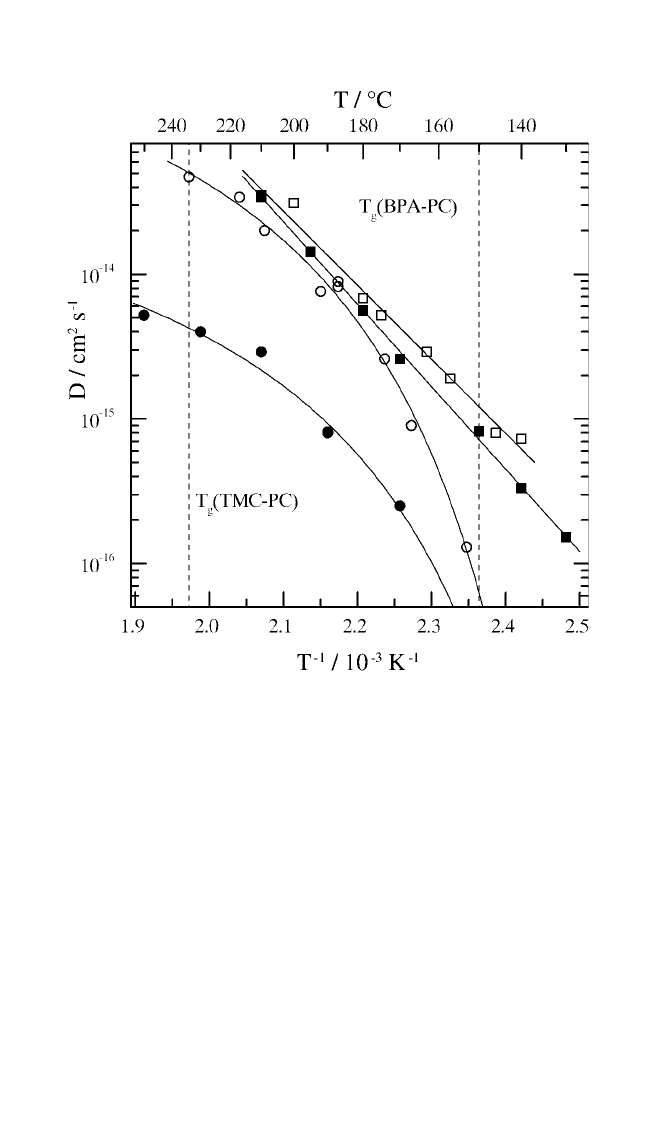
interaction. Diffusivities for Au and Ag in polycarbonates are displayed
in Fig. 7.8.
Note in Fig. 7.7 that the diffusivities of noble-metal atoms in poly-
imide are many orders of magnitude smaller than those of simple gas mol-
ecules of comparable size. This was interpreted in terms of significant
contributions from metal interactions with the polymer even for noble
metals. Substantial interactions between noble metal atoms and polymers
are also reflected in the relatively high adsorption energies discussed in
Sec. 7.2 and listed in Table 7.1. Based on a detailed analysis of the metal
diffusion behavior in BPApolycarbonate,
[9]
which turned out to be completely
decoupled from the polymer dynamics, for example, we have attributed
the drastic slowing down of the atomic mobility of metals in compari-
son to noninteracting gas molecules to metal-atom-induced temporary
METAL DIFFUSION IN POLYMERS, FAUPEL ET AL. 349
Figure 7.7 Diffusion coefficients versus reciprocal temperature for diffusion of
67
Cu
and
110m
Ag in PMDA-ODA polyimide, Cu in Kapton
®
, and
67
Cu in BPDA-PDA poly-
imide. Arrhenius plots for diffusion of O
2
, CO
2
, and H
2
O in Kapton
®[85]
are shown
for comparison. Legend: open circles,
[75, 78]
open squares,
[79, 86]
solid squares,
[45]
solid circles,
[87]
open triangles from RBS combined with ion implantation,
[88]
and
crosses.
[79, 86]
Ch_07.qxd 11/12/04 4:10 PM Page 349
350 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
cross-linking.
[24, 80]
It was reported, for example, that polyimide-
polyamide copolymers could no longer be dissolved in typical solvents
after a fine dispersion of Ag and Pd had been incorporated (by reduction
of the corresponding metal salts).
[89]
Despite the available evidence of substantial interaction of noble
metal atoms with polymers, we cannot exclude the possibilty that the dif-
fusivities determined from the linear tails of the radiotracer profiles reflect
diffusion of small clusters rather than atomic diffusion. This particularly
holds for the measured Ag diffusivities because the tracer concentration in
Figure 7.8 Arrhenius plots for atomic diffusion of
198
Au (solid symbols) and
110m
Ag
(open symbols) in the polycarbonates BPA-PC and TMC-PC. The dashed lines
indicate the glass transition temperatures measured by means of DSC at a heat-
ing rate of 20°C/min. Diffusion in BPA-PC is well described by linear Arrhenius fits
with activation energies of Q 98 and 109 kJ/mole and pre-exponential factors of
D
0
0.0016 and 0.022 cm
2
/s, respectively.
[9, 80, 87]
The data for TMC-PC exhibit a
downward curvature.
[81, 87]
Ch_07.qxd 11/12/04 4:10 PM Page 350
the experiments with Ag typically corresponds to several monolayers
(while only ≈14 monolayer was used in the Cu tracer studies). As dis-
cussed in Sec. 7.2, the differences in the ratios of D
Cu
D
Ag
for surface and
bulk diffusion also point to this possibility. Our recent radiotracer meas-
urements of
110m
Ag in TMC polycarbonate
[23]
indeed suggest that the dif-
fusivities reported by Willecke and Faupel
[81]
are those of small clusters
and that single Ag atoms, which are only present in trace amounts, are
mobile even at room temperature.
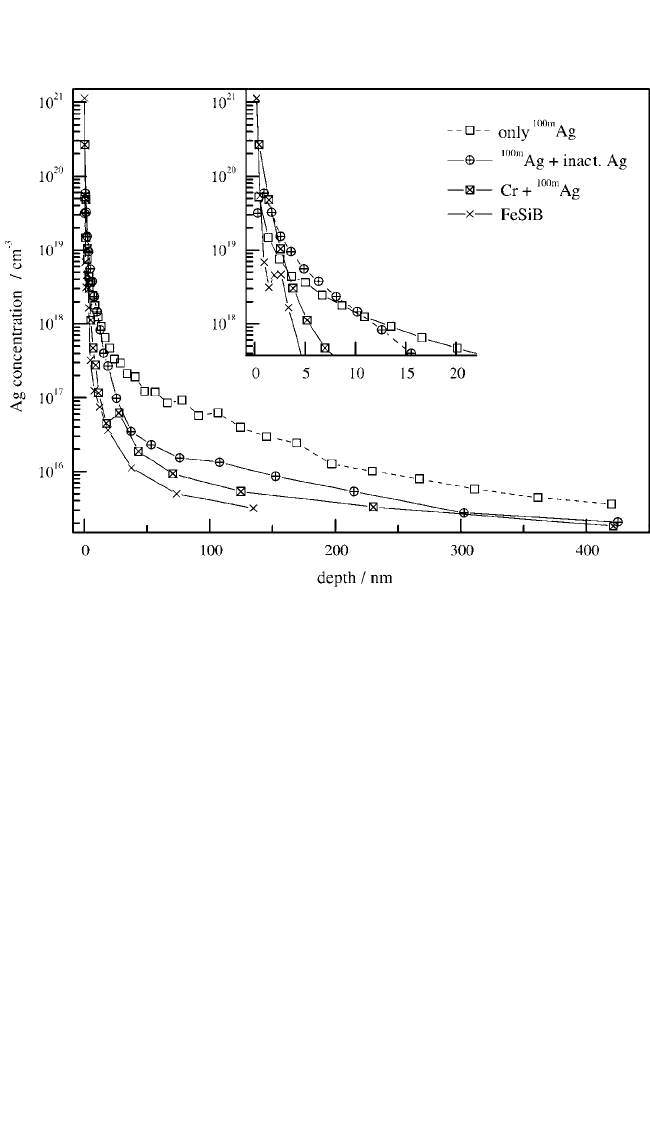
In these experiments, several diffusion profiles were measured in
samples where a thin layer of
110m
Ag was deposited at 35°C over periods
of 12 to 15 minutes, and annealing was performed for up to 11 days.
[82]
A representative
110m
Ag profile is shown in Fig. 7.9 (open squares). As
expected from the aggregation-induced immobilization, the Ag concen-
tration drops by several orders of magnitude over very short penetration
METAL DIFFUSION IN POLYMERS, FAUPEL ET AL. 351
Figure 7.9 Penetration profiles obtained after
110m
Ag deposition and annealing at
35°C, with a predeposited layer of 0.08 ML Cr, and with co-deposition of ≈3 ML
of nonradioactive silver after 3 min. of tracer evaporation (12 min. total deposi-
tion time). The resolution function, obtained from a thin layer of
110m
Ag evapo-
rated on a Fe-Si-B glass without annealing, is shown for comparison. The depth
scale is linear.
Ch_07.qxd 11/12/04 4:10 PM Page 351
