Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

The formation of a phosphorimidazolide intermediate provides better reactivity toward
amine nucleophiles than the EDC phosphodiester intermediate if EDC is used without added
imidazole. The EDC phosphodiester intermediate also has a shorter half-life in aqueous condi-
tions due to hydrolysis than the phosphorimidazolide. Although EDC alone will create nucle-
otide phosphoramidate conjugates with amine-containing molecules (Shabarova, 1988), the
result of forming the secondary phosphorimidazolide-activated species is increased derivatiza-
tion yield over carbodiimide-only reactions.
The downside of EDC conjugation with oligonucleotides is the potential for reaction of the
carbodiimide at the guanosine N-1 site or with thymidine residues (von der Haar et al., 1971).
In practice, however, this cross-reactivity appears to be low enough to maintain complete bio-
logical activity and hybridization effi ciency in the fi nal conjugate, indicating most of the deriva-
tization occurs at the 5 phosphate group (Chu et al ., 1983).
The following protocol describes the modifi cation of DNA or RNA probes at their 5 -phos-
phate ends with a bis-hydrazide compound, such as adipic acid dihydrazide or carbohydrazide.
A similar procedure for coupling the diamine compound cystamine can be found in Section 2.2
(this chapter).
Protocol
1. Weigh out 1.25 mg of the carbodiimide EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodii
mide hydrochloride; Thermo Fisher) into a microfuge tube.
2. Add to the tube 7.5 l of RNA or DNA containing a 5 phosphate group. The concentra-
tion of the oligonucleotide should be 7.5–15 nmol or total of about 57–115.5 g. Also
immediately add 5 l of 0.25 M bis-hydrazide compound dissolved in 0.1 M imidazole,
pH 6.0. Because EDC is labile in aqueous solutions, the addition of the oligo and bis-
hydrazide/imidazole solutions should be done quickly.
3. Mix by vortexing, then place the tube in a microcentrifuge and spin for 5 minutes at
maximal rpm.
4. Add an additional 20 l of 0.1 M imidazole, pH 6.0. Mix and react for 30 minutes at
room temperature.
6. Purify the hydrazide-labeled oligo by gel fi ltration on desalting resin using 10 mM sodium
phosphate, 0.15 M NaCl, 10 mM EDTA, pH 7.2. The probe now may be used to conju-
gate with an aldehyde-containing molecule.
2.2. Sulfhydryl Modifi cation of DNA
Creating a sulfhydryl group on nucleic acid probes allows conjugation reactions to be done
with sulfhydryl-reactive heterobifunctional crosslinkers (Chapter 5), providing increased
control over the derivatization process. Proteins can be activated with a crosslinking agent
containing an amine-reactive and a sulfhydryl-reactive end, such as N-succinimidyl 3-(2-
pyridyldithio)propionate (SPDP) (Chapter 5, Section 1.1), leaving the sulfhydryl-reactive por-
tion free to couple with the modifi ed DNA probe. Having a sulfhydryl group on the probe
directs the coupling reaction to a discrete site on the nucleotide strand, thus better preserving
hybridization ability in the fi nal conjugate. In addition, heterobifunctional crosslinkers of this
type allow two- or three-step conjugation procedures to be done, which result in better yield of
the desired conjugate than when using homobifunctional reagents.
980 27. Nucleic Acid and Oligonucleotide Modifi cation and Conjugation
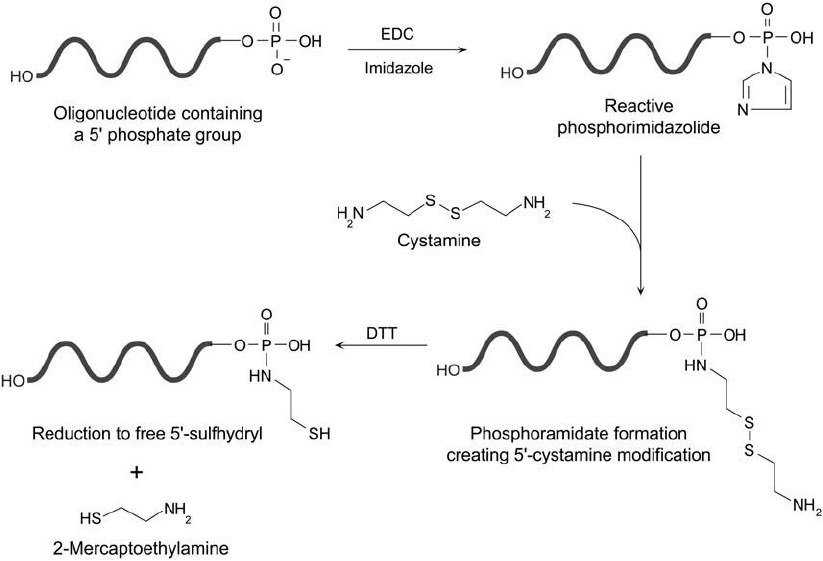
Cystamine Modifi cation of 5 Phosphate Groups Using EDC
DNA or RNA may be modifi ed with cystamine at the 5 phosphate group using a carbodiimide
reaction identical to that described previously (Section 2.1, this chapter). In some procedures,
the reaction is carried out in a two-step process by fi rst forming a reactive phosphorimidazolide
by EDC conjugation in an imidazole buffer. Next, cystamine is reacted with the activated oligo-
nucleotide, causing the imidazole to be replaced by the amine and creating a phosphoramidate
linkage (Chu et al., 1986). An easier protocol was described by Ghosh et al. (1990) in which
the oligo, cystamine, and EDC were all reacted together in an imidazole buffer. A modifi cation
of this method developed by Zanocco et al . (1993) is described below.
Reduction of the cystamine-labeled oligo using a disulfi de reducing agent releases 2-mer-
captoethylamine and creates a thiol group for conjugation ( Figure 27.6 ). DNA probes labeled
in this manner have been successfully coupled with SPDP-activated alkaline phosphatase
(Chapter 26, Sections 1.2 and 2.5), maleimide-activated horseradish peroxidase (HRP)
(Chapter 26, Section 1.1), NHS-LC-biotin (Chapter 11, Section 1 and Chapter 27, Section 2.3),
and the fl uo rescent tag AMCA–HPDP (Chapter 9, Section 3 and Chapter 27, Section 2.5).
A kit designed specifi cally to perform 5 -phosphate labeling on DNA probes is available
from Thermo Fisher.
Figure 27.6 The 5 -phosphate group of oligonucleotides may be labeled with cystamine using the EDC/imid-
azole reaction. This results in the formation of an amine-terminal spacer containing an internal disulfi de group.
Reduction of the disulfi de provides a route to creating a free thiol for further derivatization.
2. Chemical Modifi cation of Nucleic Acids and Oligonucleotides 981
Protocol
1. Weigh out 1.25 mg of the carbodiimide EDC (1-ethyl-3-(3-dimethylaminopropyl)
carbodiimide hydrochloride; Thermo Fisher) into a microfuge tube.
2. Add to the tube 7.5 l of RNA or DNA containing a 5 phosphate group. The concentra-
tion of the oligonucleotide should be 7.5–15 nmol/ l or total of about 57–115.5 g. Also
immediately add 5 l of 0.25 M cystamine in 0.1 M imidazole, pH 6.0. Because EDC is
labile in aqueous solutions, the addition of the oligo and cystamine/imidazole solutions
should be done quickly.
3. Mix by vortexing, then place the tube in a microcentrifuge and spin for 5 minutes at
maximal rpm.
4. Add an additional 20 l of 0.1 M imidazole, pH 6.0. Mix and react for 30 minutes at
room temperature.
5. For reduction of the cystamine disulfi des, add 20 l of 1.0 M DTT and incubate at room
temperature for 15 minutes. This will release 2-mercaptoethylamine from the cystamine
modifi cation site and create the free sulfhydryl on the 5 terminus of the oligonucleotide.
6. Purify the SH-labeled oligo by gel fi ltration on a desalting resin using 10 mM sodium
phosphate, 0.15 M NaCl, 10 mM EDTA, pH 7.2. The probe now may be used to con-
jugate with an activated enzyme, biotin, fl uorescent tag, or other molecules containing a
sulfhydryl-reactive group.
SPDP Modifi cation of Amines on Nucleotides
Oligonucleotide probes that have been modifi ed with an amine-terminal spacer arm using any
of the methods discussed in Sections 1 and 2 of this chapter may be thiolated to contain a
sulfhydryl residue. Theoretically, any of the amine-reactive thiolation reagents described in
Chapter 1, Section 4.1 may be used to convert an amino group on a DNA molecule into a
thiol. One of the more common choices, both for crosslinking and for thiolation reactions, is
the heterobifunctional reagent, SPDP (Chapter 5, Section 1.1). The NHS ester end of SPDP
reacts with primary amine groups to produce stable amide bonds. The other end of the
crosslinker contains a thiol-reactive pyridyl disulfi de group that also can be reduced with DTT
to create a free sulfhydryl.
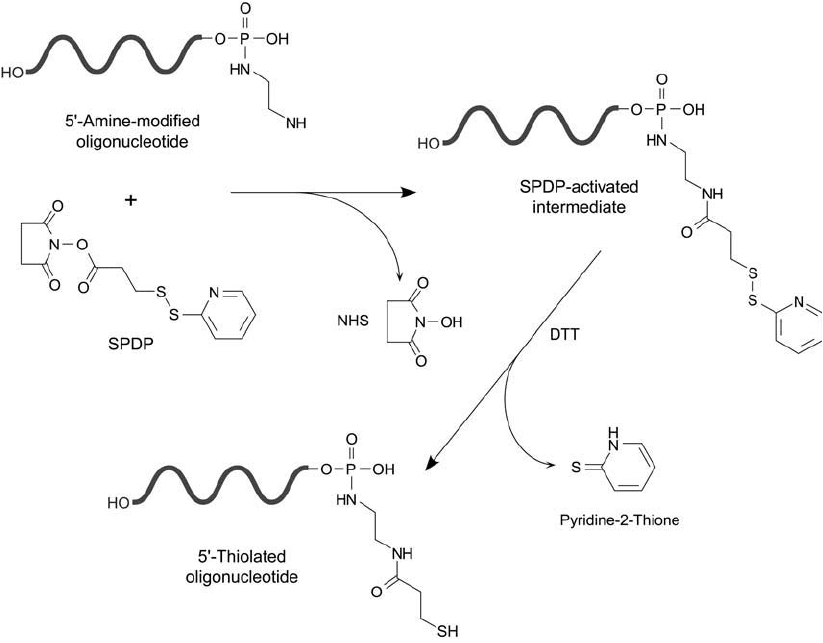
The reaction of a 5 -diamine-modifi ed oligonucleotide probe with SPDP proceeds under
mildly alkaline conditions (optimal pH 7–9) to give the pyridyl disulfi de-activated intermedi-
ate ( Figure 27.7 ). This derivative has dual functionality. It can be used to couple directly with
sulfhydryl-containing detection reagents or enzymes, or it may be converted into a free sulfhy-
dryl for coupling to thiol-reactive compounds (Gaur et al., 1989; Gaur, 1991). In an alternative
approach, Chu and Orgel (1988) used 2,2 -dipyridyldisulfi de (Chapter 1, Section 5.2) to cre-
ate reactive pyridyl disulfi de groups on a reduced 5 -cystamine-labeled oligonucleotide probe.
This derivative then can be used to couple with sulfhydryl-containing molecules, forming a
disulfi de bond.
Reduction of the pyridyl disulfi de end after SPDP modifi cation releases the pyridine-2-thione
leaving group and generates a terminal––SH group. This procedure allows sulfhydryl-reactive
derivatives such as maleimide-activated enzymes (Chapter 26, Section 2.3) to be conjugated
with DNA probes for use in hybridization assays (Malcolm and Nicolas, 1984).
982 27. Nucleic Acid and Oligonucleotide Modifi cation and Conjugation
Protocol
1. Dissolve the amine-modifi ed oligonucleotide to be thiolated in 250 l of 50 mM sodium
phosphate, pH 7.5.
2. Dissolve SPDP (Thermo Fisher) at a concentration of 6.2 mg/ml in DMSO (makes a
20 mM stock solution). Alternatively, LC-SPDP may be used and dissolved at a concen-
tration of 8.5 mg/ml in DMSO (also makes a 20 mM solution). The “ LC ” form of the
crosslinker provides a longer spacer arm that often results in better probe activity after
modifi cation. If the water-soluble sulfo-LC-SPDP is used, a stock solution in water may
be prepared just prior to adding an aliquot to the thiolation reaction. In this case, prepare
a 10 mM solution of sulfo-LC-SPDP by dissolving 5.2 mg/ml in water. Since an aqueous
solution of the crosslinker will degrade by hydrolysis of the sulfo-NHS ester, it should be
used quickly to prevent signifi cant loss of activity.
2. Chemical Modifi cation of Nucleic Acids and Oligonucleotides 983
Figure 27.7 A oligonucleotide modifi ed at its 5 -phosphate with a diamine compound may be reacted with
SPDP and subsequently reduced to create a free sulfhydryl.
3. Add 50 l of the SPDP (or LC-SPDP) solution to the oligo solution. Add 100 l of the
sulfo-LC-SPDP solution, if the water-soluble crosslinker is used. Mix.
4. React for 1 hour at room temperature.
5. Remove excess reagents from the modifi ed oligo by gel fi ltration on a desalting resin. The
modifi ed probe now may be used to conjugate with a sulfhydryl-containing molecule, or
it may be reduced to create a thiol for conjugation with sulfhydryl-reactive molecules.
6. To release the pyridine-2-thione leaving group and form the free sulfhydryl, add 20 l
of 1.0 M DTT and incubate at room temperature for 15 minutes. If present in suffi cient
quantity, the release of pyridine-2-thione can be followed by its characteristic absorb-
ance at 343 nm ( 8.08 10
3
M
1
cm
1
). For many oligonucleotide modifi cation
applications, however, the leaving group will be present in too low a concentration to be
detectable.
7. Purify the thiolated oligonucleotide from excess DTT by dialysis or gel fi ltration using
50 mM sodium phosphate, 1 mM EDTA, pH 7.2. The modifi ed probe should be used
immediately in a conjugation reaction to prevent sulfhydryl oxidation and formation of
disulfi de crosslinks.
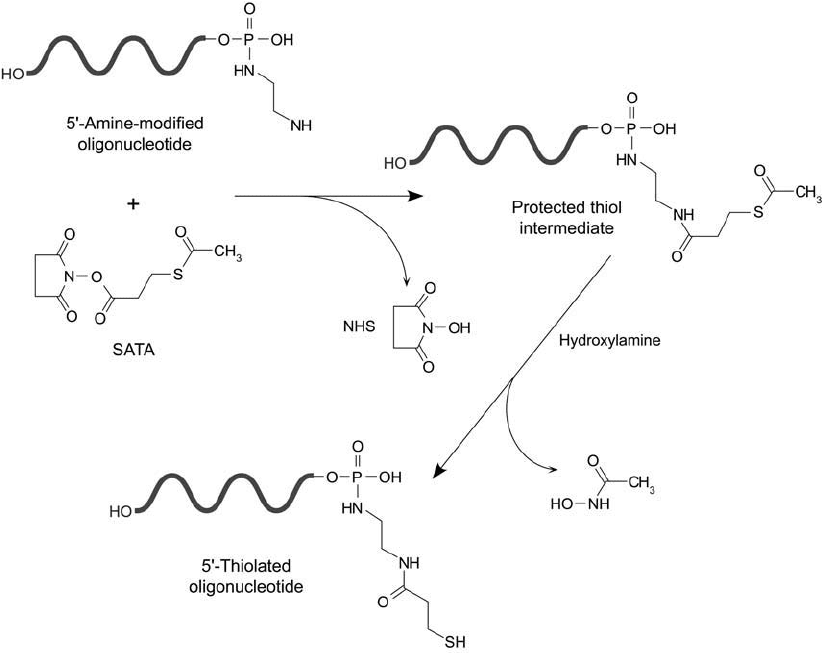
SATA Modifi cation of Amines on Nucleotides
Oligonucleotides containing amine groups introduced by enzymatic or chemical means may be
modifi ed with N-succinimidyl S-acetylthioacetate (SATA) (Chapter 1, Section 4.1), to produce
protected sulfhydryl derivatives. The NHS ester end of SATA reacts with a primary amine to
form a stable amide bond. After modifi cation, the acetyl protecting group can be removed as
needed by treatment with hydroxylamine under mildly alkaline conditions ( Figure 27.8 ). The
result is terminal sulfhydryl groups that can be used for subsequent labeling with thiol-reactive
probes or activated-enzyme derivatives (Kumar and Malhotra, 1992).
The advantage of using SATA over disulfi de-containing thiolation reagents such as SPDP
(previous section) is that the introduction of sulfhydryl residues does not include the use of a
disulfi de reducing agent. Typically, the pyridyl dithiol group resulting from an SPDP thiolation
must be reduced with a sulfhydryl-containing disulfi de reducing compound like DTT to free
the––SH group. With SATA, the sulfhydryl is freed by hydroxylamine cleavage, thus eliminat-
ing the need for removal of sulfhydryl reductants prior to a conjugation reaction.
Protocol
1. Dissolve the amine-modifi ed oligonucleotide to be thiolated in 250 l of 50 mM sodium
phosphate, pH 8.0.
2. Dissolve SATA in DMF at a concentration of 8 mg/ml.
3. Add 250 l of the SATA solution to the oligo solution. Mix.
4. React for 3 hours at 37 °C.
5. Remove excess reagents from the modifi ed oligo by gel fi ltration.
6. To deprotect the acetylated thiol group, add 100 l of 50 mM hydroxylamine hydrochlo-
ride, 2.5 mM EDTA, pH 7.5.
7. React for 2 hours.
8. The sulfhydryl-containing oligonucleotide may be used immediately to conjugate with
a sulfhydryl-reactive label, or it can be purifi ed from excess hydroxylamine by gel
fi ltration.
984 27. Nucleic Acid and Oligonucleotide Modifi cation and Conjugation
2.3. Biotin Labeling of DNA
Biotinylation of oligonucleotide probes provides a highly specifi c biological recognition site
for detection of DNA using (strept)avidin conjugates. The preparation of biotin-labeled DNA
can be done either by enzymatic or chemical means. Enzyme-catalyzed reactions utilize bioti-
nylated nucleoside triphosphates that can be incorporated into an oligonucleotide randomly or
at the 3 terminus (Section 1, this chapter). Chemical derivatization methods make use of cer-
tain reactive biotin compounds that can couple to functionally modifi ed probes or react with
DNA with the use of an activating reagent. For a description of the wide range of biotinylation
compounds available, see Chapter 11. The preparation and use of avidin and streptavidin con-
jugates is discussed in Chapter 23.
Biotin-LC-dUTP
Perhaps the most common method of DNA biotinylation is through enzymatic incorporation
with the use of a biotin-labeled deoxynucleoside triphosphate. First reported by Langer et al. in
2. Chemical Modifi cation of Nucleic Acids and Oligonucleotides 985
Figure 27.8 SATA may be used to modify a 5 -amine derivative of an oligonucleotide, forming a protected sulf-
hydryl. Deprotection with hydroxylamine results in generation of a free thiol.
1981, the procedure is probably the most popular nonradioactive labeling technique reported
for oligonucleotide probes. Although biotinylated derivatives of dCTP and dATP are reported
in the literature, by far the most frequently employed derivative is biotin–dUTP prepared from
the reaction of an amine-modifi ed dUTP with an amine-reactive biotinylation reagent, such as
NHS-LC-biotin (Chapter 11, Section 3.1).
Biotin–dUTP derivatives are formed by modifi cation of the C-5 position of uridine. This
location is not involved in hydrogen bonding activity with complementary DNA strands, thus
hybridization effi ciency is not immediately compromised. By contrast, biotin–dCTP or biotin–
dATP derivatives involve modifi cation of the bases at the N-4 position of cytosine and the N-6
position of adenine, locations directly involved in hydrogen bond formation with complemen-
tary bases. Thus, DNA biotinylation through the use of modifi ed deoxynucleoside triphos-
phates to be incorporated into existing DNA strands may result in better activity of the probe
if dUTP is used over dATP or dCTP.
The length of the spacer arm between the C-5 position of uridine and the biotin group is
another important parameter for activity of the resulting conjugate. The spacer affects the
incorporation effi ciency into existing probes using DNA polymerases, and it also affects the
ability of an (strept)avidin conjugate to effectively bind the biotinylated probe. Designation of
spacer length is usually expressed as the number of atoms separating the nucleotide base from
the biotin component. Thus, biotin- n-dUTP would describe a biotinylated deoxynucleoside tri-
phosphate having a spacer arm n atoms long. The shorter the spacer arm, the better the deriva-
tive is able to be recognized and incorporated into DNA polymers using polymerase enzymes.
Conversely, the longer the spacer arm, the better the biotinylated probe is able to hybridize
to its target and still maintain the capacity to have a streptavidin conjugate be complexed
with it. Thus, there is an optimal trade-off in spacer length between enzymatic incorporation
effi ciency and labeled-probe detectability. Studies have determined that this optimal range is
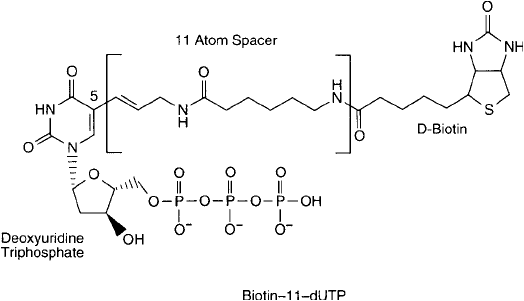
rather broad—between 7 atoms and 21 atoms in length. Perhaps the most common derivative
is biotin-11-dUTP, wherein an 11-atom spacer is employed ( Figure 27.9 ).
General protocols for the enzymatic incorporation of biotin-11-dUTP into DNA probes can be
found in Section 1 (this chapter). A particularly interesting modifi cation of the typical enzymatic
986 27. Nucleic Acid and Oligonucleotide Modifi cation and Conjugation
Figure 27.9 Biotin-11-dUTP is perhaps the most popular nucleotide derivative used for enzymatic biotinylation
of oligonucleotides. The “11” designation refers to the number of atoms in its spacer arm.
incorporation protocol for biotin is described by Didenko (1993). The single strand template
is immobilized by adsorption onto membranes before synthesis of the biotinylated probe. After
polymerase incorporation of biotin-11-dUTP, the labeled probe is removed by brief heating to
90 ° C in water. The result is highly pure probe with no contaminating complementary DNA
strands.
Photobiotin Modifi cation of DNA
The photoreactive biotin derivative, N -(4-azido-2-nitrophenyl)-aminopropyl- N -( N -d-biotinyl-
3-aminopropyl)-N-methyl-1,3-propanediamine, simply called photoactivatable biotin or pho-
tobiotin (Forster et al., 1985) contains a 9-atom diamine spacer group on the biotin valeric
acid side chain on one end, while the other end of the spacer terminates in an phenyl azide
group. The phenyl azide group can be photolyzed with UV light (350 nm) resulting in the for-
mation of a highly reactive nitrene intermediate. In most instances, this nitrene rapidly reacts
via ring expansion to form a dehydroazepine that is reactive with nucleophiles, such as amine
groups (Chapter 5, Section 3).
When photobiotin is irradiated in the presence of DNA the reaction process nonselectively
couples a biotin label to every 100–200 base residues. The result is an oligonucleotide probe
detectable by the use of (strept)avidin conjugates. The uses of photobiotin for DNA or RNA
modifi cation are summarized in Chapter 11, Section 4.
The following protocol is based on the method of Forster et al. (1985). Some optimization may
be necessary to obtain the best signal and activity for particular probes in hybridization assays.
Protocol for Labeling DNA Probes with Photobiotin
1. In subdued lighting conditions, dissolve photobiotin in water at a concentration of
1 g/ 1. Protect from light.
2. Dissolve the oligonucleotide probe in water or 0.1 mM EDTA, pH 7.0, at a concentration
of 1 g/ l.
3. Mix an equal volume of the photobiotin solution with the DNA probe solution.
4. Place the solution in an ice bath and irradiate from above (about 10 cm away) for
15 minutes using a sunlamp (such as Philips Ultrapnil MLU 300 W, General Electric
sunlamp RSM 275 W, or National Self-Ballasted BHRF 240–250 V 250 W W-P lamp).
5. Add 50 l of 0.1 M Tris, pH 9, and increase the total volume of the solution to 100 l (if
it is less than this amount).
6. To extract excess photobiotin, add 100 l of 2-butanol. Mix well and centrifuge. Discard
the upper phase. Repeat this process two more times.
7. To recover the biotinylated DNA, add 75 l of 4 M NaCl and mix.
8. Add 100
l of ethanol and cool the sample in dry ice (CO
2
) for 15 minutes.
9. Centrifuge to collect the precipitated, biotinylated DNA.
Reaction of NHS-LC-Biotin with Diamine-Modifi ed DNA Probes
NHS-LC-biotin is an extended spacer arm derivative of biotin containing an amine-reactive
NHS ester (Chapter 11, Section 1). The compound is a popular choice for biotinylating a wide
2. Chemical Modifi cation of Nucleic Acids and Oligonucleotides 987
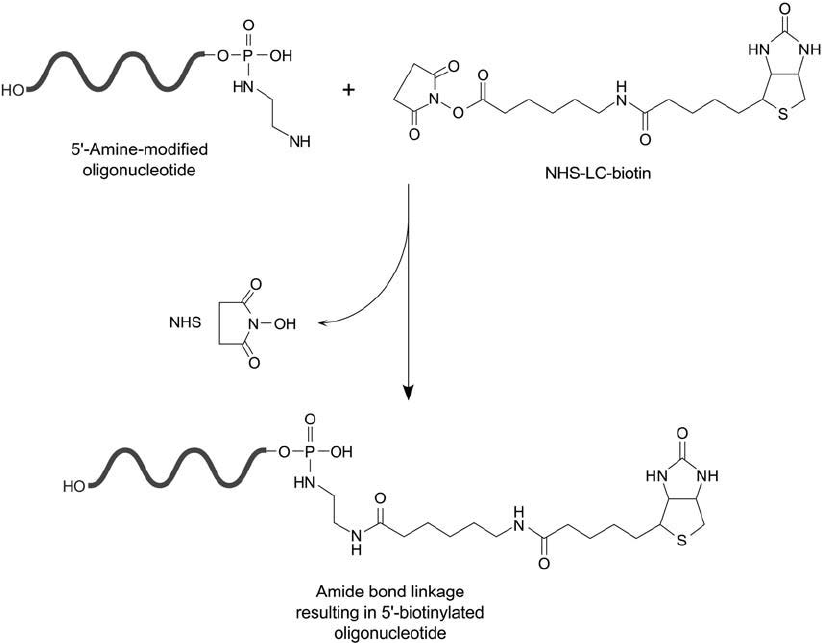
range of molecules containing primary amine groups, especially proteins. Oligonucleotides mod-
ifi ed to contain amine-terminal spacer arms also can be modifi ed with NHS-LC-biotin to create
stable amide bond derivatives. Alternatively, a hydrophilic biotinylation compound containing
a PEG spacer arm can be used to form a biotin-oligo derivative without the hydrophobic
character of the alkyl chain within NHS-LC-biotin. Chapter 18, Section 3 describes the amine-
reactive NHS–PEG
n
–biotin compounds suitable for this purpose.
Whether an amine is incorporated into an oligo by enzymatic means or chemical deriva-
tization, an NHS-ester-containing biotinylation reagent can be used to label the derivative in
high yield. If an amine group is added to the 5 end of a DNA probe by phosphoramidate
formation (Section 2.1, this chapter), then biotinylation of such molecules directs the label to a
region totally removed from interfering in subsequent hybridization with a target DNA strand
(Figure 27.10 ).
The following protocol assumes that the amine-containing oligo has already been synthe-
sized by any of the methods discussed in Section 2.1, this chapter.
988 27. Nucleic Acid and Oligonucleotide Modifi cation and Conjugation
Figure 27.10 Biotinylation of oligonucleotides may be done at the 5 -phosphate end using a diamine derivative
and reacting with NHS-LC-biotin.
Protocol
1. Prepare 10–20 g of amine-containing oligonucleotide in 200 l of water. Add to this
solution, 20 l of 1 M sodium bicarbonate, pH 9.0.
2. Dissolve NHS-LC-biotin (Thermo Fisher) in DMSO at a concentration of 10 mg/ml. Add
50 l of the biotinylation solution to the oligo solution. Mix well.
3. React for 2 hours at room temperature.
4. Isolate the biotinylated probe by ethanol/salt precipitation as described in Section 1 (this
chapter) for nick-translation modifi cation of DNA probes. Alternatively, dialysis, gel fi l-
tration, or n -butanol extraction may be used to remove excess reagents.
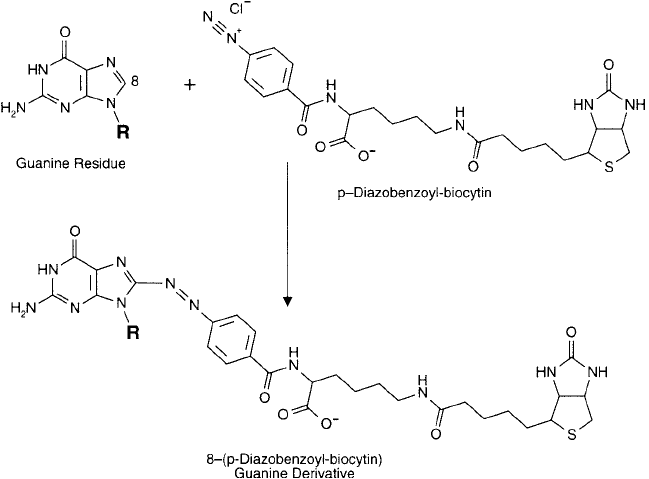
Biotin–Diazonium Modifi cation of DNA
Diazonium groups are able to couple at the C-8 position of adenosine or guanosine residues,
forming diazo bonds. -Aminobenzoyl biocytin can be used in this reaction to add a biotin
handle to purine bases within oligonucleotides (Chapter 11, Section 5). This biotinylation rea-
gent contains a 4-aminobenzoic acid amide derivative off the -amino group of biocytin ’s lysine
residue (Thermo Fisher). The aromatic amine can be treated with sodium nitrite in dilute HCl
to form a highly reactive diazonium derivative, which is able to couple with active hydrogen-
containing compounds. A diazonium reacts rapidly with histidine or tyrosine residues within
proteins, forming covalent diazo bonds (Wilchek et al., 1986). It also can react with purine
residues within DNA at position 8 of the bases (Rothenberg and Wilchek, 1988; Lowe, 1979)
(Figure 27.11 ).
2. Chemical Modifi cation of Nucleic Acids and Oligonucleotides 989
Figure 27.11 This diazo derivative of biocytin may be used to modify guanine bases at the C-8 position.
