Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

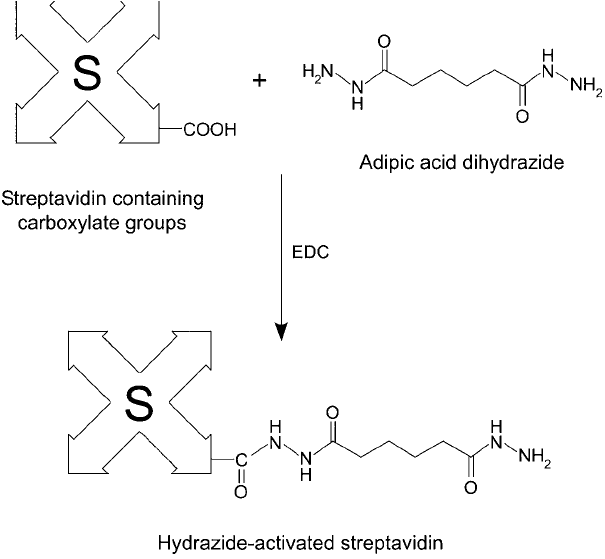
920 23. Avidin–Biotin Systems
3. Add 160 mg of the water-soluble carbodiimide EDC (Thermo Fisher) (Chapter 3, Section
1.1) to the solution, and mix to dissolve.
4. React for 4 hours at room temperature.
5. Dialyze against PBS, pH 7.2 to remove excess reagent and reaction by-products.
Hydrazide-activated (strept)avidin may be stored as a freeze-dried preparation without loss
of activity.
6. Biotinylation Techniques
In addition to preparing the (strept)avidin conjugates necessary to develop (strept)avidin–biotin-
based systems, the process of modifying targeting molecules with a biotin tag is just as critical
and forms the other key component of the interacting complex. Since biotin is a relatively small
molecule (MW 244.31), coupling it to macromolecules usually can be done without disturbing
the activity or binding capability of either the targeting molecule or the biotin handle. Proteins,
carbohydrates, lipid molecules, and nucleic acids can be modifi ed to contain one or more bio-
tins able to strongly interact with (strept)avidin. The technique of biotinylation is made easy
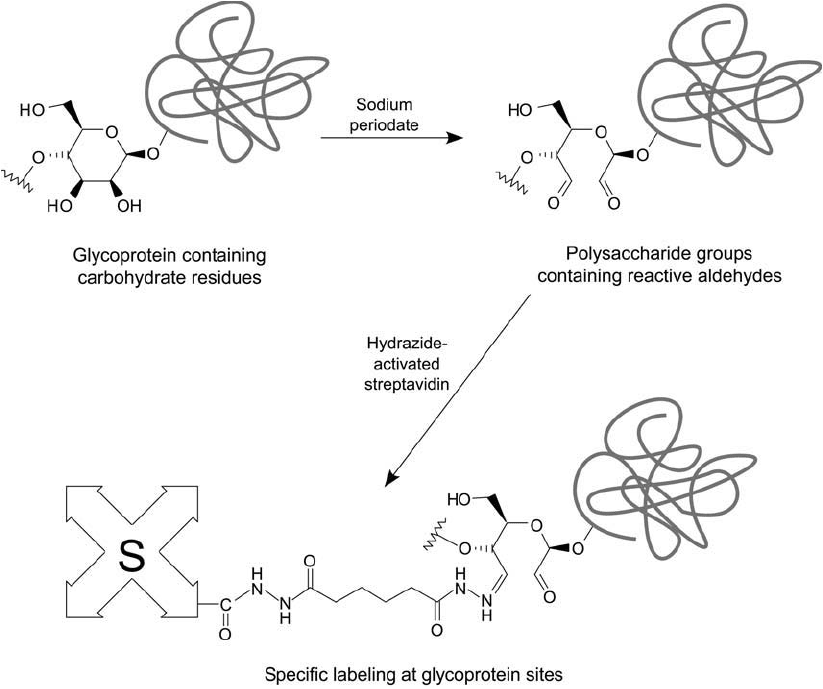
Figure 23.9 Reaction of adipic acid dihydrazide with (strept)avidin produces a hydrazide derivative that is
highly reactive toward periodate-oxidized polysaccharides.
through the commercial availability of a range of different biotin derivatives having a number
of important reactivity and property characteristics useful in (strept)avidin–biotin chemistry.
Chapter 11 and Chapter 18, Section 3, describe the major biotinylation compounds and their
properties. Also provided in these sections are suggested protocols for reacting each of these
reagents with specifi c functionalities on macromolecules.
7. Determination of the Level of Biotinylation
It is often important to determine the extent of biotin modifi cation after a biotinylation reac-
tion is complete. Measuring biotin incorporation into macromolecules can aid in optimizing
a particular (strept)avidin–biotin assay system. It also can be used to assure reproducibility in
Figure 23.10 Glycoproteins may be oxidized with sodium periodate to generate aldehyde residues. These may
be specifi cally labeled using a hydrazide-streptavidin derivative through hydrazone bond formation. Subsequent
detection may be done using biotinylated enzymes.
7. Determination of the Level of Biotinylation 921
922 23. Avidin–Biotin Systems
the biotinylation process. The most common method of measuring the degree of biotinylation
makes use of the HABA-dye assay (Green, 1965). HABA is 4 -hydroxyazobenzene-2-carboxylic
acid. In the absence of biotin, the dye is capable of specifi cally forming noncovalent complexes
with (strept)avidin at its biotin binding sites. Upon binding to (strept)avidin in aqueous solution,
HABA exhibits a characteristic absorption band at 500 nm ( 35,500 M
1
cm
1
, expressed
as per mole of HABA bound). The addition of biotin to this complex results in displacement
of HABA from the binding site, since the affi nity constant of the (strept)avidin–biotin inter-
action (1.3 10
15
M
1
) is much greater than that for (strept)avidin–HABA (6 10
6
M
1
). As
HABA is displaced, the absorbance of the complex decreases proportionally. Thus, the amount
of biotin present in the solution can be determined by plotting the (strept)avidin–HABA absorb-
ance at 500 nm versus the absorbance modulation with increasing concentrations of added
biotin. Comparing an unknown biotin-containing sample to this standard response curve can
result in the determination of the biotin concentration in the sample.
Since a biotinylated molecule potentially is able to interact with (strept)avidin at its biotin
binding sites just as strongly as biotin in solution, the degree of biotinylation may be deter-
mined using the HABA method as well. Comparison of the response of a biotinylated protein,
for example, with a standard curve of various biotin concentrations allows calculation of the
molar ratio of biotin incorporation.
Two variations of the HABA-dye assay for biotinylated proteins are possible. In one
approach, the biotinylated protein is digested using the enzyme pronase prior to doing the
assay. The digestion process breaks the protein into small fragments, some of which possess
biotin modifi cations. The digestion is done to eliminate any sterically hindered biotinylation
sites from not being able to interact with (strept)avidin. The second approach merely uses the
intact biotinylated protein in the assay, assuming that the HABA assay results then will provide
a truer picture of the level of accessible biotin sites on the molecule. Pronase addition obviously
is not necessary for assessing biotinylated molecules which are not proteins.
The following protocol describes both of these HABA-based tests for determining the level
of biotinylation.
Protocol
1. Dissolve (strept)avidin in 0.05 M sodium phosphate, 0.15 M NaCl, pH 6.0, at a concen-
tration of 0.5 mg/ml. A total of 3 ml of the (strept)avidin solution is required to create a
standard curve using known concentrations of biotin and an additional 3 ml is needed
for each sample determination.
2. Dissolve the HABA dye (Sigma) in 10 mM NaOH at a concentration of 2.42 mg/ml
(10 mM). Prepare about 100 l of the HABA solution for each 3 ml portion of (strept)avidin
solution required.
3. Dissolve the biotinylated protein to be measured in 0.05 M sodium phosphate, 0.15 M
NaCl, pH 6.0, at a concentration of 10–20 mg/ml. The amount required is about 100 l
of sample per determination.
4. Dissolve D-biotin in 0.05 M sodium phosphate, 0.15 M NaCl, pH 6.0, at a concentration
of 0.5 mM.
5. For the proteolytic digestion procedure, dissolve pronase in water at a concentration of
1 percent (w/v).
6. If pronase digestion of the biotinylated protein is to be done, heat 100 l of the sample at
56 ° C for 10 minutes, then add 10 l of the pronase solution. Allow the sample to digest
enzymatically at room temperature overnight. If no pronase digestion is desired, simply
use the biotinylated protein solution prepared in step 3 without further treatment.
7. To construct a standard curve of various biotin concentrations, fi rst zero a spectropho-
tometer at an absorbance setting of 500 nm with sample and reference cuvettes fi lled
with 0.05 M sodium phosphate, 0.15 M NaCl, pH 6.0. Remove the buffer solution from
the sample cuvette and add 3 ml of the (strept)avidin solution plus 75 l of the HABA-
dye solution. Mix well and measure the absorbance of the solution at 500 nm. Next add
2l aliquots of the biotin solution to this (strept)avidin–HABA solution, mix well after
each addition, and measure and record the resultant absorbance change at 500 nm. With
each addition of biotin, the absorbance of the (strept)avidin–HABA complex at 500 nm
decreases. The absorbance readings are plotted against the amount of biotin added to
construct the standard curve.
8. To measure the response of the biotinylated protein sample, add 3 ml of the (strept)avidin
solution plus 75 l of the HABA dye to a cuvette. Mix well and measure the absorbance
of the solution at 500 nm. Next, add a small amount of sample to this solution and mix.
Record the absorbance at 500 nm. If the change in absorbance due to sample addition
was not suffi cient to obtain a signifi cant difference from the initial (strept)avidin–HABA
solution, add another portion of sample and measure again. Determine the amount of
biotin present in the protein sample by using the standard curve. The number of moles of
biotin divided by the moles of protein present gives the number of biotin modifi cations
on each protein molecule.
7. Determination of the Level of Biotinylation 923

924
24
As early as the fi rst decade of the twentieth century colloidal gold sols containing particles of
less than 10 nm were produced by chemical means (Zsigmondy, 1905). However, the application
of these inorganic suspensions to protein labeling didn ’t occur until 1971 when Faulk and Taylor
invented the immunogold staining procedure. Since that time, the labeling of targeting mole-
cules, especially proteins, with gold nanoparticles has revolutionized the visualization of cellular
or tissue components by electron microscopy (Horisberger et al., 1975; Horisberger, 1979). The
silver enhancement technique further broadened the application of gold labeling to include light
microscopy (Holgate et al., 1983). The electron-dense and visually opaque nature of gold labels
also provided excellent detection qualities for such techniques as blotting, fl ow cytometry, cyto-
chemical staining, and hybridization assays (Jackson et al., 1990; Gee et al., 1991). Double- or
triple-labeling systems have been constructed using immunogold methods in tandem with immu-
noenzymatic techniques to detect more than one antigen at the same time (Gillitzer et al., 1990).
This section discusses the properties of gold particles as well as the common methods of
labeling proteins and other biomolecules with them. The cited references should be consulted
to obtain protocols for using these protein–gold complexes in assay and detection systems.
1. Properties and Use of Gold Conjugates
Colloidal gold suspensions consist of small granules of this transition metal in a stable, uni-
form dispersion. Viewed under the light or electron microscope, they appear as solid spheres
of dense material. In electron microscopy the gold particles are visible as dense, dark markers
usually black in appearance. In light microscopy, they can appear as light dots on a darker
background due to the high refl ectance of the particles or as an orange-red coating where they
are localized in large conglomerates on cells or tissues. Colloidal gold particles act as effi cient
nuclei for deposition of silver, thus markedly enhancing their detection under light micros-
copy (Danscher and Rytter-Nörgaard, 1983). The same silver–gold combination also provides
increased sensitivity in blotting applications (Moeremans et al ., 1984).
Most preparations of colloidal gold consist of particles varying in diameter from about 5 nm
to around 150 nm. The methods of forming small particle gold suspensions of known diameter
are discussed in Section 2.
Preparation of Colloidal Gold-Labeled Proteins
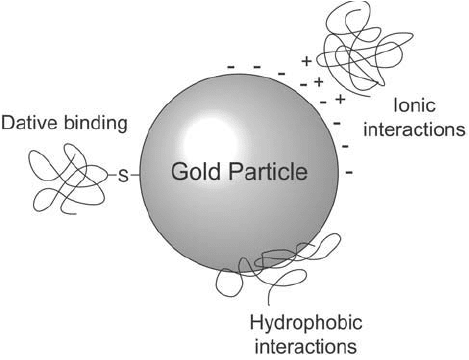
The labeling of macromolecules with gold particles proceeds through a number of rather
poorly understood processes. Preparing stable protein–gold complexes depends on several
interactions: (a) the electronic attraction between the negatively charged gold particles and
the abundant positively charged sites on the protein molecule, (b) an adsorption phenomena
involving hydrophobic pockets on the protein binding to the metal surface, and (c) the potential
for covalent binding of gold to free sulfhydryl groups, if present (dative binding, see Chapter 2,
Section 2.8) ( Figure 24.1 ).
Deryagin and Landau (1941) and Verwey and Overbeek (1948) working independently
developed a theory of the behavior of colloidal systems that aids in understanding macro-
molecular labeling with gold particles. Called the DLVO theory from the initials of the four
authors, it views the particles in a sol as consisting of two components producing opposite
effects in aqueous suspension. The overlap of the electrical double layer of each particle causes
a negative charge on the surface, leading to particle–particle repulsion and stabilizing the sol
from aggregation. The other phenomenon is electromagnetic in nature and leads to the poten-
tial for Van der Waals attraction between the metal surface and other molecules.
In the colloidal suspension, there exists a balance between the negative charge repulsion
and the attractive forces which could cause coagulation. As particles approach each other, an
energy barrier must be traversed to overcome the repulsive character of the negative surface
and enter the region of Van der Waals attraction. This barrier can be breached by the addition
of electrolytes to the solution that can mask the negative charge on each particle. At a certain
concentration of electrolytes, the colloid will begin to collapse as the gold particles adsorb onto
one another, forming large aggregates and ultimately falling out of suspension.
Electrolyte-mediated coagulation forms the basis for creating all gold conjugates with other
molecules. If macromolecules such as proteins are present in the colloidal suspension as the
electrolyte concentration is raised to surpass the negative repulsion effects, then adsorption will
occur with the protein molecules instead of with other gold particles. Thus, in place of aggre-
gation and collapse of the suspension, labeling occurs.
Figure 24.1 Protein binding to gold particles can occur through several types of interactions.
1. Properties and Use of Gold Conjugates 925
926 24. Preparation of Colloidal Gold-Labeled Proteins
The most common electrolyte additions in protein–gold labeling are NaCl or buffer salts. If
no macromolecules are present, the addition of NaCl would itself cause gold particle coagula-
tion. The aggregation is accompanied by a color change from orange-red to red-violet or blue
(Roth and Binder, 1978), and it may be quantifi ed spectrophotometrically by the change in
absorbance at 580 nm (Horisberger et al ., 1975).
In practice, the addition of a protein to a gold sol will result in spontaneous adsorption on the
surface of the gold particles due to electrostatic, hydrophobic, and Van der Waals interactions. To
prepare labeled proteins, initially the gold suspension is rapidly mixed while a quantity of protein
is added. As the gold is bound to the protein molecules, a decrease in the absorbance at 580 nm
occurs as the gold particles become stabilized and less coagulated. To check for the completeness
of the adsorption process and to determine if the gold particles are totally blocked, a portion of
the sol can be removed and an aliquot of NaCl added. If coagulation occurs upon addition of
salt (increase in A
580
nm
), more protein should be added to completely stabilize the sol. Finally,
many protocols further stabilize the colloidal suspension after protein binding by the addition of
polyethylene glycol (PEG) or an immunochemical blocking agent, such as BSA or a solution of
dried milk. These blocking agents completely mask any remaining sites of potential gold–gold or
gold–protein interactions, thus preventing aggregation or nonspecifi c binding during assays.
To produce acceptable gold probes, it is often a common practice to add the minimum
quantity of protein needed to prevent NaCl-induced aggregation plus about 10–20 percent
excess (Horisberger and Rosset, 1977; De Mey et al., 1981). Other investigators have reported
that the addition of large excesses of protein to the amount of gold present yields conjugates
of higher specifi c activity (Tokuyasu, 1983; Tinglu et al., 1984). However, there is some evidence
that overloading may cause leaching of loosely bound protein (Horisberger and Clerc, 1985).
As in any conjugation procedure, optimization of the ratios of reactants must be done to
obtain the best probes. In labeling proteins with gold particles, several parameters should be
considered: (a) the pI of the protein, (b) the pH of the adsorption process, and (c) the quan-
tity of protein charged to the labeling reaction. It is generally believed that most proteins can
be made to adsorb maximally at or near their isoelectric point (Norde, 1986). This is the pH
of net electrical neutrality for a protein, wherein any electrically induced repulsive or attrac-
tive forces are balanced. For many proteins, especially antiserum-derived immunoglobulins, the
average pI is a broad band encompassing a range of pH values. Thus, a polyclonal antibody
preparation may possess an average pI much different than a particular purifi ed monoclonal.
Geoghegan (1988) determined that as the pH of the adsorption reaction increased beyond
the pI range, the percentage of IgG bound to gold particles decreased. However, for high-pI
immunoglobulins, coupling at basic pH values increased the coupling yield. Geoghegan also
noted that the more immunoglobulin that was charged to the adsorption process, the more
ended up being coupled, although the percent bound would decrease.
Thus, while defi nite standards for the ratio of protein-to-gold are not universally agreed
upon, the effi ciency of the process can be improved by following these general guidelines: (a)
perform the adsorption reaction at a pH within the range of the pI of the protein being modifi ed
or at slightly higher pH, (b) charge an amount of protein to the gold particles that is slightly
more (by about 10 percent) than necessary to maintain colloidal stability upon addition of
NaCl, (c) avoid high overloads of protein, since this may promote subsequent leaching of
bound material, (d) evaluate the degree of adsorption and the relative coagulation of the gold
particles by measuring the absorbance of the solution at 580 nm, and (e) each protein–gold
conjugate should be optimized as to colloidal stability and retention of activity.
An approximation of the correct amount of protein to be added to a gold sol to maintain
stability of the colloid can be done using the following protocol (Slot and Geuze, 1984).
Protocol
1. Add 0.25 ml of the gold suspension to separate tubes containing 25 l of different con-
centrations of the protein to be adsorbed. The amount of protein required to stabilize
1 ml of most gold sols is in the microgram range. The protein concentrations should be
from about 10 g/100 l to about 150 g/100 l. Mix well.
2. After about 1 minute, add 0.25 ml of 10 percent NaCl to the gold/protein suspension.
Mix well.
3. Monitor the stability of the gold sol by its color or by the absorbance of the mixture at
580 nm. As long as the colloid continues to turn blue, and thus forms gold aggregates
with addition of electrolyte, the amount of protein added is not suffi cient to stabilize the
suspension. This condition translates into a decrease in the absorbance at 580 nm. When
the concentration of protein added is enough to stabilize the colloidal suspension, the
solution no longer changes color (the absorbance at 580 nm no longer decreases).
4. The amount of protein added at the stabilization point plus 10 percent should be used to
produce the fi nal protein–gold conjugate.
The use of gold probes in detection systems has a number of advantages. The ability to label
macromolecules with a range of gold particle sizes makes it possible to visualize the probe
under a variety of microscopic conditions. Gold avoids all the disadvantages of radioactive
labels, while being much more stable to quenching or fading than fl uorescent probes or enzy-
matically developed substrate chromophores. A gold-labeled tissue, cell, or blot will maintain
its record of staining on a permanent basis. Under suffi cient magnifi cation, an assessment of the
degree of antigen labeling can be made simply by counting the number of gold particles present
per unit area of cell or tissue mass. This cannot be done with other labeling systems, since
chemical stains develop an amorphous quality that does not allow differentiation of individual
molecules. Finally, gold probes are essentially non-toxic and relatively inexpensive to use.
A variety of biological molecules can be labeled with gold particles. Proteins are perhaps the
most common gold probes; toxins, antibodies, immunoglobulin binding proteins such as pro-
tein A, enzymes, lectins, avidin and streptavidin, lipoproteins, and glycoproteins all have been
labeled with colloidal gold to form highly sensitive reagents. In addition, polymers, hormones,
carbohydrates, and lipids have been gold-labeled for various applications. Small hapten mol-
ecules co-adsorbed with adjuvant peptides to gold particles make extraordinary immunogen
complexes, producing polyclonal antibody responses having very high titers (Pow and Crook,
1993).
Very small gold particles can even be derivatized to contain specifi c chemical reactive groups
for covalent coupling to macromolecules. For instance, an NHS ester-containing gold particle
of 1.4 nm is manufactured by Nanoprobes (Stony Brook, NY). Presumably, such derivatives are
formed by adsorption of chemically reactive polymers or by dative binding with a sulfhydryl-
containing modifi cation reagent.
The following sections discuss the preparation of colloidal gold suspensions of various par-
ticle sizes and their use in labeling proteins for detection purposes. Gold-labeled molecules and
proteins are available from a number of manufacturers (Janssen, E-Y Labs, and Nanoprobes).
1. Properties and Use of Gold Conjugates 927
928 24. Preparation of Colloidal Gold-Labeled Proteins
2. Preparation of Mono-Disperse Gold Suspensions for Protein Labeling
Mono-disperse colloidal gold suspensions useful for labeling macromolecules can be produced
by a variety of chemical methods. Three main procedures have become common for making
particles which fall into predictable particle-size ranges. All of them use reductive processes on
chloroauric acid (HAuCl
4
) to create the spheroidal gold particles. In general, the greater the
power and concentration of the reducing agent, the smaller the resultant particles.
To create large-particle colloidal gold dispersions, chloroauric acid normally is treated with
sodium citrate. The result is a particle range of about 15–150 nm, depending on the concentra-
tion of citrate utilized (Honsberger, 1979; Horisberger and Rosset, 1977; Pow and Morris, 1991).
Medium-sized gold particles of diameter between 6 nm and 15 nm (average 12 nm) are formed by
treatment with sodium ascorbate as the reductant (although some procedures use trisodium cit-
rate at higher concentrations than the sodium citrate used for making large particles) (Honsberger
and Tacchini-Vonlanthen, 1983; Albrechte et al., 1989). The smallest gold particles ( 5 nm diam-
eter) are created by reduction with either yellow or white phosphorus (Zsigmondy, 1905; Faulk
and Taylor, 1971; Horisberger and Rosset, 1977; Pawley and Albrecht, 1988). Particles as small
as 2 nm may be created by reduction with sodium borohydride (Bonnard et al., 1984).
The following protocols for creating colloidal gold sols are adaptations from the above cited
articles. To obtain reproducible preparations, extreme care should be taken in making each
batch to maintain the same reagent concentrations, temperatures, and times for the reactions.
In each preparation, a color change is noted as the chloroauric acid is reduced from its ini-
tial state to the fi nal gold sol. The initial color is typically a brown, purple-red, or dark blue,
depending on the reductant used and other conditions. The fi nal color of the mono-disperse
colloidal gold preparation is typically red.
2.1. Preparation of 2 nm Gold Particle Sols
1. Prepare 1 ml of a 4 percent HAuCl
4
solution in deionized water.
2. Add 375 l of the chloroauric acid solution plus 500 l of 0.2 M K
2
CO
3
to 100 ml deion-
ized water, cooled on ice to 4°C. Mix well.
3. Dissolve sodium borohydride (NaBH
4
) in 5 ml of water at a concentration of 0.5 mg/ml.
Prepare fresh.
4. Add fi ve 1-ml aliquots of the sodium borohydride solution to the chloroauric acid/
carbonate suspension with rapid stirring. A color change from bluish-purple to reddish-
orange will be noted as the additions take place.
5. Stir for 5 minutes on ice after the completion of sodium borohydride addition.
2.2. Preparation of 5 nm Gold Particle Sols
1. Prepare 7 ml of a 1 percent HAuCl
4
solution in deionized water.
2. Add 6.25 ml of the chloroauric acid solution plus 5.8 ml of 0.1 M K
2
CO
3
to 500 ml
deionized water. Mix well.
3. In a fume hood, prepare a saturated solution of white phosphorus in diethyl ether, then
dilute 1 part of the saturated phosphorus solution with 4 parts of diethyl ether.
4. Add 4.16 ml of the diluted phosphorus solution to the chloroauric acid/carbonate solu-
tion with mixing.
5. React at room temperature for 15 minutes.
6. Bring the mixture to a boil and refl ux until the color of the suspension turns from brown-
ish to red. This should take no more than about 5 minutes.
7. Cool the sol to room temperature.
8. The pH of the suspension will be around 6. Adjustments to more alkaline conditions for
adsorbing macromolecules of higher pI may be done by addition of 0.1 M K
2
CO
3
with
stirring. Monitor pH of the sol using a gel-fi lled electrode (Orion Research, No. 9115,
Cambridge, MA) (Geoghegan et al., 1980). After pH adjustment, the gold should be used
immediately for complexing with a protein or other macromolecule.
2.3. Preparation of 12 nm Gold Particle Sols
1. Prepare 5 ml of a 1 percent HAuCl
4
solution in deionized water.
2. Add 4 ml of the chloroauric acid solution plus 4 ml of 0.1 M K
2
CO
3
to 100 ml deionized
water. Mix well and cool the solution on ice.
3. With rapid mixing of the chloroauric acid/carbonate solution, quickly add 1 ml of a
7 percent sodium ascorbate solution prepared in water. Maintain the solution cooling in
an ice bath. Higher temperatures will create larger particle sizes. The color of the solution
at this point will turn to a purple-red.
4. Adjust the volume of the reaction to 400 ml with deionized water.
5. Bring the mixture to a boil and refl ux until the color of the suspension turns from purple-
red to red.
6. Cool the sol to room temperature.
7. The pH of the suspension will be around 6. Adjustments to more alkaline conditions for
adsorbing macromolecules of higher pI may be done by addition of 0.1 M K
2
CO
3
with
stirring. Monitor pH of the sol using a gel-fi lled electrode (Geoghegan et al., 1980). After
pH adjustment, the gold should be used immediately for complexing with a protein or
other macromolecule.
2.4. Preparation of 30 nm Gold Particle Sols
1. Prepare 1 ml of a 4 percent HAuCl
4
solution in deionized water.
2. Add 0.5 ml of the chloroauric acid solution to 200 ml of deionized water and bring to a
boil while mixing.
3. Add to the boiling, rapidly mixing solution of chloroauric acid, 3 ml of a 1 percent
sodium citrate solution.
4. Refl ux for 30 minutes. The color of the suspension will change from a dark blue to a red
as the mono-disperse colloidal gold particles are formed.
5. Cool to room temperature.
Any of the particle sols prepared above may be used to adsorb macromolecules to create
gold probes. To concentrate the suspensions, the solutions may be fi ltered through a small-pore
2. Preparation of Mono-Disperse Gold Suspensions for Protein Labeling 929
