Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.

3.1.2
Environment
The microbial flora of the pharmacy environment is a reflection of the general hospital
environment and the activities undertaken there. Free-living opportunist pathogens,
such as Ps. aeruginosa can normally be found in all wet sites, such as drains, sinks and
taps. Cleaning equipment, such as mops, buckets, cloths and scrubbing machines, may
be responsible for distributing these organisms around the pharmacy; if stored wet
they provide a convenient niche for microbial growth, resulting in heavy contamination
of equipment. Contamination levels in the production environment may, however, be
minimized by observing good manufacturing practices, by installing heating traps in
sink U-bends, thus destroying one of the main reservoirs of contaminants, and by proper
maintenance and storage of equipment, including cleaning equipment. Additionally,
cleaning of production units by contractors should be carried out to a pharmaceutical
specification.
3.1.3 Packaging
Sacking, cardboard, card liners, corks and paper are unsuitable for packaging
pharmaceuticals, as they are heavily contaminated, for example with bacterial or
fungal spores. These have now been replaced by non-biodegradable plastic materials.
Packaging in hospitals is frequently re-used for economic reasons. Large numbers of
containers may be returned to the pharmacy, bringing with them microbial contaminants
introduced during use in the wards. Particular problems have been encountered in
the past with disinfectant solutions where residues of old stock have been 'topped
up' with fresh supplies, resulting in the issue of contaminated solutions to wards.
Re-usable containers must, therefore, be thoroughly washed and dried, and never
refilled directly.
Another common practice in hospitals is the repackaging of products purchased in
bulk into smaller containers. Increased handling of the product inevitably increases the
risk of contamination, as shown by one survey when hospital-repacked items were
found to be contaminated twice as often as those in the original pack (Public Health
Laboratory Service Report 1971).
3.2 In use
Pharmaceutical manufacturers may justly argue that their responsibility ends with
the supply of a well-preserved product of high microbiological standard in a
suitable pack and that the subsequent use, or indeed abuse, of the product is of
little concern to them. Although much less is known about how products become
contaminated during use, there is reasonable evidence that continued use of such products
is undesirable, particularly in hospitals where it may result in the spread of cross-
infection.
All multidose products are subject to contamination from a number of sources
during use. The sources of contamination are the same whether products are used in
hospital or in the community environment, but opportunities for observing it are, of
course, greater in the former.
Contamination of non-sterile pharmaceuticals 377
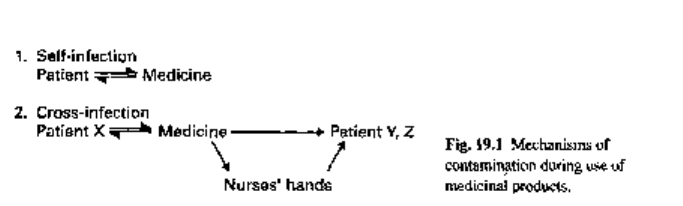
3.2.1 Human sources
During normal usage, the patient may contaminate his/her medicine with his/her
own microbial flora; subsequent use of the product may or may not result in self-
infection (Fig. 19.1). Topical products are considered to be most at risk, since the
product will probably be applied by hand thus introducing contaminants from the
resident skin flora of staphylococci, Micrococcus spp. and diphtheroids but also perhaps
transient contaminants, such as Pseudomonas, which would normally be removed during
effective handwashing. Opportunities for contamination may be reduced by using
disposable applicators for topical products or by taking oral products by disposable
spoon.
In hospitals, multidose products, once contaminated, may serve as a vehicle of
cross-contamination or cross-infection between patients. Zinc-based products packed
in large stock-pots and used in the treatment and prevention of bed-sores in long-stay
and geriatric patients may become contaminated during use with organisms such as
Ps. aeruginosa and Staphylococcus aureus. These unpreserved products will allow
multiplication of contaminants, especially if water is present either as part of the
formulation, for example in oil/water (o/w) emulsions, as a film in w/o emulsions
which have undergone local cracking, or as a condensed film from atmospheric water,
and appreciable numbers may then be transferred to other patients when re-used. Clearly
the economics and convenience of using stock-pots need to be balanced against the
risk of spreading cross-infection between patients and the inevitable increase in length
of the patients' stay in hospital. The use of stock-pots in hospitals has noticeably declined
over the past decade or so.
A further potential source of contamination in hospitals is the nursing staff
responsible for medicament administration. During the course of their work, nurses'
hands become contaminated with opportunist pathogens which are not part of the normal
skin flora but are easily removed by thorough handwashing and drying. In busy wards,
handwashing between attending to patients may be omitted and any contaminants may
subsequently be transferred to medicaments during administration. Hand lotions and
creams used to prevent chapping of nurses' hands may similarly become contaminated,
especially when packaged in multidose containers and left at the side of the handbasin,
frequently without a lid. The importance of thorough handwashing cannot be over-
emphasized in the control of hospital cross-infection. Hand lotions and creams should
be well preserved and, ideally, packaged in disposable dispensers. Other effective control
methods include the supply of products in individual patient's packs and the use of a
non-touch technique for medicament administration.
378 Chapter 19
3.2.2
Environmental sources
Small numbers of airborne contaminants may settle out in products left open to the
atmosphere. Some of these will die during storage, with the rest probably remaining
at a static level of about 10
2
-10
3
colony forming units (cfu) g
_I
or ml
-1
. Larger
numbers of water-borne contaminants may be accidentally introduced into topical
products by wet hands or by a 'splash-back mechanism', if left at the side of a basin.
Such contaminants generally have simple nutritional requirements and, following
multiplication, levels of contamination may often exceed 10
6
cfug~' or ml
-1
. This
problem is encountered particularly when the product is stored in warm hospital wards
or in hot steamy bathroom cupboards at home. Products used in hospitals as soap
substitutes for bathing patients are particularly at risk, and soon not only become
contaminated with opportunist pathogens such as Pseudomonas spp., but also provide
conditions conducive for their multiplication. The problem is compounded by using
stocks in multidose pots for use by several patients in the same ward over an extended
period of time.
The indigenous microbial population is quite different in the home and in hospitals.
Pathogenic organisms are found much more frequently in the latter and consequently
are isolated more often from medicines used in hospital. Usually, there are fewer
opportunities for contamination in the home, as patients are generally issued with
individual supplies in small quantities.
3.2.3 Equipment sources
Patients and nursing staff may use a range of applicators (pads, sponges, brushes,
spatulas) during medicament administration, particularly for topical products. If re-
used, these easily become contaminated and may be responsible for perpetuating
contamination between fresh stocks of product, as has indeed been shown in
studies of cosmetic products. Disposable applicators or swabs should therefore always
be used.
In hospitals today a wide variety of complex equipment is used in the course
of patient treatment. Humidifiers, incubators, ventilators, resuscitators and other
apparatus require proper maintenance and decontamination after use. Chemical
disinfectants used for this purpose have in the past through misuse become contaminated
with opportunist pathogens, such as Ps. aeruginosa, and ironically have contributed
to, rather than reduced, the spread of cross-infection in hospital patients. Disinfectants
should only be used for their intended purpose and directions for use must be followed
at all times.
4 The extent of microbial contamination
Detailed examination of reports in the literature of medicament-borne contamination
reveals that the majority of these are anecdotal in nature, referring to a specific product
and isolated incident. Little information is available, however, as to the overall risk of
products becoming contaminated and causing patient infections when subsequently
used. As with risk analysis in food microbiology (assessment of the hazards of
Contamination of non-sterile pharmaceuticals 379
consumption of a contaminated preparation) this information is considered invaluable
not only because it indicates the effectiveness of existing practices and standards, but
also because the value of potential improvements in quality from a patient's point of
view can be balanced against the inevitable cost of such processes. Thus, the old
argument that all pharmaceutical products, regardless of their use, should be produced
as sterile products, although sound in principle, is kept in perspective by the fact that it
cannot be justified on economic grounds alone.
Contamination in manufacture
Investigations carried out by the Swedish National Board of Health in 1965 revealed
some startling findings on the overall microbiological quality immediately after
manufacture of non-sterile products made in Sweden. A wide range of products was
routinely found to be contaminated with Bacillus subtilis, Staph, albus, yeasts and
moulds, and in addition large numbers of coliforms were found in a variety of tablets.
Furthermore, two nationwide outbreaks of infection were subsequently traced to the
inadvertent use of contaminated products. Two hundred patients were involved in an
outbreak of salmonellosis, caused by thyroid tablets contaminated with Salmonella
bareilly and Sal. muenchen; and eight patients had severe eye infections following
the use of hydrocortisone eye ointment contaminated with Ps. aeruginosa. The results
of this investigation have not only been used as a yardstick for comparing the
microbiological quality of non-sterile products made in other countries, but also as a
baseline upon which international standards could be founded.
In the UK, the microbiological and chemical quality of pharmaceutical products
made by industry has since been governed by the Medicines Act 1968. The majority of
products have been found to be made to a high standard, although spot checks have
occasionally revealed medicines of unacceptable quality and so necessitated product
recall. By contrast, the manufacture of pharmaceutical products in hospitals has in the
past been much less rigorously controlled, as shown by the results of surveys in the
1970s in which significant numbers of preparations were found to be contaminated
with Ps. aeruginosa. In 1974, hospital manufacture also came under the terms of the
Medicines Act and, as a consequence, considerable improvements have been seen in
recent years not only in the conditions and standard of manufacture, but also in the
chemical and microbiological quality of finished products.
Furthermore, in the past decade hospital manufacturing operations have been
rationalized. Economic restraints have resulted in a critical evaluation of the true cost
of these activities; competitive purchasing from industry has in many cases produced
cheaper alternatives and small-scale manufacturing has been largely discouraged. Where
licensed products are available, NHS policy now dictates that these are purchased from
a commercial source and not made locally. Hospital manufacturing is at present
concentrated on the supply of bespoke products from a regional centre or small-scale
specialist manufacture of those items currently unobtainable from industry. Repacking
of commercial products into more convenient pack sizes is still, however, common
practice.
Removal of Crown immunity from the NHS in 1991 meant that manufacturing
operations in hospitals were then subject to the full licensing provisions of the Medicines
Act 1968, i.e. hospital pharmacies intending to manufacture were required to obtain a
manufacturing licence and to comply fully with the EC Guide to Good Pharmaceutical
Manufacturing Practice (1989, revised in 1992); amongst other requirements, this
included the use of appropriate environmental manufacturing conditions and associated
environmental monitoring. Subsequently, the Medicines Control Agency (MCA) issued
guidance in 1992 on certain manufacturing exemptions, by virtue of the product batch
size or frequency of manufacture. At the same time the need for extemporaneous
dispensing of 'one-off' special formulae continues in hospital pharmacies today, although
this work has largely been transferred from the dispensing bench to dedicated preparative
facilities with appropriate environmental control.
Contamination in use
Higher rates of contamination are invariably seen in products after opening and use,
and, amongst these, medicines used in hospitals are more likely to be contaminated
than those used in the general community. The Public Health Laboratory Service Report
of 1971 expressed concern at the overall incidence of contamination in non-sterile
products used on hospital wards (327 of 1220 samples) and the proportion of samples
found to be heavily contaminated (18% in excess of 10
4
cfug
-1
or ml
-1
). The presence
of Ps. aeruginosa in 2.7% of samples (mainly oral alkaline mixtures) was considered
to be highly undesirable.
By contrast, medicines used in the home are not only less often contaminated but
also contain lower levels of contaminants and fewer pathogenic organisms. Generally,
there are fewer opportunities for contamination here since smaller quantities are used
by individual patients. Medicines in the home may, however, be hoarded and used for
extended periods of time. Additionally, storage conditions may be unsuitable and expiry
dates ignored and thus problems other than those of microbial contamination may be
seen in the home.
Factors determining the outcome of a
medicament-borne infection
A patient's response to the microbial challenge of a contaminated medicine may be
diverse and unpredictable, perhaps with serious consequences. In one patient, no clinical
reactions may be evident, yet in another these may be indisputable, illustrating one
problem in the recognition of medicament-borne infections. Clinical reactions may
range from inconvenient local infections of wounds or broken skin, caused possibly
from contact with a contaminated cream, to gastrointestinal infections from the ingestion
of contaminated oral products, to serious widespread infections, such as a bacteraemia
or septicaemia, leading perhaps to death, as have resulted from infusion of contaminated
fluids. Undoubtedly, the most serious outbreaks of infection have been seen in the past
where contaminated products have been injected directly into the bloodstream of patients
whose immunity is already compromised by their underlying disease or therapy. The
outcome of any episode is determined by a combination of several factors, amongst
which the type and degree of microbial contamination, the route of administration and
the patient's resistance are of particular importance.
Contamination of non-sterile pharmaceuticals 381
5.1 Type and degree of microbial contamination
Microorganisms that contaminate medicines and cause disease in patients may be
classified as true pathogens or opportunist pathogens. Pathogenic organisms like
Clostridium tetani and Salmonella spp. rarely occur in products, but when present
cause serious problems. Wound infections from using contaminated dusting powders
have been reported, including several cases of neonatal death from talcum powder
containing CI. tetani. Outbreaks of salmonellosis have followed the inadvertent ingestion
of contaminated thyroid and pancreatic powders. On the other hand, opportunist
pathogens like Ps. aeruginosa, Klebsiella, Serratia and other free-living organisms
are more frequently isolated from medicinal products and, as their name suggests,
may be pathogenic if given the opportunity. The main concern with these organisms is
that their simple nutritional requirements enable them to survive in a wide range of
pharmaceuticals, and thus they tend to be present in high numbers, perhaps in excess
of 10
6
-10
7
cfug"' or ml
-1
; nevertheless, the product itself may show no visible sign of
contamination. Opportunist pathogens can survive in disinfectants and antiseptic
solutions which are normally used in the control of hospital cross-infection but which
when contaminated may even perpetuate the spread of infection. Compromised hospital
patients, i.e. the elderly, burned, traumatized or immunosuppressed, are considered to
be particularly at risk from infection with these organisms, whereas healthy patients in
the general community have given little cause for concern.
The critical dose of microorganisms which will initiate an infection is largely
unknown and varies not only between species but also within a species. Animal and
human volunteer studies have indicated that the infecting dose may be reduced
significantly in the presence of trauma or foreign bodies or if accompanied by a drug
having a local vasoconstrictive action.
5.2 The route of administration
As stated previously, contaminated products injected directly into the bloodstream or
instilled into the eye cause the most serious problems. Intrathecal and epidural injections
are potentially hazardous procedures. In practice, epidural injections are frequently
given through a bacterial filter. Injectable and ophthalmic solutions are often simple
solutions and provide Gram-negative opportunist pathogens with sufficient nutrients
to multiply during storage; if contaminated, numbers in excess of 10
6
cfu and endotoxins
should be expected. Total parenteral nutrition fluids, formulated for individual patients'
nutritional requirements, can also provide more than adequate nutritional support for
invading contaminants. Pseudomonas aeruginosa, the notorious contaminant of eye-
drops, has caused serious ophthalmic infections, including the loss of sight in some
cases. The problem is compounded when the eye is damaged through the improper use
of contact lenses or scratched by fingernails or cosmetic applicators.
The fate of contaminants ingested orally in medicines may be determined by several
factors, as is seen with contaminated food. The acidity of the stomach may provide a
successful barrier, depending on whether the medicine is taken on an empty or full
stomach and also on the gastric emptying time. Contaminants in topical products may
cause little harm when deposited on intact skin. Not only does the skin itself provide an
382 Chapter 19
excellent mechanical barrier but, furthermore, few contaminants normally survive in
competition with its resident microbial flora. Skin damaged during surgery or trauma
or in patients with burns or pressure sores may, however, be rapidly colonized and
subsequently infected by opportunist pathogens. Patients treated with topical steroids
are also prone to local infections, particularly if contaminated steroid drugs are
inadvertently used.
Resistance of the patient
A patient's resistance is crucial in determining the outcome of a medicament-borne
infection. Hospital patients are more exposed and susceptible to infection than
those treated in the general community. Neonates, the elderly, diabetics and patients
traumatized by surgery or accident may have impaired defence mechanisms. People
suffering from leukaemia and those treated with immunosuppressants are most
vulnerable to infection; there is a strong case for providing all medicines in a sterile
form for these patients.
Prevention and control of contamination
Prevention is undoubtedly better than cure in minimizing the risk of medicament-borne
infections. In manufacture the principles of good manufacturing practice must be
observed, and control measures must be built in at all stages. Initial stability tests should
show that the proposed formulation can withstand an appropriate microbial challenge;
raw materials from an authorized supplier should comply with in-house microbial
specifications; environmental conditions, appropriate to the production process, require
regular microbiological monitoring; finally, end-product analysis should indicate that
the product is microbiologically suitable for its intended use and conforms to accepted
in-house and international standards.
Based on present knowledge, contaminants, by virtue of their type or number, should
not present a potential health hazard to patients when used.
Contamination during use is less easily controlled. Successful measures in the
hospital pharmacy have included the packaging of products as individual units, thereby
discouraging the use of multidose containers. Unit packaging (one dose per patient)
has clear advantages, but economic constraints prevent this desirable procedure from
being realized. Ultimately, the most fruitful approach is through the training and
education of patients and hospital staff, so that medicines are used only for their intended
purpose. The task of implementing this approach inevitably rests with the clinical and
community pharmacists of the future.
Further reading
Baird R.M. (1981) Drugs and cosmetics. In: Microbial Biodeterioration (ed. A.H. Rose), pp. 387-426.
London: Academic Press.
Baird R.M. (1985) Microbial contamination of pharmaceutical products made in a hospital pharmacy.
Pharm J, 234, 54-55.
Baird R.M. (1985) Microbial contamination of non-sterile pharmaceutical products made in hospitals
in the North East Regional Health Authority. J Clin Hosp Pharm, 10, 95-100.
Contamination of non-sterile pharmaceuticals 383
Baird R.M. & Shooter R.A. (1976) Pseudomonas aeruginosa infections associated with the use of
contaminated medicaments. Br Med J, 2, 349-350.
Baird R.M., Brown W.R.L. & Shooter R.A. (1976) Pseudomonas aeruginosa in hospital pharmacies.
Br Med J, 1,511-512.
Baird R.M., Elhag K.M. & Shaw E.J. (1976) Pseudomonas thomasii in a hospital distilled water supply.
J Med Microbiol, 9, 493-495.
Baird R.M., Parks A. & Awad Z.A. (1977) Control of Pseudomonas aeruginosa in pharmacy
environments and medicaments. Pharm J, 119, 164-165.
Baird R.M., Crowden C.A., O'Farrell S.M. & Shooter R.A. (1979) Microbial contamination of
pharmaceutical products in the home. J Hyg, 83, 277-283.
Baird R.M. & Bloomfield S.F.L. (eds) (1996) Microbial Quality Assurance of Cosmetics, Toiletries
and Non-sterile Pharmaceuticals. London: Taylor and Francis.
Bassett D.C.J. (1971) Causes and prevention of sepsis due to Gram-negative bacteria: common sources
of outbreaks. Proc R Soc Med, 64, 980-986.
Crompton D.O. (1962) Ophthalmic prescribing. Australas J Pharm, 43, 1020-1028.
Denyer S.P. & Baird R.M. (eds) (1990) Guide to Microbiological Control in Pharmaceuticals. Chichester:
Ellis Horwood.
EC Guide to Good Manufacturing Practice (1992).
Hills S. (1946) The isolation of CI. tetani from infected talc. NZMedJ, 45, 419-423.
Kallings L.O., Ringertz O., Silverstolpe L. & Ernerfeldt F. (1966) Microbiological contamination of
medicinal preparations. 1965 Report to the Swedish National Board of Health. Acta Pharm Suecica,
3, 219-228.
Maurer I.M. (1985) Hospital Hygiene, 3rd edn. London: Edward Arnold.
Meers P.D., Calder M.W., Mazhar M.M. & Lawrie G.M. (1973) Intravenous infusion of contaminated
dextrose solution: the Devonport incident. Lancet, ii, 1189-1192.
Morse L.J., Williams H.I., Grenn F.P., Eldridge E.F. & Rotta J.R. (1967) Septicaemia due to Klebsiella
pneumoniae originating from a handcream dispenser. N Engl J Med, 277, 472-473.
Myers G.E. & Pasutto F.M. (1973) Microbial contamination of cosmetics and toiletries. Can J Pharm
Sci, 8, 19-23.
Noble W.C. & Savin J. A. (1966) Steroid cream contaminated with Pseudomonas aeruginosa. Lancet,
i, 347-349.
Parker M.T. (1972) The clinical significance of the presence of microorganisms in pharmaceutical and
cosmetic preparations. J Soc Cosm Chem, 23, 415-426.
Report of the Public Health Laboratory Service Working Party (1971) Microbial contamination of
medicines administered to hospital patients. Pharm J, 207, 96-99.
Russell A.D., Hugo W.G. & Ayliffe G.A.J, (eds) (1998) Principles and Practice of Disinfection,
Preservation and Sterilization, 3rd edn. Oxford: Blackwell Science.
Smart R. & Spooner D.F. (1972) Microbiological spoilage in pharmaceuticals and cosmetics. / Soc
Cosm Chem, 23, 721-737.
Principles and practice of sterilization
1 Introduction
2 Sensitivity of microorganisms
2.1 Survivor curves
2.2 Expressions of resistance
2.2.1 D-value
2.2.2 z-value
2.3 Sterility assurance
3 Sterilization methods
4 Heat sterilization
4.1 Sterilization process
4.2 Moist heat sterilization
4.2.1 Steam as a sterilizing agent
4.2.2 Sterilizer design and operation
4.3 Dry heat sterilization
4.3.1 Sterilizer design
4.3.2 Sterilizer operation
5 Gaseous sterilization
5.1 Ethylene oxide
Introduction
Sterilization is an essential stage in the processing of any product destined for parenteral
administration, or for contact with broken skin, mucosal surfaces or internal organs,
where the threat of infection exists. In addition, the sterilization of microbiological
materials, soiled dressings and other contaminated items is necessary to minimize the
health hazard associated with these articles.
Sterilization processes involve the application of a biocidal agent or physical
microbial removal process to a product or preparation with the object of killing or
removing all microorganisms. These processes may involve elevated temperature,
reactive gas, irradiation or filtration through a microorganism-proof filter. The success
of the process depends upon a suitable choice of treatment conditions, e.g. temperature
and duration of exposure. It must be remembered, however, that with all articles to be
sterilized there is a potential risk of product damage, which for a pharmaceutical
preparation may result in reduced therapeutic efficacy or patient acceptability. Thus,
there is a need to achieve a balance between the maximum acceptable risk of failing to
achieve sterility and the maximum level of product damage which is acceptable. This
is best determined from a knowledge of the properties of the sterilizing agent, the
properties of the product to be sterilized and the nature of the likely contaminants. A
suitable sterilization process may then be selected to ensure maximum microbial kill/
removal with minimum product deterioration.
Principles and practice of sterilization 385
5.1.1 Sterilizer design and operation
5.2 Formaldehyde
5.2.1 Sterilizer design and operation
6 Radiation sterilization
6.1 Sterilizer design and operation
6.1.1 Gamma-ray sterilizers
6.1.2 Electron accelerators
6.1.3 Ultraviolet irradiation
7 Filtration sterilization
7.1 Filtration sterilization of liquids
7.2 Filtration sterilization of gases
8 Conclusions
9 Acknowledgements
10 Appendix
11 Further reading
20
1 Sensitivity of microorganisms
The general pattern of resistance of microorganisms to biocidal sterilization processes
is independent of the type of agent employed (heat, radiation or gas), with vegetative
forms of bacteria and fungi, along with the larger viruses, showing a greater sensitivity
to sterilization processes than small viruses and bacterial or fungal spores. The choice
of suitable reference organisms for testing the efficiency of sterilization processes (see
Chapter 23) is therefore made from the most durable bacterial spores, usually represented
by Bacillus stearothermophilus for moist heat, certain strains of B. subtilis for dry heat
and gaseous sterilization, and B. pumilus for ionizing radiation.
Ideally, when considering the level of treatment necessary to achieve sterility a
knowledge of the type and total number of microorganisms present in a product, together
with their likely response to the proposed treatment, is necessary. Without this
information, however, it is usually assumed that organisms within the load are no more
resistant than the reference spores or than specific resistant product isolates. In the
latter case, it must be remembered that resistance may be altered or lost entirely by
laboratory subculture and the resistance characteristics of the maintained strain must
be regularly checked.
A sterilization process may thus be developed without a full microbiological
background to the product, instead being based on the ability to deal with a 'worst
case' condition. This is indeed the situation for official sterilization methods which
must be capable of general application, and modern pharmacopoeial recommendations
are derived from a careful analysis of experimental data on bacterial spore survival
following treatments with heat, ionizing radiation or gas.
However, the infectious agents responsible for spongiform encephalopathies such
as bovine spongiform encehalopathy (BSE) and Creutzfeldt-Jacob disease (CJD) exhibit
exceptional degrees of resistance to all known lethal agents. Recent work has even cast
doubts on the adequacy of the process of 18min exposure to steam at 134-138°C
which has been officially recommended for the destruction of these agents (and which
far exceeds the lethal treatment required to achieve adequate destruction of bacterial
spores).
2.1 Survivor curves
When exposed to a killing process, populations of microorganisms generally lose their
viability in an exponential fashion, independent of the initial number of organisms.
This can be represented graphically with a 'survivor curve' drawn from a plot of the
logarithm of the fraction of survivors against the exposure time or dose (Fig. 20.1). Of
the typical curves obtained, all have a linear portion which may be continuous (plot A),
or may be modified by an initial shoulder (B) or by a reduced rate of kill at low survivor
levels (C). Furthermore, a short activation phase, representing an initial increase in
viable count, may be seen during the heat treatment of certain bacterial spores. Survivor
curves have been employed principally in the examination of heat sterilization methods,
but can equally well be applied to any biocidal process.
386 Chapter 20
