Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

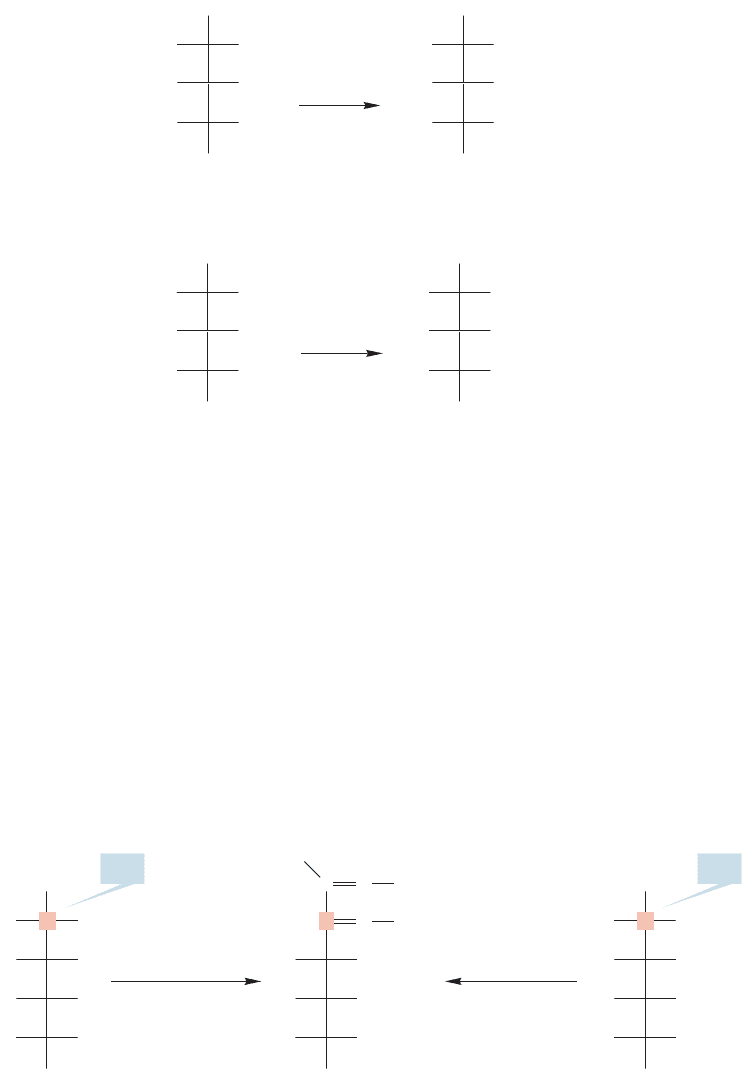
22.5 The Fischer Determination of the Structure of D-Glucose 1159
Now we know all four stereoisomers of the D-aldopentoses, and we can construct
the
L series by drawing the mirror images of the D series. Doing so gives us all eight
aldopentoses.
Let’s complete the determination of the structures of the D-aldohexoses. D-Gulose
and
D-idose give the same osazone and must differ only in their configurations at
C(2) (Fig. 22.56).
There are only two remaining
D-aldopentoses. One of them, D-xylose, gives a
meso diacid on oxidation,whereas the other,
D-lyxose,gives an optically active diacid.
The structures must be as shown in Figure 22.55.
CH
2
OH
HNO
3
HNO
3
OHH
OHH
H
CHO
HO
COOH
OHH
H
COOH
HO
meso Diacid
D-Xylose
Optically active
diacid
D-Lyxose
OHH
CH
2
OH
OHH
HHO
H
CHO
HO
COOH
HHO
H
COOH
HO
OHH
FIGURE 22.55 D-Xylose gives a meso diacid and
D-lyxose gives an optically active diacid, which fixes
the structures of the remaining
D-aldopentoses.
CH
2
OH
OHH
OHH
OH
CHO
C
H
D-Gulose
H
HO
CH
2
OH
OHH
HHO
OH
CHO
H
D-Idose
H
HO
CH
2
OH
OHH
OH
C
C
H
H
Same osazone
H
HO
3 equiv.
PhNHNH
2
3 equiv.
PhNHNH
2
N NHPh
N NHPh
C
C(2) C(2)
FIGURE 22.56 D-Gulose and D-idose give the same osazone, which determines the structure of
D-idose and its mirror image L-idose.
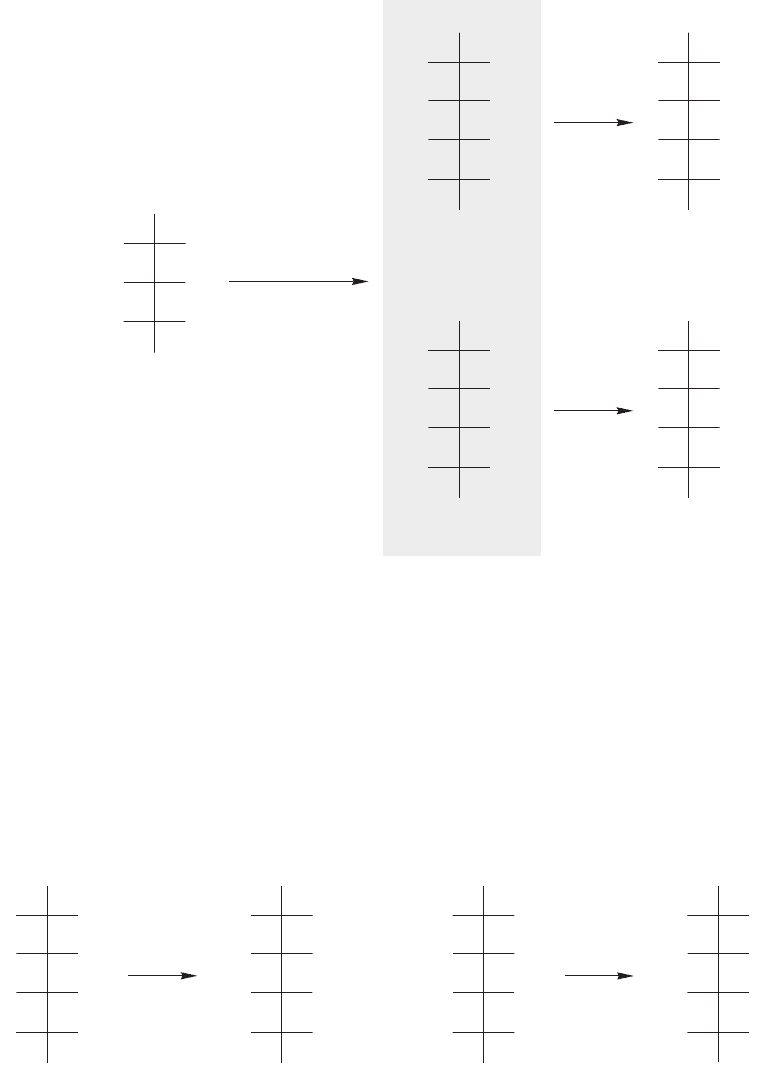
1160 CHAPTER 22 Carbohydrates
CH
2
OH
OHH
OHH
OH
CHO
H
D-Allose
OH
H
HNO
3
CH
2
OH
OHH
HHO
OH
CHO
H
D-Altrose
OH
H
OHH
OHH
OH
COOH
COOH
H
A meso diacid
OH
H
OHH
HHO
OH
COOH
COOH
H
An optically
active diacid
OH
H
CH
2
OH
OHH
OH
CHO
H
D-Ribose
OH
H
HNO
3
+
Kiliani–Fischer
synthesis
FIGURE 22.57 The Kiliani–Fischer synthesis used to determine the structures of
D-allose, D-altrose, and their mirror-image L isomers.
CH
2
OH
OHH
OHH
H
CHO
HO
D-Galactose
H
HO
HNO
3
CH
2
OH
OHH
HHO
H
CHO
HO
D-Talose
H
HO
COOH
OHH
OHH
H
COOH
HO
A meso diacid
H
HO
OHH
HHO
H
COOH
COOH
HO
An optically
active diacid
H
HO
HNO
3
FIGURE 22.58 D-Galactose gives a meso diacid, and D-talose gives an optically active diacid.
The Kiliani–Fischer synthesis applied to D-ribose gives D-allose and
D-altrose, which must have the two structures shown in Figure 22.57. The two
structures can be assigned by noting that
D-allose gives an optically inactive,
meso diacid on treatment with nitric acid, whereas
D-altrose gives an optically
active diacid.
Of the two remaining
D-aldohexoses, D-galactose gives a meso diacid on
oxidation with nitric acid, but
D-talose gives an optically active diacid (Fig. 22.58).
Now we know the structures of all the aldohexoses.The structures of the eight
L isomers are simply the mirror images of the D series, and we have at last com-
pleted our task.
22.6 Special Topic: An Introduction to Di- and Polysaccharides 1161
22.6 Special Topic: An Introduction to Di- and
Polysaccharides
A great many sugars containing 12,18, and other multiples of 6 carbons are known.
Sugars that have the formula C
n
H
2n
O
n
are called monosaccharides because they
are composed of a single sugar molecule. Sugars that come from the combination
of two sugar units, the most common being C
12
carbohydrates, are called disaccha-
rides and they have the formula of C
n
H
2n2
O
n1
because the two sugar units are
always joined by a dehydration process. Some of the disaccharides have common
names. For example, sucrose, lactose, and maltose are all disaccharides. Starch and
cellulose are polymers of simple repeating monosaccharide units, less many mol-
ecules of water. We call such biopolymers polysaccharides.
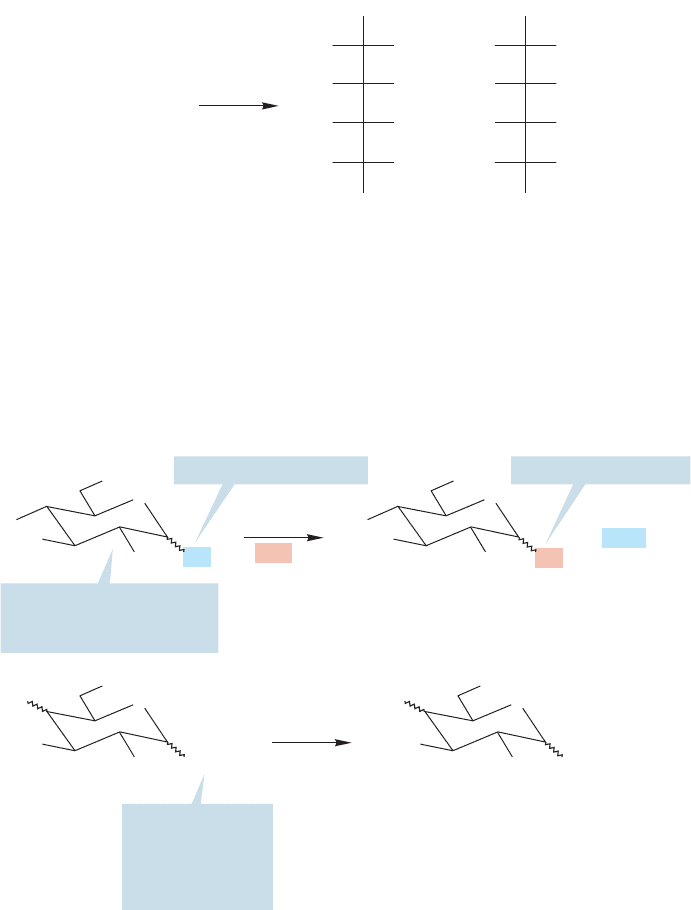
We will use lactose as an example of how the structure of a disaccharide can be
unraveled.The first important observation is that ()-lactose, a disaccharide having
the formula C
12
H
22
O
11
, can be hydrolyzed in acid to a pair of simple aldohexoses:
D-glucose and D-galactose (Fig. 22.59). This observation identifies the two mono-
saccharides making up ()-lactose.
Only this CH
3
O group...
Dilute acid does not affect
the other simple ethers
such as this one
The two sugars
must be attached
here, at the
glycosidic link
to C(1)
H
2
O
cat.
HCl
+
...is hydrolyzed to HO
RO
RO
OR
O
OR
OR
RO
RO
OR
O
OH
ROH
OR
H
2
O
cat.
HCl
+
HO
HO
OH
O
OSugar
OH
HO
HO
OH
O
OH
OH
HOSugar
FIGURE 22.60 The fact that
()-lactose is hydrolyzed in dilute
acid to give two monosaccharides
means the monosaccharides must be
joined via a glycosidic linkage.
CH
2
OH
H
2
O
(+)-Lactose
C
12
H
22
O
11
OHH
OHH
H
CHO
HO
D-Galactose
H
HO
H
3
O
+
CH
2
OH
OHH
OHH
H
CHO
HO
D-Glucose
OH
H
+
FIGURE 22.59 In acid, ()-lactose
is hydrolyzed to
D-glucose and
D-galactose.These sugars are C(4)
epimers.
But this simple experiment gives us another piece of less obvious information.
Remember that only glycosidic linkages, the acetals,are cleaved in acid—simple ether
connections are not touched. We saw this distinction first in the hydrolysis of only
the glycosidic methoxy group of fully methylated
D-glucose (p. 1150). D-Glucose
and
D-galactose must therefore be attached via the C(1) hydroxyl group of one of
the sugars (Fig. 22.60). If the attachment were at any other position, dilute acid
would not break up the disaccharide.
1162 CHAPTER 22 Carbohydrates
We don’t (yet) know which
O is used to make this bond
Hemiacetal
link
H
2
O
cat.
HCl
+
Br
H
2
O
(+)-Lactose in open form
HO
HO
OH
OH
O
OH
HO
OH
OH
O
OH
O
O
O
O
O
HO
HO
OH
O
O
OH
O
O
O
O
O
OH
HO
HO
OH
COOH
O
OH
O
O
O
OH
D-Gluconic acid
Lactobionic acid
D-Galactopyranose
CH
2
OH
OHH
OHH
OHH
H
COOH
HO
HO
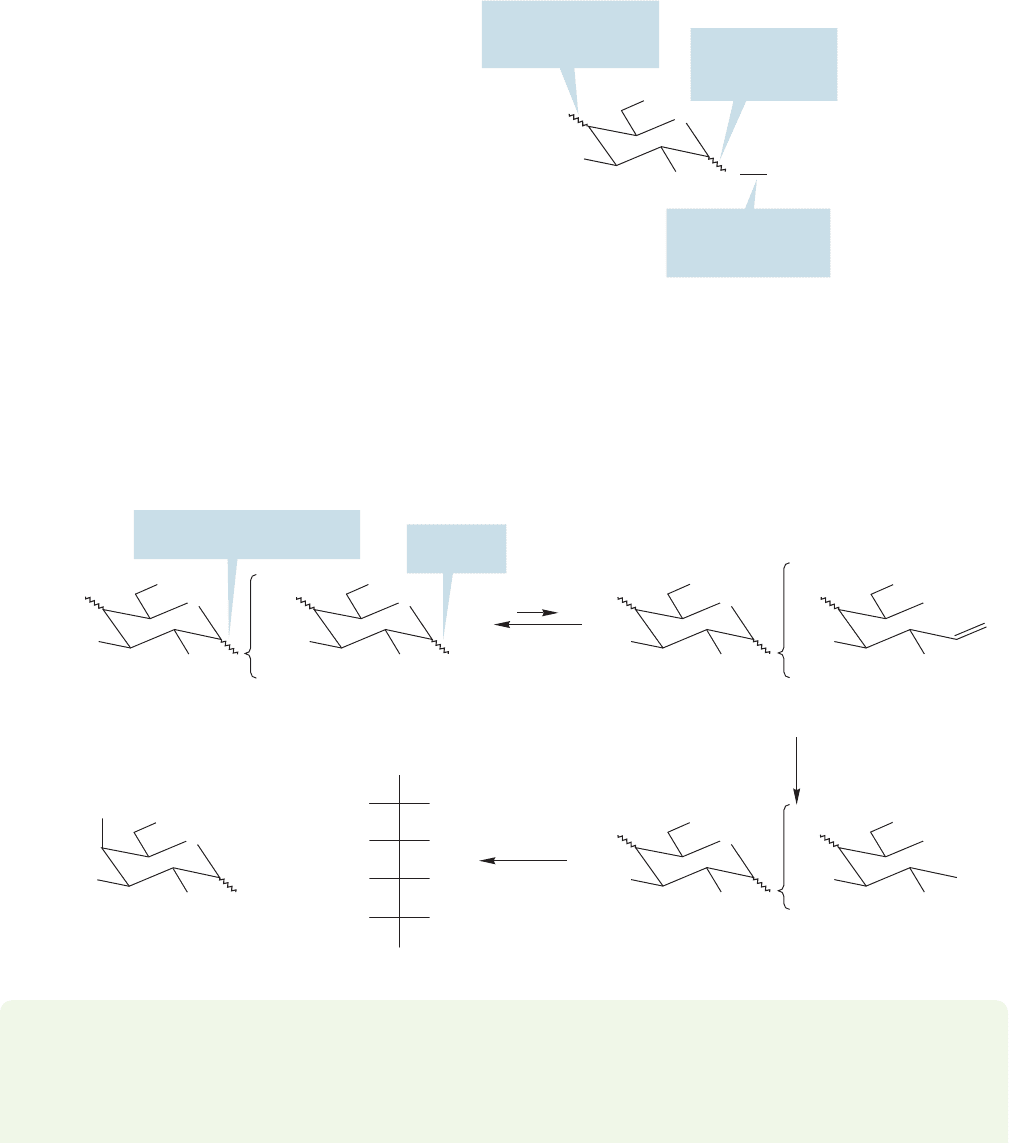
FIGURE 22.62 The carboxylic acid
group in lactobionic acid marks the
position of the aldehyde/hemiacetal
carbon in ()-lactose. The fact that
hydrolysis of lactobionic acid gives a
gluconic acid and not a galactonic
acid tells us that it is glucose that has
the aldehyde/hemiacetal group in
()-lactose.
FIGURE 22.61 The issues that must
be addressed to determine the
structure of ()-lactose.
We now need to know three details about the dimeric structure of lactose
(Fig. 22.61). The issues are:
1. Which sugar is the “attacher” (the ROH) and which is the “attachee” (receives
ROH at its anomeric carbon)?
2. Which hydroxyl group of the attacher is used to make the linkage to the anomeric
carbon of the attachee?
3. Are the two sugars linked with stereochemistry that is α or β?
1. Is this glucose
(equatorial OH) or
galactose (axial OH)?
2. To which
carbon is this bond
attached?
3. Is this linkage
α or β (axial or
equatorial)?
HO
HO
OH
O
OSugar
OH
WORKED PROBLEM 22.22 If the two monosaccharides in ()-lactose were
attached with C(1) of glucose as the acetal linkage and the galactose having the
free aldehyde/hemiacetal, what would the products of the experiment shown in
Figure 22.62 be?
()-Lactose can be oxidized by bromine in water to the improbably named
lactobionic acid (Fig. 22.62). Bromine in water oxidizes only the aldehydic or hemiac-
etal carbon of a sugar to give the carboxylic acid (p. 1144). In one of the partners in the
disaccharide there is no hemiacetal, and therefore no free aldehyde to be oxidized by
bromine/water. Acid hydrolysis of the lactobionic acid gives
D-galactopyranose and
D-gluconic acid.This result tells us that the aldehyde/hemiacetal group in ()-lactose
must belong to glucose,because it was the monosaccharide that was oxidized.Therefore
the galactose must be attached to glucose via an acetal linkage at the galactose C(1).
(continued)
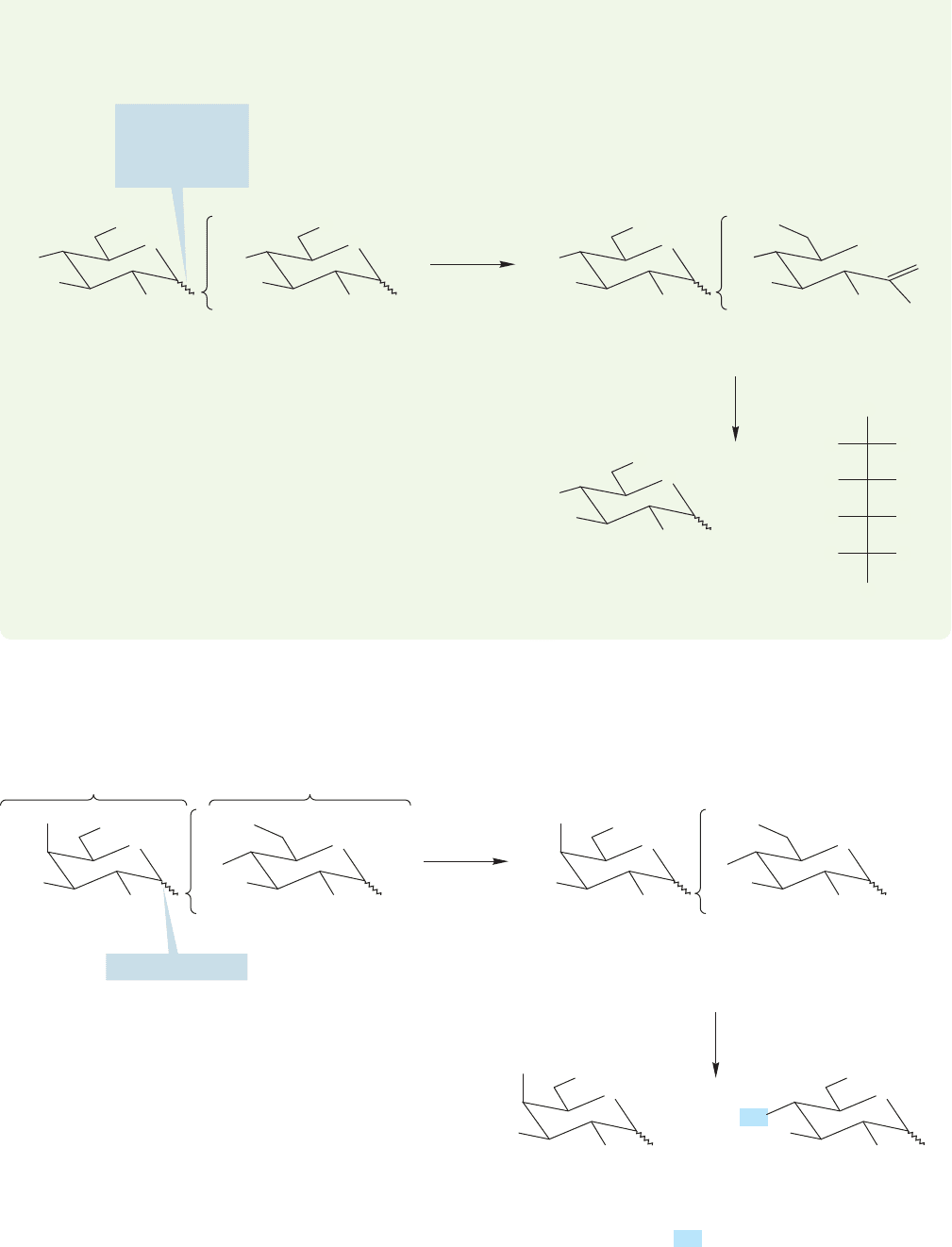
22.6 Special Topic: An Introduction to Di- and Polysaccharides 1163
We do not yet
know which O
group is used to
make this bond
HO
HO
OH
OH
O
OH
O
O
O
O
O
cat.
HCl
H
2
O
Hypothetical “reverse” lactobionic acid
Hypothetical “reverse” lactose
HO
OH
O
O
OH
O
O
O
OH
OH
H
2
O
Br
2
D-Glucose part D-Galactose part
D-Galactonic acid
CH
2
OH
OHH
HHO
OHH
H
COOH
HO
+
HO
OH
O
OH
D-Glucopyranose
O
OH
HO
HO
Our next task is to determine which carbon of glucose is attached to C(1) of galac-
tose.To address this issue, we can use a Williamson ether synthesis to methylate all free
hydroxyl groups in ()-lactose (Fig.22.63). After methylation, the lactose is hydrolyzed
in acid to 2,3,4,6-tetramethyl
D-galactopyranose and 2,3,6-trimethyl D-glucopyranose.
ANSWER If the monosaccharides were attached this way, the galactose aldehyde
would be oxidized to , and hydrolysis of this hypothetical lactobionic acid
would give
D-galactonic acid and D-glucopyranose.
COOH
cat.
HCl
H
2
O
Three ?’s are methyl groups, but
one oxygen is used to make
the attachment to glucose
HO
OH
OH
O
OH
O
O
O
OCH
3
CH
3
O
Ag
2
O
excess
CH
3
I
OCH
3
The C(4) OH group was used
to attach the two sugars
O
OCH
3
?O
O?
O
2,3,4,6-Tetramethyl
D-galactopyranose
HO CH
3
O
C(1) of galactose
One of the oxygens in
glucose is used to make the
bond to C(1) of galactose
Galactose Glucose
O ?O
O
?O
CH
3
O
OCH
3
2,3,6-Trimethyl
D-glucopyranose
O
OCH
3
CH
3
O
CH
3
O
OCH
3
O
OCH
3
HO+
OH
OH
FIGURE 22.63 The position of the OH used to attach
glucose to C(1) of galactose can be determined
through a methylation followed by hydrolysis.

1164 CHAPTER 22 Carbohydrates
SUGAR SUBSTITUTES
The four major sugar substitutes vary widely in structure,
but all four are united by a single thread: Saccharin, cycla-
mate, aspartame, and sucralose were found not by a directed
search but by accident. Indeed, each of these four was dis-
covered when a chemist tasted a compound made for other
purposes. In fact, cyclamates were discovered because a ciga-
rette smoked in the lab tasted sweet. This observation may
seem like courting disaster to you—deliberately ingesting an
unknown laboratory chemical, not to mention smoking in a
chemistry lab—but it was quite common in the old days.
Even as recently as the 1960s it was normal to smell—
closely and carefully—just about everything one made. How
many chemists destroyed their sense of smell or succumbed
to the odor because of this practice, we will never know.
Now the laboratory is better ventilated and we gingerly waft
the fumes from a flask if we must test for an odor.
After 100 years of research and debate concerning sac-
charin (discovered in 1879), the consensus is that no ill
health effects are associated with moderate consumption.
Although cyclamate (discovered in 1937) is banned in the
United States, there is no evidence implicating it as a health
hazard according to the Food and Drug Administration
(FDA). Aspartame (discovered in 1965) may be the single
most studied food additive. It has been cleared for use,
except for phenylketonurics. People with this disease must
avoid phenylalanine, which is formed from the hydrolysis of
aspartame. Aspartame also releases methanol when heated,
but only extremely small amounts are produced. Sucralose
(discovered in 1976) has cleared all toxicology tests.
Although halogenated alkanes are known carcinogens, the
chlorinated sucralose is not lipophilic enough to be retained
in the body.
6
Lactose is found in milk and other dairy products, so it is often called milk sugar. Disaccharides such as lactose
need to be cleaved to monosaccharides in order to be absorbed into the bloodstream.The acetal bond in lactose is
cleaved by the enzyme lactase. Many adults are lactose intolerant because their bodies don’t make lactase.For such
individuals, consumed lactose doesn’t get absorbed into the bloodstream and the result is abdominal discomfort.
This hydrolysis liberates only the OH groups that in ()-lactose were tied up in
acetals; it leaves simple ether groups unchanged.It is the OH at C(4) of glucose that
is unmethylated, telling us that this is the glucose position con-
nected to galactose in the lactose molecule.
To determine whether the ()-lactose glycoside linkage is made
as the α or β anomer we turn to enzymes such as lactase
6
that have
evolved to cleave only β-glycosidic bonds,and to which α-linked dis-
accharides are inert. ()-Lactose is cleaved by lactase and therefore
must be β-linked.
1
H NMR spectroscopy can also be used to iden-
tify whether a disaccharide is linked as the α or β anomer.The hydro-
gen on the anomeric carbon is more deshielded than any of the other
hydrogens,and the C(1) equatorial hydrogen (δ 5.2 ppm) is further
downfield than the C(1) axial hydrogen (δ 4.6 ppm). Figure 22.64
shows the final three-dimensional representation of ()-lactose.
'
'
(+)-Lactose β linkage
HO
OH
O
O
OH
HO
HO
OH
O
OH
OH
WEB 3D
4
6
5
3
1
2
4
6
5
3
1
2
D-Galactose D-Glucose
FIGURE 22.64 The structure of the disaccharide
()-lactose.The monosaccharides
D-galactose and
D-glucose are joined by a β-glycosidic linkage between
C(1) of galactose and C(4) of glucose.
Cyclamate Aspartame
O
H
N
S
O
O
O
Saccharin Sucralose
O
O
NH
S
O
O
N
H
HOOC
NH
2
–
CH
2
OH
CH
2
Cl
CH
2
Cl
OCH
3
Ph
HO
HO
O
OH
OH
O
Cl
O
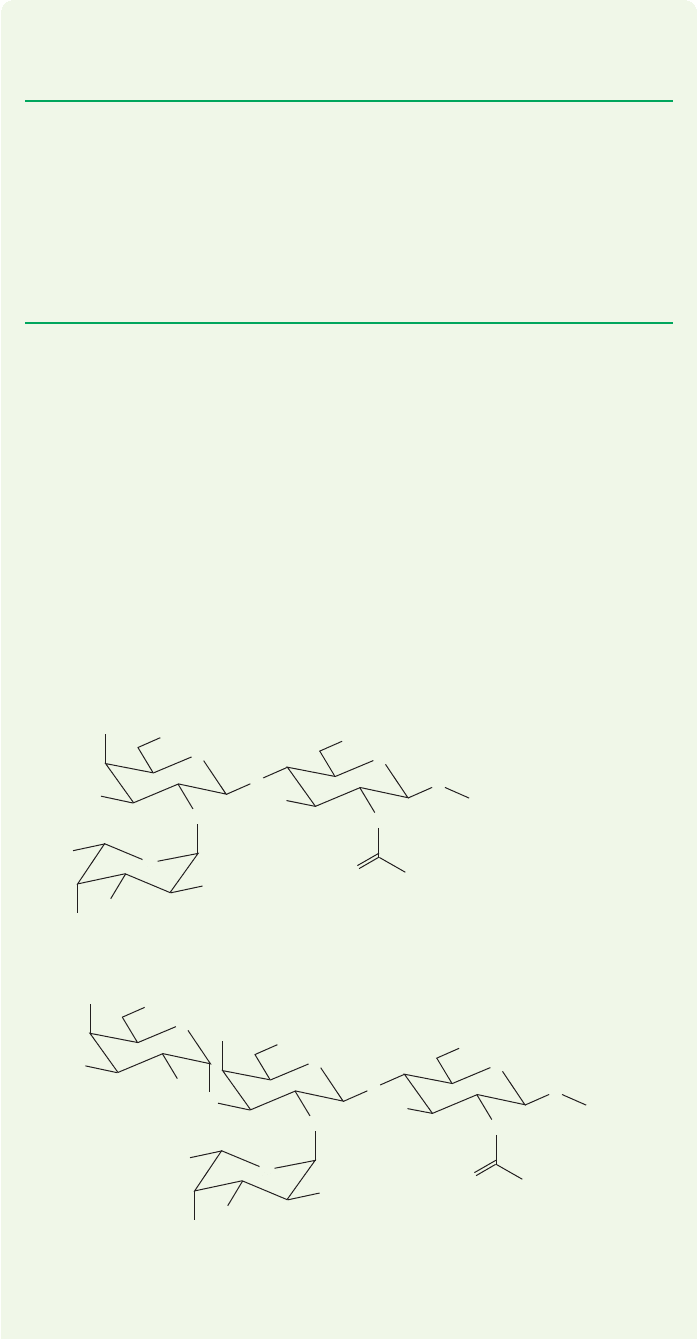
22.6 Special Topic: An Introduction to Di- and Polysaccharides 1165
PROBLEM 22.23 Draw a Fischer projection for ()-lactose. Hint: You will need
several long, “around the corner” bonds as illustrated in Figure 22.16.
PROBLEM 22.24 Cellobiose is a disaccharide cleaved by hydrolysis into two mol-
ecules of D-glucose. Methylation of cellobiose leads to an octamethyl derivative.
Hydrolysis with acid converts the octamethyl derivative into 2,3,6-trimethyl-
D-glucopyranose and 2,3,4,6-tetramethyl-D-glucopyranose. Cellobiose can be
cleaved enzymatically with lactase. There are actually two possible structures for
cellobiose. One has
1
H NMR signals at δ 4.5 and δ 4.7 ppm. The other isomer
has signals at δ 4.5 and δ 5.2 ppm. Draw the two structures of cellobiose.
PROBLEM 22.25 Red blood cells have glycoproteins (carbohydrates covalently
attached to a protein, Chapter 23) on the cell surface that play an important role
in a person’s immune system. There are three versions of the glycoproteins found
in humans. These variations lead to what are called blood types. You might have
blood type A, B, AB, or O.The differences are simply a matter of the number and
kinds of carbohydrates in the glycoprotein of your red blood cells. Other animals
have different glycoproteins associated with their red blood cells. Horses, for
example, have eight blood types.The carbohydrates involved in the human blood
types are shown. Type O is a trisaccharide composed of N-acetyl-
D-glucosamine,
D-galactose, and L-fucose. (Fucose is the common name for 6-deoxygalactose.)
Type B is a tetrasaccharide made up of the three monosaccharides of type O plus
a
D-galactose.Type A is also a tetrasaccharide that has the basic structure of type
O plus an N-acetyl-D-galactosamine.
HO
O
O
O
Type O
HO
HO
OH
NH
O
O
Protein
O
OH
HO
HO
OH
O
O
O
O
O
Type B
OH
HO
OH
HO
HO
O
HO
OH
NH
O
O
Protein
O
OH
HO
OH
O
HO
(continued)
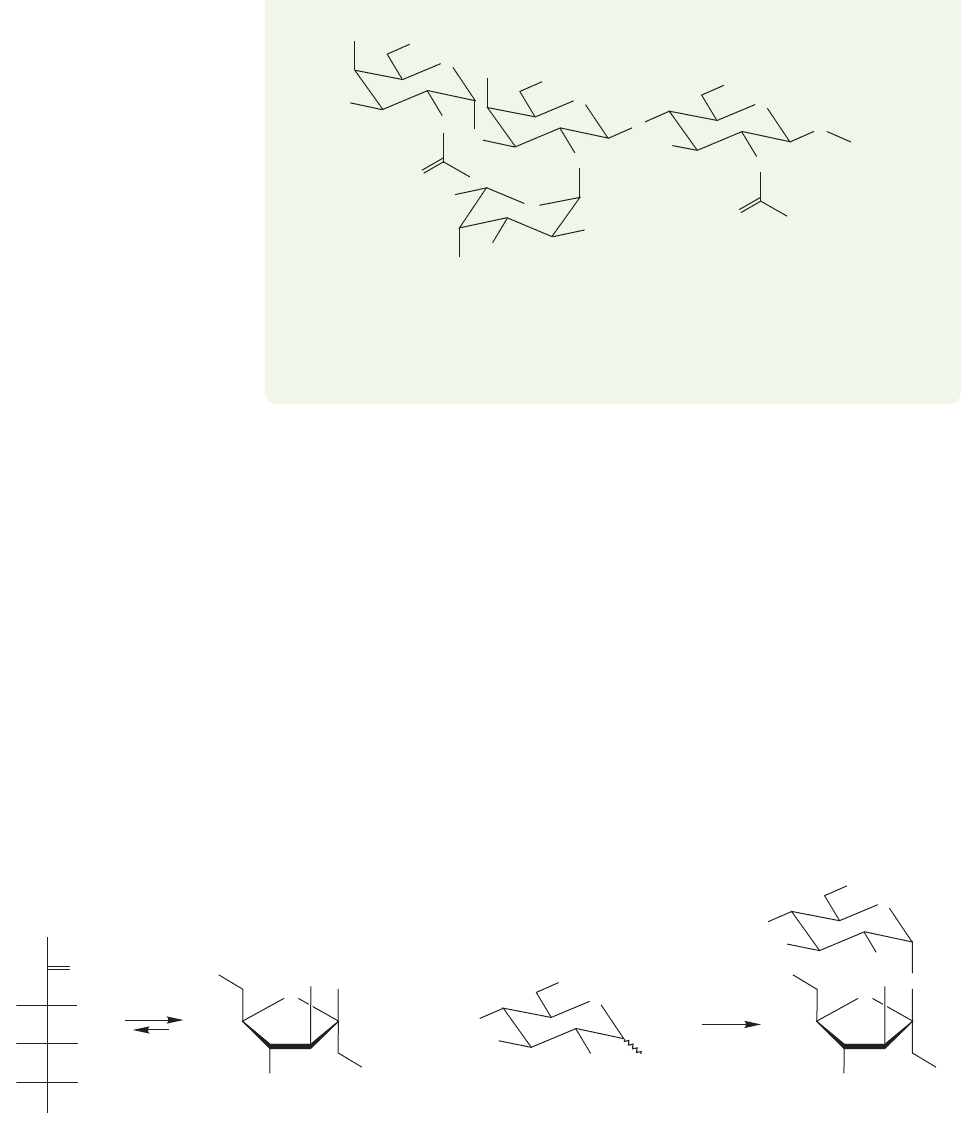
1166 CHAPTER 22 Carbohydrates
O
O
O
O
Type A
OH
HO
OH
HO
HN
HO
O
HO
OH
NH
O
O
Protein
O
OH
HO
OH
O
O
(a) Draw the Fischer projection of L-fucose. (b) Label the anomeric carbons as α
or β. (c) Are these saccharides reducing sugars? Why or why not?
α
β
CH
2
OH
CH
2
OH
D-Fructose
H
+
OH
OHH
H
HO
O
D-Glucopyranose (+)-Sucrose-D-Fructofuranose
HO
HO
OH
O
OH
OH
4
6
5
3
1
2
HO
HO
OH
O
OH
O
OH
OH
OH
OH
HO
6
1
2
3
5
4
O
OH
O
OH
OH
HO
1
2
3
4
5
6
FIGURE 22.65 In nonreducing sugars, there is no free aldehyde group. In the case of sucrose, attachment between the sugars is
between the anomeric carbons.This kind of attachment makes sucrose, which is table sugar, a nonreducing sugar.
Synthesis of disaccharides is possible using the protecting group methods dis-
cussed earlier (Fig. 22.43). We can use the free alcohol of one monosaccharide to
link with the hemiacetal of a protected second monosaccharide. The acetate-
In addition to lactase,the enzyme that cleaves the glycosidic bond in lactose, there
are many other glycosidase enzymes in Nature.They play a critical role in a variety
of processes related to our diet and our environment. For example, glycosidases are
necessary for the degradation of the polysaccharide biomass found in plants. The
chemistry a glycosidase facilitates is hydrolysis of the acetal linkage connecting two
sugars. The result is a disconnection at the anomeric carbon.
Sometimes two sugars are linked via an oxygen connecting the two anomeric car-
bons. Because these disaccharides are acetals on both sugar units, oxidation to an
aldonic acid (Fig.22.28) is not possible because there is no free aldehyde. Remember:
In aqueous conditions the cyclic acetals are not in equilibrium with the open form.
In contrast to reducing sugars,in which at least a small amount of an oxidizable free
aldehyde is present in solution, sugars containing no free aldehyde groups are nonre-
ducing sugars. An example is sucrose (common table sugar) shown in Figure 22.65,
in which the anomeric carbon C(1) of
D-glucose is linked via O to the anomeric
carbon C(2) of
D-fructose.
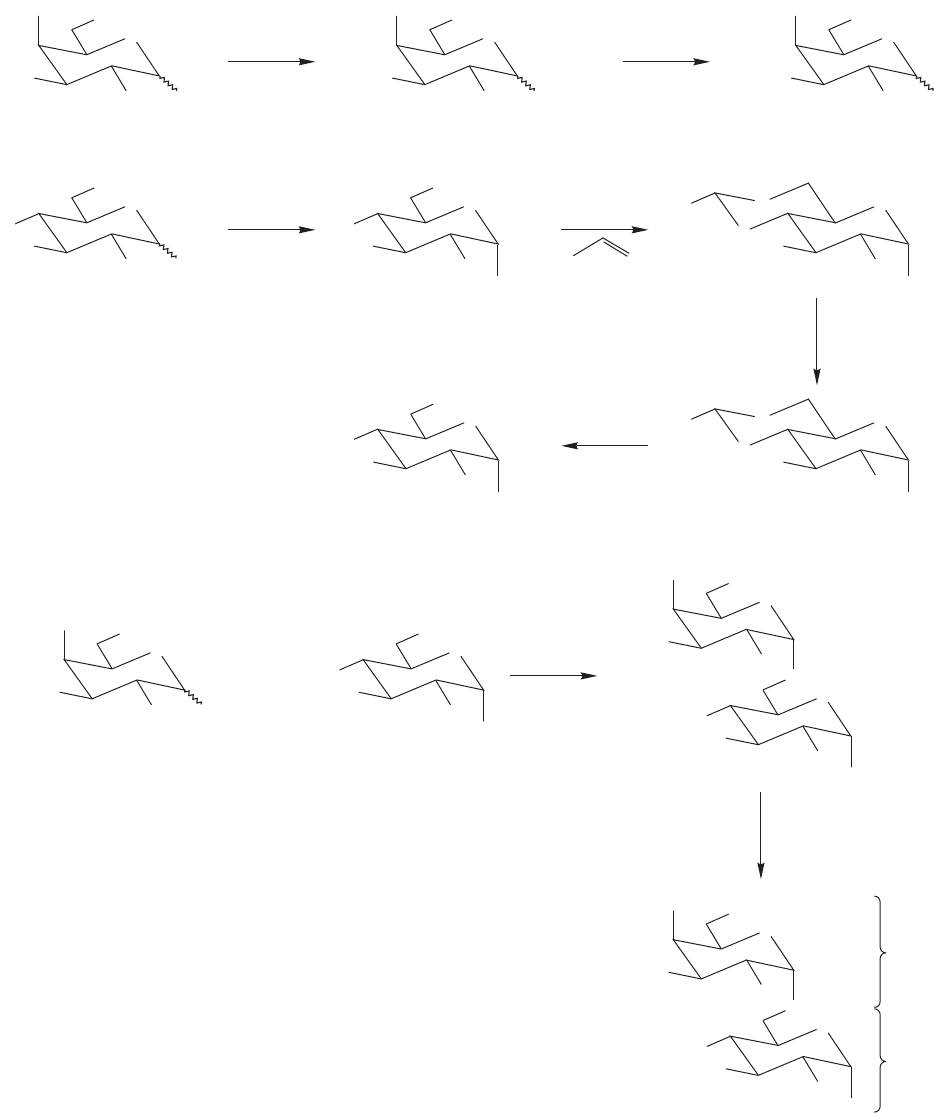
22.6 Special Topic: An Introduction to Di- and Polysaccharides 1167
protected sugar discussed in Problem 22.20 can be used to make disaccharides.
Benzyl protecting groups can also be used. An advantage to employing the benzyl
protecting group ( ) is that it can be removed from an oxygen (or nitrogen)
under the fairly mild conditions of hydrogenation (H
2
/Pd). Figure 22.66 uses this
protecting group method to make melibiose, a natural isomer of lactose. Melibiose
is a galactose–glucose disaccharide that uses the C(6) OH of D-glucose to attach to
C(1) of
D-galactose.
PhCH
2
cat.
HCl
PhCH
2
OH
cat.
HCl
D-Glucopyranose
HO
HO
HO
O
OH
4
6
5
3
1
2
HO
HO
HO
O
OH
OH
OCH
2
Ph
HO
HO
O
OCH
2
Ph
Ph
O
O
LiAlH
4
O
OCH
2
Ph
Ph
O
O
Ph O
excess
NaH
PhCH
2
Br
excess
NaH
PhCH
2
Br
Pd
Galactose
Glucose
H
2
H
2
O
cat.
HCl
D-Galactopyranose
HO
HO
O
OH
OH
4
6
5
3
1
2
4
6
5
3
1
2
4
6
5
3
1
2
OH
cat.
HCl
Benzylated
galactopyranose
O
OH
+
O
OCH
2
Ph
OCH
2
Ph
OCH
2
Ph
PhCH
2
O
PhCH
2
O
PhCH
2
O
O
OH
OCH
2
Ph
PhCH
2
O
PhCH
2
O
PhCH
2
O
PhCH
2
O
PhCH
2
O
O
OCH
2
Ph
OCH
2
Ph
PhCH
2
O
PhCH
2
O
PhCH
2
O
PhCH
2
O
PhCH
2
O
PhCH
2
O
O
OCH
2
Ph
O
PhCH
2
O
PhCH
2
O
Benzylated
glucopyranoside
Benzylated
glucopyranoside
Benzylated
glucopyranose
Melibiose
O
OH
PhCH
2
O
PhCH
2
O
PhCH
2
O
OCH
2
Ph
O
PhCH
2
O
PhCH
2
O
PhCH
2
O
PhCH
2
O
O
OH
O
HO
HO
OH
O
HO
HO
HO
HO
OH
FIGURE 22.66 Use of the benzyl
protecting group to make the
disaccharide melibiose.
1168 CHAPTER 22 Carbohydrates
22.7 Summary
New Concepts
O
(b) Starch amylose, which is poly-
D-glucose linked α
α
α
α
HO
OH
OH
OH
OH
OH
O
OH
OH
OH
OH
OH
OH
O
HO
O
O
HO
O
O
HO
O
O
HO
(a) Cellulose is poly-
D-glucose linked β
OH
O
O
HO
O
O
O
β
β
β
C(1)
C(4)
FIGURE 22.67 The two most
common natural polysaccharides:
(a) cellulose and (b) amylose.
right in the Fischer projection, the molecule is a
D sugar; if it is
on the left, the molecule is an
L sugar (Fig. 22.3).
When Emil Fischer worked out the structure of glucose in
the late 1800s, there was no way to tell absolute configuration.
Fischer could not tell the
D series from the enantiomeric L
series, so he assumed that the OH at C(5), the configurational
carbon, was on the right and worked out the relative configura-
tions of the other groups. As it turned out, he guessed correctly.
A number of times in this chapter we encounter reactions
in which a minor partner in an equilibrium reacts to give the
product. As long as equilibrium is reestablished, removal of the
minor partner can drive the reaction to completion (Fig. 22.14).
Carbohydrates, also called sugars or saccharides, are molecules
with the general formula of C
n
H
2n
O
n
. The simplest carbohy-
drates, the monosaccharides, have an aldehyde at one end in the
open form of the molecule and a primary alcohol at the other
end. In solution, carbohydrates exist mainly as cyclic hemiacetals.
The stereochemical complications lead to the simplifying
tool of a Fischer projection in which a stylized drawing is used
to reduce complexity. The molecule is drawn vertically with the
aldehyde group at the top. Horizontal bonds are taken as com-
ing toward you and vertical bonds as retreating from you. The
stereogenic carbon closest to the primary alcohol group is called
the configurational carbon. If the OH on this carbon is on the
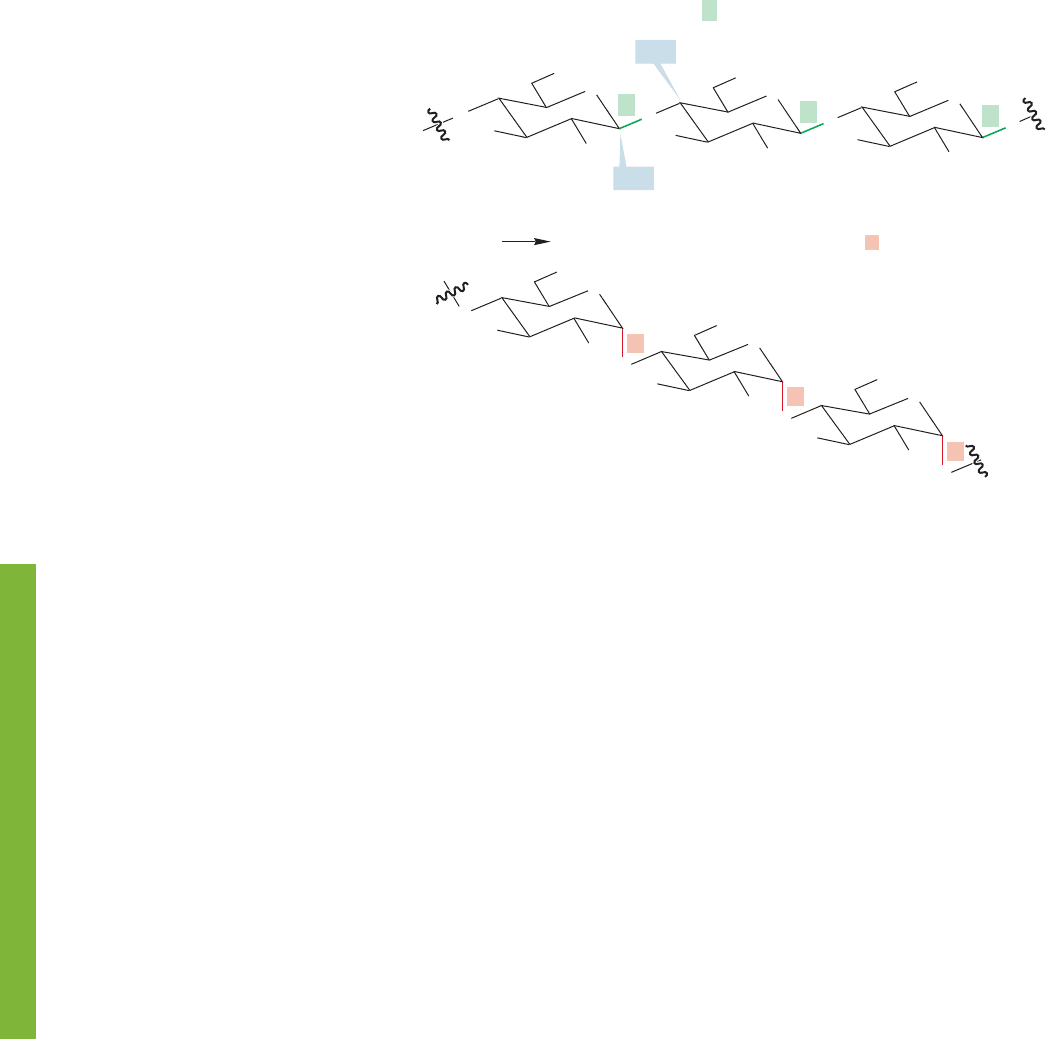
There is no reason that more than two sugars cannot be connected by glycosidic
bonds, and larger polysaccharides are common. The carbohydrate cellulose,for
instance, is a polysaccharide in which thousands of glucose units are connected lin-
early by β-glycosidic bonds between C(4) of one glucose and the anomeric C(1) of
a neighboring glucose (Fig. 22.67a). Cellulose, the stuff plants are made of, contains
most of the carbon on Earth! Cotton is 90% cellulose, and wood is about 50%.Most
mammals, including humans, are unable to break down cellulose. Starch is another
polysaccharide made up of glucose units. Starch is the main saccharide in our diet.
Corn, rice, potatoes, and wheat are sources of starch. Not all of the linkages in starch
are between C(4) and C(1), so it is described as a branched polysaccharide or as a
complex sugar. However, the linear polysaccharide amylose can be isolated from
starch (Fig.22.67b). Amylose has the same structure as cellulose except that the sug-
ars are connected through α linkages.
