Jung Han. Innovations in Food Packaging
Подождите немного. Документ загружается.


Plasticizers in edible films and coatings
405
Common plasticizers for edible films and coatings are monosaccharides, oligosac-
charides, polyols, lipids, and derivatives (Guilbert, 1986; Baldwin
et
al., 1997). Water
is also an important plasticizer for edible
films
and coatings. Water content is dependent
on the polymer and external plasticizer selected.
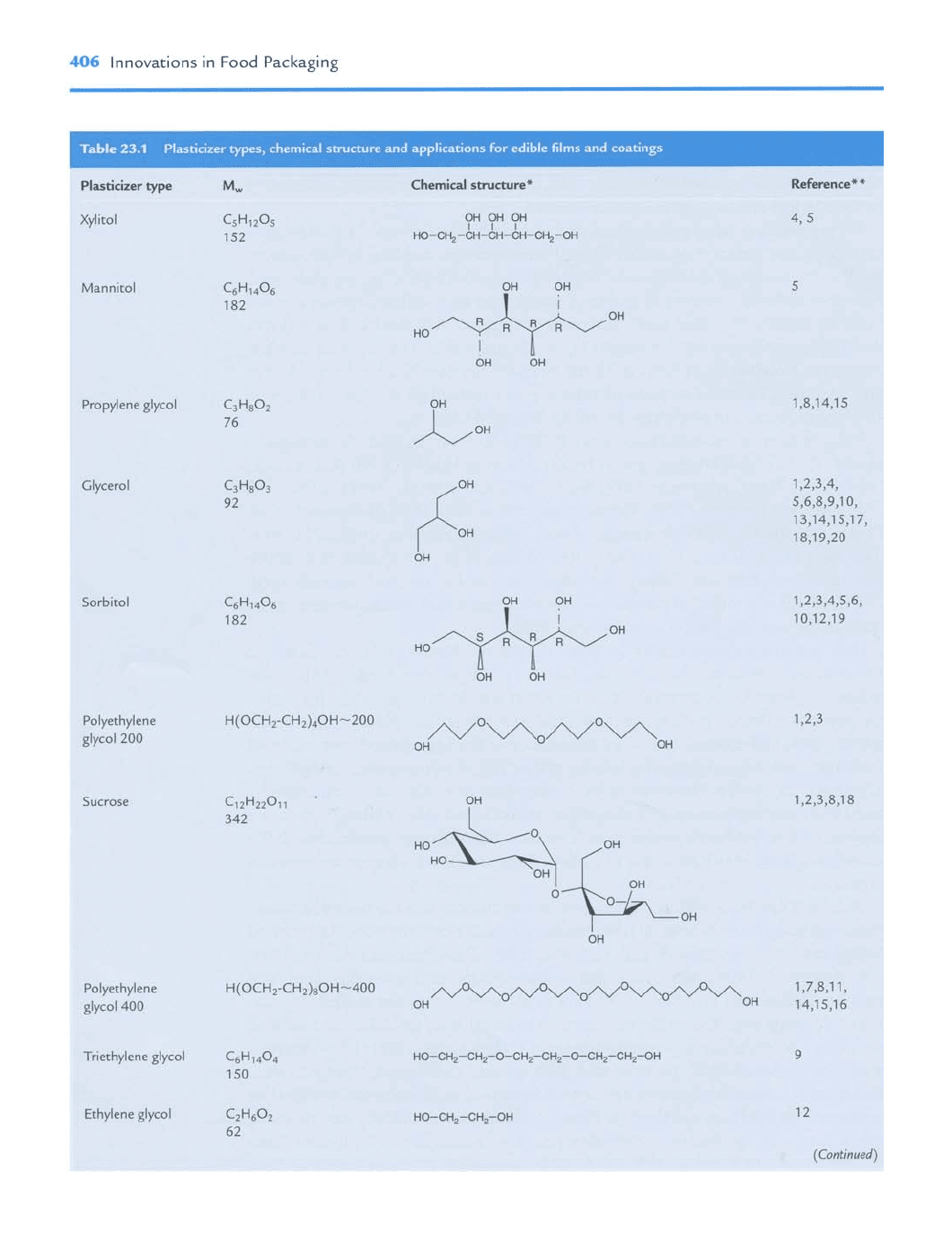
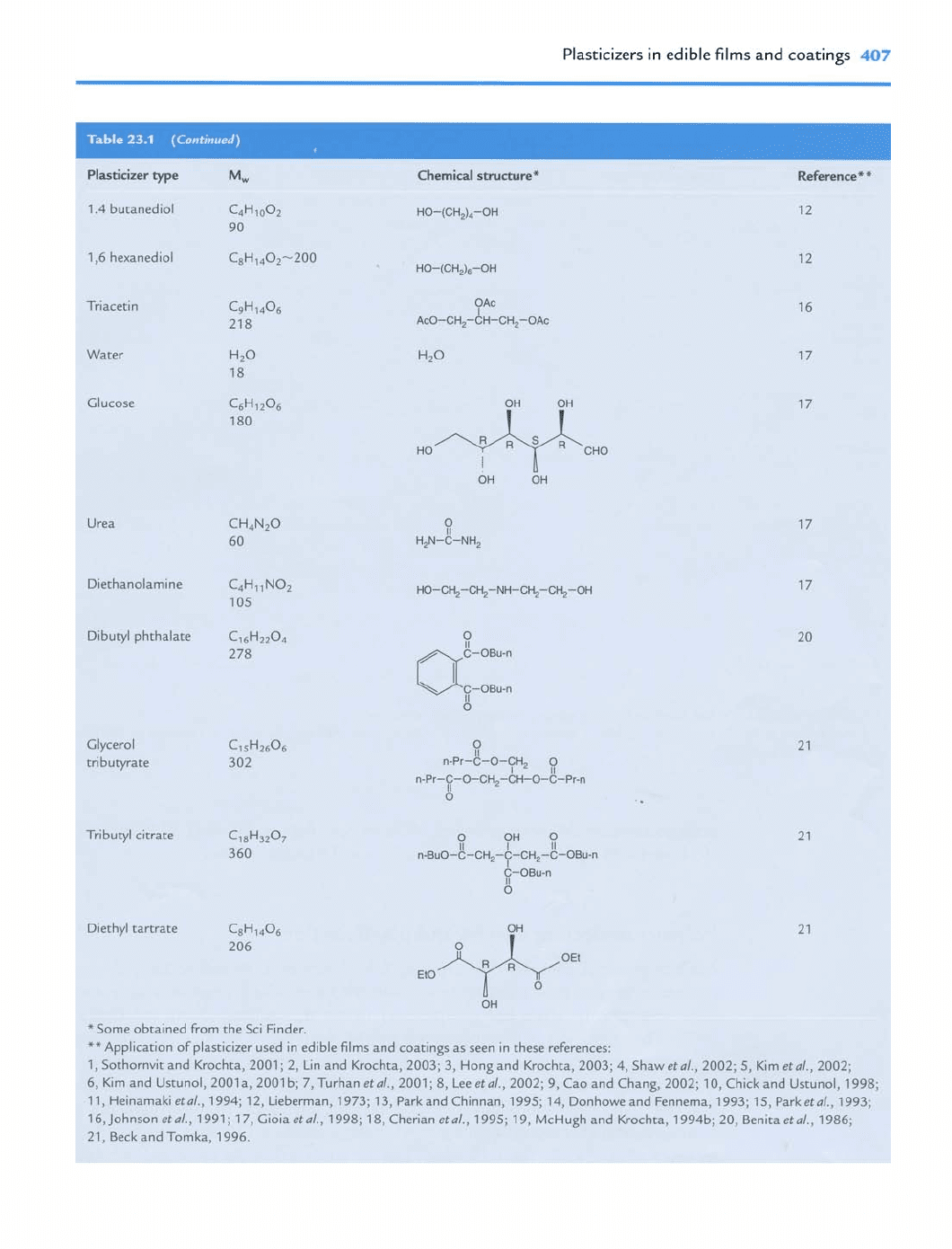
Some plasticizer types and chemical structures are shown in Table 23.1. Most plas-
ticizers contain hydroxyl groups which will form hydrogen bonds with biopolymers,
and thus increase the free volume and flexibility of the film matrix. Various plasticizers
consist of different numbers of hydroxyl groups and have different physical states
(solid or liquid); thus they show differences in degree of softening film stiffness.
Water is the smallest molecular weight (M,
=
18), and it is well known as an excellent
plasticizer. However, most films still need an additional plasticizer to obtain desired
film flexibility. Moisture sorption of various plasticizers plays an important role in
affecting different film properties, as will be discussed later.
Other plasticizers besides those shown in Table 23.1 can be used; for example, a
variety of M, of polyethylene glycol (PEG). These include PEG 300 (Gioia
et
al.,
1998), PEG 600 (Lieberman, 1973; Guo, 1993; Gioia
et
al., 1998), PEG 1450
(Donhowe and Fennema, 1993; Turhan
et
al., 2001), PEG 1500 (Heinamaki
et
al.,
1994), PEG 4000 (Guo, 1993; Heinamaki
et
al., 1994; Turhan
et
al., 2001), PEG 8000
(Donhowe and Fennema, 1993; Guo, 1993; Turhan
et
al., 2001), and PEG 20000
(Donhowe and Fennema, 1993), depending on the biopolymer materials used.
Combinations of a variety of plasticizers have also been used to obtain averaged prop-
erties (Cherian
et
al., 1995; Cao and Chang, 2002).
Lipid plasticizers such as fatty acids and derivatives, lecithin, oils, and waxes are
also commonly used in edible coatings. Generally, the purpose of adding lipid is to
reduce film water vapor permeability, since lipids are non-polar nature or hydropho-
bic, and thus provide a good barrier against moisture migration. Moreover, lipids can
provide gloss and enhance the visual appearance of food products. However, lipids
exhibit poor mechanical properties because of their lack of cohesive structural integrity
(Gontard
et
al., 1995). Therefore, incorporating lipid in protein- or polysaccharide-
based films may produce a plasticizing effect, including reduction of film strength and
increase of film flexibility, as seen in milk protein (Shellhammer and Krochta, 1997)
and wheat gluten films (Gontard
et
al., 1994). Common fatty acids used are shown in
Table 23.2.
Oleic acid has been used as a plasticizer in zein films to provide modified atmos-
phere packaging for fiesh broccoli (Rakotonirainy
et
al., 2001). PaImitic acid-plasticized
methylcellulose films (Kester and Fennema, 1989; Rico-Pena and Torres, 199 1;
J. W. Park
et
al., 1994), lauric acid- and stearic-palmitic acid-plasticized laminated
methyl cellulose/corn zein films (J. W. Park
et
al., 1994) have been studied. In addi-
tion, fatty acids from CI4 to C22 (myristic, palmitic, stearic, arachidic, and behenic
acids) have been studied as plasticizers in whey protein isolate (WPI) films (Sherwin
et
al., 1998). Stearic acid, palmitic acid, myristic acid, lauric acid, stearyl alcohol,
hexadecanol, tetradecanol, and beeswax have been used as plasticizers in WPI-lipid
emulsion films (McHugh and Krochta, 1994a). Carnauba wax, candelilla wax, beeswax,
and a hard milkfat fraction have been found to plasticize WPI-glycerol films
(Shellhammer and Krochta, 1997). Generally, increasing the chain length of fatty
$06
Innovations in Food Packaging
rable
23.1
Plasticizer types, chemical structure and applications for edible films and coatings
Plasticizer
typ
zhemical structure
Reference*
Mannitol
PropyIene glycol
C3Hs02
76
Glycerol
Sorbitol
Polyethylene
H(OCH2-CH2),0H-200
glycol
200
OH
OH
Sucrose
Polyethylene
H(OCH2-CH2)80H-400 1,7,8,11,
glycol
400
OH
14,15,16
Triethylene glycol
C6Hl4O4
150
Ethylene glycol
C~~602
62
(Continued)
Plasticizers
in
edible
films
and
coatings
407
OAc
I
AcO-CH2-CH-CH,-OAc
Urea
Diethanolamine C4HllN02
105
Dibutyl phthalate C16H2204
278
Glycerol C1sH2606
tributyrate 302
Tributyl citrate
C18H3207
Plasticizer
type
4
Chemical
structure*
1.4 butaned~ol
C4H~002
HO-(CH2)4-OH
90
1,6 hexaned~ol C8H1402-200
"'
by+$:
--
@$2
I
r~k*etlti
"bfi,,?
*'
b
!?%a
21 8
Water H2O
18
Glucose CsHlzOs
R
PH
R
n-BuO-C-CH,-C-CH,-C-OBu-n
I
$-OBu-n
0
EtO
/,&oEt
0
OH
*Some obtalned from the SCI Flnder.
**Appllcatlon of plastlclzer used In ed~ble films and coatlngs as seen In these references.
1, Sothornvlt and Krochta, 2001; 2, Lln and Krochta, 2003; 3, Hong and Krochta, 2003; 4, Shaw
etal.,
2002;
5,
Klm
etal.,
2002,
6, Klm and Ustunol, 2001a, 2001 b; 7, Turhan
etal.,
2001,8, Lee
etal.,
2002; 9, Cao and Chang, 2002, 10, Chlck and Ustunol, 1998;
11, Helnamak~
etal
,
1994; 12, heberman, 1973; 13, Park and Chlnnan, 1995, 14, Donhowe and Fennema, 1993, 15, Park
etal.,
1993;
16, Johnson
etal.,
1991
;
17, Glola
etal.,
1998, 18, Cherlan
etal.,
1995; 19, McHugh and Krochta, 1994b, 20, Ben~ta
et
a/.,
1986;
21, Beck and Tomka, 1996.
Diethyl tartrate C8H140s
206

408
Innovations
in
Food Packaging
Table
23.2
Fatty acids used in edible films and coatings
r
I
Lpeof
M,
Chemical
structure
ttty
acid
auric
C12H2402
cid 200
Reference*
1
tearic
C18~36~2
0
COOH
1,2,6
cid 284
Ialmitic
C16H3202
COOH
1,3,4,6
cid 256
.inoleic
C18H3202
COOH
5
.cid 280
.inolenic
C18H3002
COOH
5
.cid 278
nyristic
C14ti2802
COOH
6
rcid 228
Neic
C~8H3402
0
-
COOH
6
~cid 282
Arachidic
CZOH4002
COOH
6
I
acid 312
*Application of fatty acids used in edible films and coatings as seen in these references:
1,
J.
W.
Park
etal.,
1994; 2, Sherwin
etal.,
1998; 3, Rico-Pena and Torres, 1991; 4, Kester and Fennema, 1989;
5,
Tanaka
etal.,
2001;
5,
Rakotonirainy
etal.,
2001.
acids decreases the
WVP
of laminated MCIcorn zein-fatty acid films
(J.
W.
Park
et
al.,
1994) and WPI-lipid emulsion films (McHugh and Krochta, 1994a).
Polymer molecular weight and plasticization
Another potential approach to increasing film flexibility is to reduce the polymer
molecular weight, thus reducing intermolecular forces along polymer chains and
increasing polymer chain end groups and polymer free volume (Sears and Darby,
1982). Therefore, polymer molecular weight (M,) can play a plasticization role for
edible films and coatings. Park
et
al. (1993) found that film oxygen permeability (OP),
water vapor permeability
(WVP),
and tensile strength (TS) increased as methylcellu-
lose (MC) and hydroxypropyl cellulose
(I-IPC)
M, increased. However, film
WVP
decreased with increasing hydroxypropyl methyl cellulose (HPMC) and
MC
Mw
(Ayranci
et
al., 1997). The differences may be attributed to the more hydrophobic

Plasticizers in edible films and coatings
409
behavior of the methyl group in HPMC compared to the HPC (Ayranci
et
al., 1997).
Reducing whey protein isolate (WPI) Mw also showed no significant effect on film
WVP and OP (Sothornvit and Krochta, 2000), but the hydrolyzed WPI showed a
significant plasticization effect by reducing film EM and TS compared to unhy-
drolyzed WPI (Sothornvit and Krochta, 2000). Chitosan Mw effect showed the same
trend as film TS and
WVP
as hydrolyzed WPI (S. Y. Park
et
al., 2002). Generally, stud-
ies have found that lowering the polymer Mw increased film flexibility without chang-
ing film
WVP.
External plasticizer requirements
Besides cost, the selection of a plasticizer requires consideration of three basic criteria:
compatibility, efficiency, and permanence.
Compatibility
It is necessary to use a plasticizer that is compatible with the intended polymer.
Compatibility depends on polarity, structural configuration (shape), and size (M,) of
plasticizer. Good compatibility results from the plasticizer and polymer having a similar
chemical structure (Immergut and Mark, 1965; Banker, 1966; Wilson, 1995). Therefore,
different polymers require different plasticizers (Sears and Darby, 1982). In addition,
plasticizers should have low volatility, as well as being non-toxic and aroma free.
Efficiency
Generally, good plasticizers provide
high
plasticization at low concentration and exhibit
rapid polymer diffusion and interaction. The "plasticizer efficiency" is defined as the
quantity of plasticizer required to produce the desired film mechanical properties. One
method to define the efficiency is the lowering of T, at a given amount or volume frac-
tion of plasticizer. There is no exact number to indicate the efficiency of each plasti-
cizer, because it depends on the polymer properties. Not only the size (M,) but also
the rate of plasticizer diffusion into the polymer matrix is another important factor in
defining plasticizer efficiency (Immergut and Mark, 1965). A higher diffusion rate
results in higher plasticizer efficiency. Small molecules have high diffusion rates, but
possess higher volatility and therefore it is easier to lose the plasticizer in the process.
Permanence
The permanence of plasticizers in polymers depends on the size of the plasticizer mol-
ecule and on the rate of diffusion in polymers. Larger plasticizer molecules possess
lower volatility, resulting in greater permanence. Moreover, polarity and hydrogen
bond capability will influence the volatility of plasticizers. Greater plasticizer effi-
ciency, defined by rapid diffusion into the polymer matrix, may also result in lower
plasticizer permanence due to diffusion out of the polymer matrix. Thus it is often nec-
essary to compromise in selecting which type of plasticizer is more suitable for each
polymer material (Immergut and Mark, 1965; Banker, 1966).

410
Innovations in Food
Packaging
The most effective plasticizers show the following properties (Sears and Darby, 1982):
1.
A
smaller plasticizer (lower
M,)
is more effective in lowering film T,
2. Plasticizer efficiency is proportional to the
T,
of plasticized films; this means that
small amounts of plasticizer are the most effective and larger amounts have less effect
3.
Lesser affinity between plasticizer and polymer compared to the affinity between
polymer and polymer gives more efficient plasticization
-
in other words, a good
plasticizer is a bad solvent
4.
Lesser affinity between plasticizer and plasticizer compared to the affinity between
polymer and polymer gives more efficient plasticization, thus low-viscosity plasti-
cizers are more efficient.
Theories
of
plasticization
There are four theories to explain plasticizing effects. The earliest are the free volume
theory, which involves the intermolecular spaces in polymer, and the coiled spring theory,
which deals with tangled macromolecules (Sears and Darby, 1982). The gel theory
and the lubricity theory were later theories introduced to explain plasticization. The
gel theory explores the rigidity in an unplasticized gel formed by weak polymer-
polymer interactions along the polymer chains (Doolittle, 1965; Sears and Darby,
1982). The lubricity theory considers the plasticizer to act as a lubricant to facilitate the
movement of polymer chains over each other, consequently lowering resistance to
deformation (Doolittle, 1965; Sears and Darby, 1982). These two theories account for
different portions of the total phenomenon of plasticization and the deformation of
plasticized polymers. For edible films, the most useful concepts are the gel theory and
the free volume theory. Thus, these two theories will be considered in greater detail.
Gel theory
The gel theory in food hydrocolloids pertains to an internal, three-dimensional rigid
structure of polymer. Plasticizer molecules attach along the polymer chains, replacing
polymer-polymer attachments at places and hindering the forces holding polymer
chains together (Van der Waals, London, Debye, hydrogen bonding, crystal, or primary
valence forces). This reduces the rigidity of the gel structure, resulting in increased
gel flexibility. In addition, plasticizer molecules that are not attached to polymer form
aggregated plasticizer domains that facilitate the movement of polymer molecules.
This also enhances the gel flexibility.
Free volume theory
The free volume of amorphous materials or polymers is the volume unoccupied by the
material's molecules (Sears and Darby, 1982). Another definition is the volume dif-
ference between the temperature at absolute zero and the temperature of interest. Free
volume can be expressed as Vf
=
V,
-
V,, where Vf is the free volume, V, is the

Plasticizers in edible films and coatings
411
specific volume at temperature T, and
V,
is the specific volume at reference tempera-
ture (e.g. absolute zero).
Approaches to increasing the free volume of polymer system could include:
1. Using low
M,
polymer, or reducing the
M,
of polymer, to increase the number of
polymer end groups
2.
Increasing the length of side chains, thus increasing steric hindrance and lowering
chain intermolecular forces to increase polymer chain motion, related to internal
plasticization
3.
Using low
M,
plasticizers that are compatible with the polymer molecules to
increase the motion of chain ends, side chains, and main chain, related to external
plasticization
4.
Increasing temperature.
Free volume theory is used to describe many things, such as plasticizing action, T,,
viscosity, cross-linking, diffusion,
film
drylng and
film
properties (Wicks, 1986; Duda
and Zielinski, 1996).
Plasticization models
Plasticization models are used to predict the effect of components on the T, of food
systems, in order to control or estimate the properties of food materials (Roos, 1995).
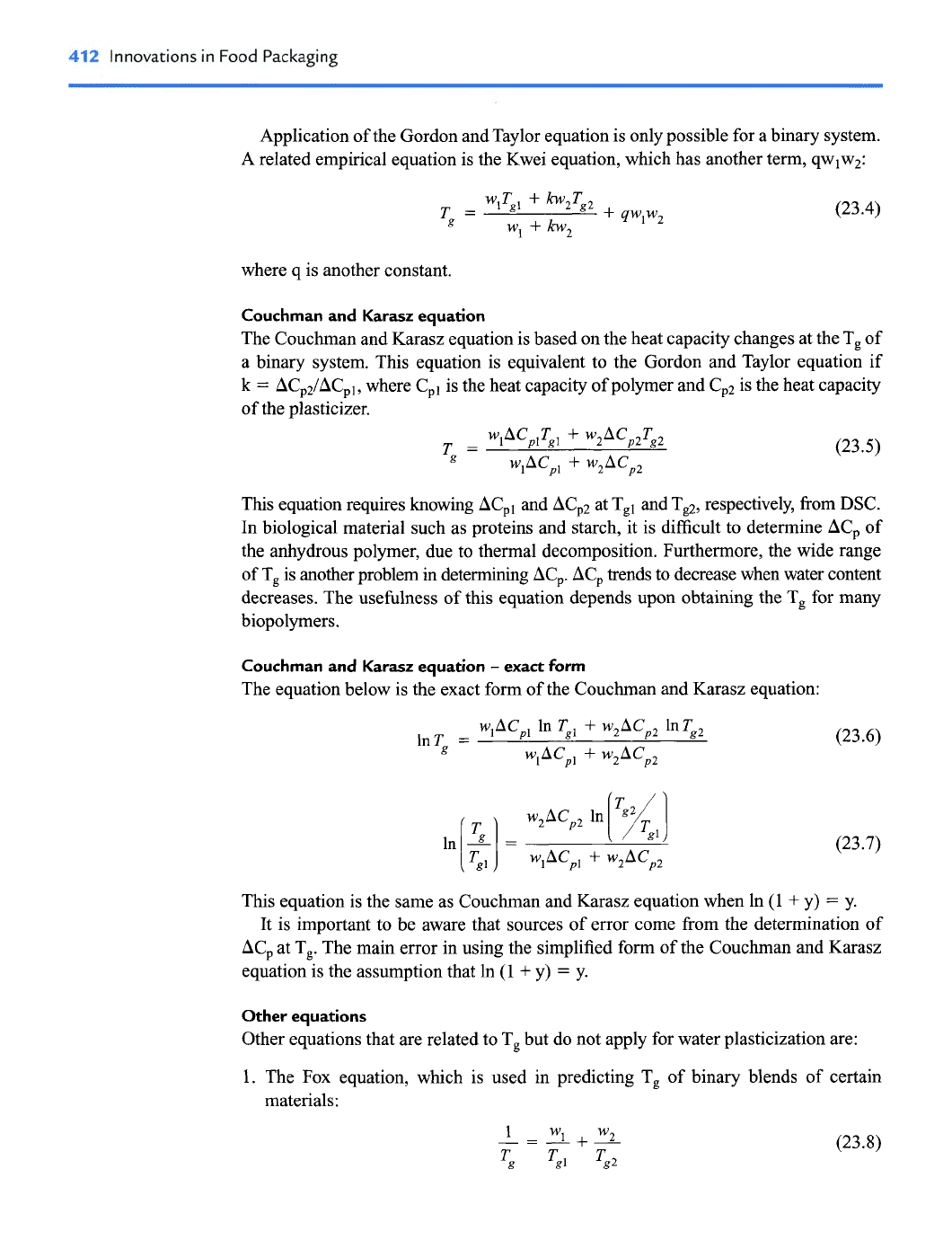
Gordon and Taylor equation
The Gordon and Taylor equation can be applied to predict plasticization of food com-
ponents such as carbohydrates and proteins, as well as of pharmaceutical materials.
This equation requires an empirical constant
(k)
from experimental data for T, at dif-
ferent plasticizer contents. For water acting as a plasticizer, the equation covers the
range of
&50%
water content.
Thus, the constant k can be obtained from the following equation:
where wl and w2 are the weight fractions of solid component and plasticizer, respec-
tively. Tg, Tgl and TgZ are the glass transition temperatures of the system, solid and
plasticizer, respectively.
If the k and Tg of the system are known, the weight fraction of solid (wl) can be
obtained with the following equation:
412
Innovations in Food Packaging
Application of the Gordon and Taylor equation is only possible for a binary system.
A related empirical equation is the Kwei equation, which has another term, qwlw2:
where q is another constant.
Couchrnan and Karasz equation
The Couchman and Karasz equation is based on the heat capacity changes at the T, of
a binary system. This equation is equivalent to the Gordon and Taylor equation if
k
=
ACp2/ACpl, where Cpl is the heat capacity of polymer and Cp2 is the heat capacity
of the plasticizer.
This equation requires knowing ACpl and ACp2 at Tgl and Tp, respectively, from DSC.
In biological material such as proteins and starch, it is difficult to determine ACp of
the anhydrous polymer, due to thermal decomposition. Furthermore, the wide range
of T, is another problem
in
determining ACp. AC,
trends
to decrease when water content
decreases. The usefulness of this equation depends upon obtaining the T, for many
biopolymers.
Couchrnan and Karasz equation
-
exact
form
The equation below is the exact form of the Couchman and Karasz equation:
This equation is the same as Couchman and Karasz equation when In (1
+
y)
=
y.
It is important to be aware that sources of error come from the determination of
ACp at T,. The main error in using the simplified form of the Couchman and Karasz
equation is the assumption that
In
(1
+
y)
=
y.
Other equations
Other equations that are related to T, but do not apply for water plasticization are:
1.
The Fox equation, which is used in predicting Tg of binary blends of certain
materials:
Plasticizers
in
edible films and coatings
413
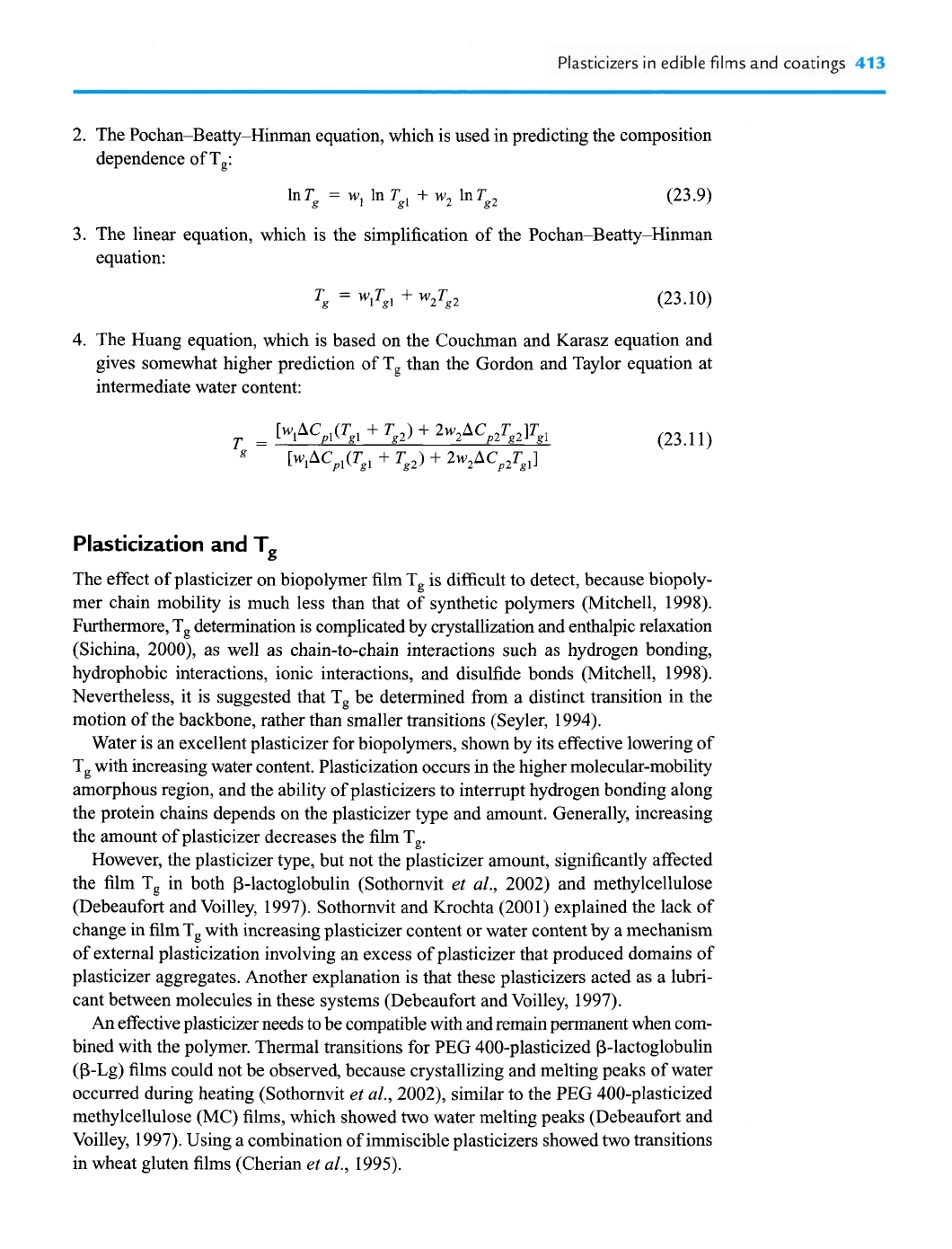
2. The Pochan-Beatty-Hinrnan equation, which is used in predicting the composition
dependence of T,:
3.
The linear equation, which is the simplification of the Pochan-Beatty-Hinman
equation:
4. The Huang equation, which is based on the Couchman and Karasz equation and
gives somewhat higher prediction of T, than the Gordon and Taylor equation at
intermediate water content:
Plasticization and
T,
The effect of plasticizer on biopolymer film Tg is difficult to detect, because biopoly-
mer chain mobility is much less than that of synthetic polymers (Mitchell, 1998).
Furthermore, Tg determination is complicated by crystallization and enthalpic relaxation
(Sichina, 2000), as well as chain-to-chain interactions such as hydrogen bonding,
hydrophobic interactions, ionic interactions, and disulfide bonds (Mitchell, 1998).
Nevertheless, it is suggested that T, be determined from a distinct transition in the
motion of the backbone, rather than smaller transitions (Seyler, 1994).
Water is an excellent plasticizer for biopolymers, shown by its effective lowering of
T, with increasing water content. Plasticization occurs in the higher molecular-mobility
amorphous region, and the ability of plasticizers to interrupt hydrogen bonding along
the protein chains depends on the plasticizer type and amount. Generally, increasing
the amount of plasticizer decreases the film T,.
However, the plasticizer type, but not the plasticizer amount, significantly affected
the film Tg in both P-lactoglobulin (Sothornvit
et
al., 2002) and methylcellulose
(Debeaufort and Voilley, 1997). Sothornvit and Krochta (2001) explained the lack of
change in film Tg with increasing plasticizer content or water content by a mechanism
of external plasticization involving
an
excess of plasticizer that produced domains of
plasticizer aggregates. Another explanation is that these plasticizers acted as a lubri-
cant between molecules in these systems (Debeaufort and Voilley, 1997).
An
effective plasticizer needs to be compatible with and remain permanent when com-
bined with the polymer. Thermal transitions for PEG 400-plasticized P-lactoglobulin
(P-Lg) films could not be observed, because crystallizing and melting peaks of water
occurred during heating (Sothornvit
et
al., 2002), similar to the PEG 400-plasticized
methylcellulose (MC) films, which showed two water melting peaks (Debeaufort and
Voilley, 1997). Using a combination of immiscible plasticizers showed two transitions
in wheat gluten films (Cherian
et
al., 1995).

414
Innovations
in
Food Packaging
The physical state of the plasticizer is another important factor that affects film T,.
For example, sorbitol (Sor)- and sucrose (Suc)-plasticized P-Lg films possessed higher
film T, than glycerol (G1y)-, PEG 200- and propylene glycol (PG)-plasticized films,
presumably because the solid state of Sor and Suc reduced their efficiency in disrupting
hydrogen bonding along protein chains compared to the liquid state plasticizers (Gly,
PEG 200 and PG) (Sothornvit
et
al., 2002). Small molecular size
(M,)
plasticizers
also appear to be more effective in reducing film T,. Furthermore, the diverse hygro-
scopicity of plasticizers affects film Tg differently. Sor is less hygroscopic and exhibits
less efficient plasticization compared to Gly. The last factor is plasticizer shape, such
as straight vs ring structure. Presumably, the bulky ring structure of Suc resulted in
higher T, than PEG 200 in P-Lg films (Sothornvit
et
al., 2002).
In summary, the effect of various plasticizers on film T, is related to film flexibility
and other properties. Thus, film T, is quite important for applications of edible films
and coatings.
Advantages and disadvantages
of
edible
films
and coatings
Most edible films and coatings are quite brittle due to extensive intermolecular forces
such as hydrogen bonding, electrostatic forces, hydrophobic bonding, and disulfide
bonding. Plasticizers are required to interrupt intermolecular forces and lower the T,,
resulting in greater flexibility and toughness. Unfortunately, plasticizers increase film
permeability to moisture, oxygen, aroma, and oils. Therefore, there are advantages
and disadvantages of using plasticizers in edible films and coatings.
Plasticizers do not only produce plasticization. They can also produce antiplasti-
cization. Plasticization is known to involve the reduction of intermolecular forces
between polymer chains, thus improving film and coating flexibility. On the other
hand, antiplasticization can occur at lower plasticizer concentrations and tempera-
tures. Antiplasticization in films has been attributed to several mechanisms, such as
reduction of polymer free volume, interaction between the polymer and plasticizer,
and film stiffness due to the presence of rigid plasticizer molecules adjacent to the
polar groups of the polymer (Guo, 1993; Seow
et
al.,
1999). In addition, adding a low
concentration of plasticizer can lead to an increase in polymer crystallinity, due to
lowering the energy barrier for a change of polymer state and thus enhancing the forma-
tion of ordered structures within amorphous regions (Wilson, 1995). Antiplasticization
decreases edible film and coating
WVP,
and increases the elastic modulus and brittle-
ness, which is the opposite behavior to plasticization. Water has been shown to have both
plasticizer and antiplasticizer effects on tapioca starch film properties over the low to
intermediate moisture range (Chang
et
al.,
2000). Moreover, triacetin, PEG 600, PEG
4000, and PEG 8000 have shown plasticization and antiplasticization effects on
WVP
of cellulose acetate films (Guo, 1993). Therefore, plasticizers must be used in the cor-
rect amount to obtain the advantage of enhancing the
film
and coating properties.
The main advantage of using plasticizers is to increase film toughness or resiliency
by increasing flexibility, decreasing tensile strength, and increasing elongation, as seen
