Kamal A.A. 1000 Solved Problems in Classical Physics: An Exercise Book
Подождите немного. Документ загружается.

408 9 Fluid Dynamics
9.28 For the first tube Q
1
=
π Pr
4
8ηl
= Q
For the second tube Q
2
=
π P(2r)
4
8ηl/2
=
32π Pr
4
8ηl
= 32Q
Total quantity of water flowing is
Q
1
+ Q
2
= Q + 32Q = 33Q
9.29 Let the pressure at the beginning of the first tube be P
1
and at the end P
2
. Since
the water flow must be continuous, the rate of flow in the two tubes must be
identical, that is, Q
1
= Q
2
. Let the atmospheric pressure be P
0
.
P
1
− P
2
=
8ηlQ
1
πr
4
(for the first tube) (1)
P
2
− P
0
=
8η(l/2)Q
2
π(2r)
4
=
8ηlQ
1
32πr
4
(for the second tube) (2)
Adding (1) and (2)
P
1
− P
0
=
8ηl
πr
4
33Q
1
32
(3)
But P
1
− P
0
=
8ηlQ
πr
4
(for single tube of length l and radius r)(4)
Comparing (3) and (4), we get Q
1
=
32Q
33
.

Chapter 10
Heat and Matter
Abstract Chapter 10 is devoted to kinetic theory of gases, collision cross-section,
mean free path, van der Waal’s equation, thermal expansion of solids, liquids and
gases; gas equation, heat conduction in composite slabs and sphere; Newton’s law of
cooling, radiation problems covering Boltzmann law and Wien’s law, specific heat
and latent heat, thermodynamics, indicator diagrams, the Carnot, Otto and Sterling’s
cycles, thermodynamic relations, elasticity and surface tension.
10.1 Basic Concepts and Formulae
The mean free path λ of a gas molecule is the average distance travelled by the
molecule between successive collisions
λ =
x
i
/N (10.1)
The average time of collision T is related to λ and <v> the mean speed by
λ = <v>T = <v>/ f (10.2)
where f = 1/T (10.3)
is the collision frequency.
N(v) dv = 4π N[m/2πkT ]
3/2
v
2
exp[−mv
2
/2kT ]dv(Maxwell’s law) (10.4)
Assuming Maxwell’s law of velocity distribution,
Most probable speed v
p
=
2kT
m
=
2RT
M
(10.5)
Average speed <v>=
8kT
πm
=
8RT
π M
(10.6)
Root-mean-square speed
<v
2
> =
8kT
m
=
8RT
M
(10.7)
409

410 10 Heat and Matter
where k is Boltzmann constant, T the absolute temperature, m the particle mass,
R the gas constant and M the molecular weight.
λ =
v
4π
√
2 r
2
N
(10.8)
where N=nV , n is the number of molecules per unit volume and V is the volume
of the gas.
Gas Laws
PV = nRT (gas equation) (10.9)
ρ
1
T
1
P
1
=
ρ
2
T
2
P
2
(10.10)
Thermal Expansion
L = L
0
[1 + αT ] (linear expansion) (10.11)
β = 2α, γ = 3α (10.12)
where α is the coefficient of linear expansion, β is the coefficient of areal expansion
and γ that of volume expansion.
Thermal Expansion and Elasticity
Force F = YaαT (10.13)
where Y is Young’s modulus and a the cross-sectional area.
The coefficient A of apparent expansion of a liquid
A = γ − g = γ − 3α (10.14)
where γ is the absolute volume coefficient of expansion of liquid and g that of the
container.
Apparent expansion of liquid =
mass expelled
(mass left)(temperature rise)
Heat Transfer
Heat conduction through a slab:
−
dQ
dr
= k
1
A
T
1
− T
2
d
(10.15)
10.1 Basic Concepts and Formulae 411
Rate of heat flow is directly proportional to the temperature gradient dT/dx and
the cross-sectional area and inversely proportional to the thickness. The constant of
proportionality k is known as thermal conductivity.
Heat conduction is through a composite slab made of two slabs of thickness d
1
and d
2
and thermal conductivity k
1
and k
2
, respectively, in series, the end tempera-
tures being T
1
and T
2
. The equivalent conductivity is given by
k
eq
=
d
1
+ d
2
d
1
k
1
+
d
2
k
2
(10.16)
The temperature of the interface is given by
T =
k
1
T
1
d
1
+
k
2
T
2
d
2
k
1
d
1
+
k
2
d
2
(10.17)
The rate of flow of heat in a composite slab made of n slabs in parallel:
−
dQ
dt
=
(T
1
− T
2
)
d
n
i=1
k
i
A
i
(10.18)
k
eq
=
k
i
A
i
A
i
(10.19)
Convection
Rate of cooling:
−
dθ
dt
= C(θ −θ
0
) (Newton’s law of cooling) (10.20)
where θ is the mean temperature, θ
0
the room temperature and C a constant.
Radiation
Rate of energy loss:
−
dE
dt
= σ A
T
4
1
− T
4
2
(Stefan–Boltzmann formula) (10.21)
where A is the area of the radiator, T
1
is its absolute temperature, T
2
is the absolute
temperature of the surroundings and σ is known as Stefan–Boltzmann constant.
Wien’s Law
λ
m
· T = b = 3 × 10
−3
m − K (10.22)

412 10 Heat and Matter
The wavelength λ
m
for the maximum intensity of black body spectrum is inversely
proportional to the absolute temperature of the body.
Thermodynamics
Process Quantity that remains constant
(i) Isobaric Pressure
(ii) Isochoric Volume
(iii) Isothermal Temperature
(iv) Adiabatic Heat
First law of Thermodynamics
Q = U + W (10.23)
Q is positive if heat is absorbed by the system and negative if heat is evolved.
Internal energy U of a system tends to increase if energy is added as heat and tends to
decrease if energy is lost as work done by the system. Both Q and W are dependent
while U is path independent.
Work done by the system:
Isobaric process: W =
P dV = P(V
1
− V
2
)
Isochoric process: W = 0, Q = U
Isothermal process: W =−nRT ln
V
2
V
1
,dU = 0, dQ = dW
Adiabatic process: W =
1
γ − 1
(P
2
V
2
− P
1
V
1
),dQ= 0, dU =−dW
where γ is the ratio of two specific heats (c
p
/c
v
).
The change in entropy
S =
dQ
T
(10.24)
Enthalpy (H) is the total heat and is defined by
H = U + PV (10.25)
Gibb’s function (G) is defined by
G = U + PV − TS (10.26)
Number of degrees of freedom
f =
2
γ − 1
(10.27)

10.1 Basic Concepts and Formulae 413
Heat Engines Thermodynamic efficiency
e =
Work done by the gas
Heat put into the system
=
W
Q
in
(10.28)
The Carnot cycle consists of two isothermal processes and two adiabatic processes.
The Sterling cycle consists of two isothermal processes and two isochoric pro-
cesses.
The Otto cycle consists of two adiabatic processes and two i sochoric processes.
e = (Q
H
− Q
C
)/Q
H
(10.29)
e = (T
H
− T
C
)T
H
(10.30)
where the symbols H and C are for hot and cold reservoirs.
Elasticity
Stress = force/area = F/A
Strain = elongation/original length = L/L
Young’s modulus(Y ) = stress/strain = FL/AL
Shear modulus (η) = shear stress/shear strain = Fy/Ax
Bulk modulus (K ) = pressure increment/volume strain =
P
−V/V
Poisson’s ratio (σ ) = lateral contraction per unit length/longitudinal elongation
per unit length.
Relations for the elastic moduli:
Y =
9ηK
3K + η
= 2n(1 + σ) = 3K (1 −2σ) (10.31)
σ =
3K − 2η
6K + 2η
(10.32)
Surface Tension
Excess pressure in a drop
P = 2S/r (10.33)
Excess pressure in a bubble
P = 4S/r (10.34)
Pressure in a bubble due to electric charges
P = σ
2
/2ε
0
(10.35)
where σ is the charge density and ε
0
is the permittivity.

414 10 Heat and Matter
Energy released in coalescing n droplets each of radius r into a large drop of
radius R
W = 4πr
2
(n − n
2/3
)S (10.36)
W = 4π R
2
(n
1
3
− 1)S (10.37)
Capillary rise:
2S cos θ = (h +r/3)rρg (10.38)
where θ is the angle of contact, r is the radius of the bore and h is the height of the
liquid column.
10.2 Problems
10.2.1 Kinetic Theory of Gases
10.1 Define the mean free path of a gas both mathematically and in words. Calcu-
late the mean free path of a molecule in a gas if the number of collisions is
2 × 10
10
/s and the mean molecular velocity is 1000 m/s.
[University of Manchester 2008]
10.2
(a) Consider a gas that has a molecular weight of 28 and a temperature of
27
◦
C. What is the rms speed of molecule of the gas if it has a Maxwellian
velocity distribution? The ideal gas constant is 8.31 J/mol K.
(b) What is the mean free path of a molecule if the pressure is 2 atm (1atm=
101.3kPa), the temperature is 27
◦
C, and the cross-section is 0.43 nm
2
?
Using the average velocity from part (a) calculate the collision frequency
for the molecule. Boltzmann’s constant is 1.38 × 10
−23
J/K.
10.3
(a) By considering a volume, V , of ideal gas, containing N spherical
molecules with radius r, show that the mean free path of the molecules
can be defined by the equation
λ =
V
4π
√
2r
2
N
(b) Hence, or otherwise, calculate the mean free path of air at 100
◦
C and
1.01 × 10
5
Pa. Assume that the molecules of air are spheres of radius
r = 2.0 ×10
−10
m.
(k = 1.38 ×10
−23
J/K)
[University of Aberystwyth, Wales]
10.4 Figure 10.1 shows the Maxwell–Boltzmann velocity distribution functions
of a gas for two different temperatures, of which first (curve f
1
)isfor
T
1
= 300 K.
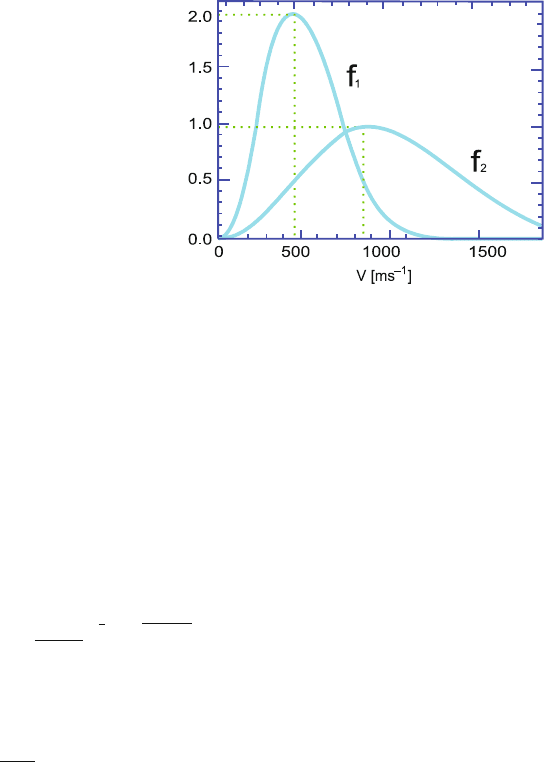
10.2 Problems 415
Fig. 10.1 Maxwell–
Boltzmann velocity
distributions
(a) Read the approximate value for the most probable speed of the molecules
from the diagram for each of the two cases.
(b) What is the temperature, T
2
, when the velocity distribution is given by f
2
?
(c) Indicate the average speeds in the diagram for each of the two
temperatures and give the ratio between the two average speeds.
(d) The gas consists of 5 mol of molecules. If the molecular velocity
distribution is given by f
2
, estimate the number of those molecules in
the gas which have a speed between v
A
= 800 m/s and v
B
= 900 m/s.
10.5 If the Maxwell–Boltzmann distribution of speeds is given by
f (v) = 4π
m
2πkT
3
2
v
2
e
−mv
2
2kT
show that the most probable speed is defined by the equation
v
mp
=
2kT
m
1/2
10.6 For carbon dioxide gas (CO
2
, molar mass = 44.0g/mol) at T = 300 K,
calculate
(i) the mean kinetic energy of one molecule
(ii) the r oot mean square speed, v
rms
(iii) the most probable speed, v
mp
(iv) the average speed, v
av
(R = 8.31 J/K/mol, k = 1.38 ×10
−23
J/K, N
A
= 6.02 ×10
23
/mol)
10.7
(a) Give three assumptions that are made when deriving the properties of an
ideal gas using a molecular model.
416 10 Heat and Matter
(b) A weather balloon is loosely filled with 2 m
3
of helium at 1 atm. and
27
◦
C. The balloon is then released, and by the time it has reached an
elevation of 7000 m the pressure has dropped to 0.5 atm. and the balloon
has expanded. If the temperature at this elevation is −48
◦
C, what is the
new volume of the balloon?
10.8 van der Waal’s equation can be written in terms of moles per volume as
n
V
=
⎛
⎜
⎜
⎝
p +a
n
2
V
2
RT
⎞
⎟
⎟
⎠
1 − b
n
V
The van der Waal’s parameters for hydrogen sulphide gas (H
2
S) are a =
0.448 J m
3
/mol
2
and b = 4.29 × 10
−5
m
3
/mol. Determine an estimate of
the number of moles per volume of H
2
S gas at 127
◦
C and a pressure of
9.80 × 10
5
Pa as follows:
(a) Calculate as a first approximation using the ideal gas equation,
n
V
=
p
RT
.
(b) Substitute this first approximation into the right-hand side of the equation
derived in part (a) to find a new approximation of
n
V
(on the left-hand
side) that takes into account real gas effects.
10.2.2 Thermal Expansion
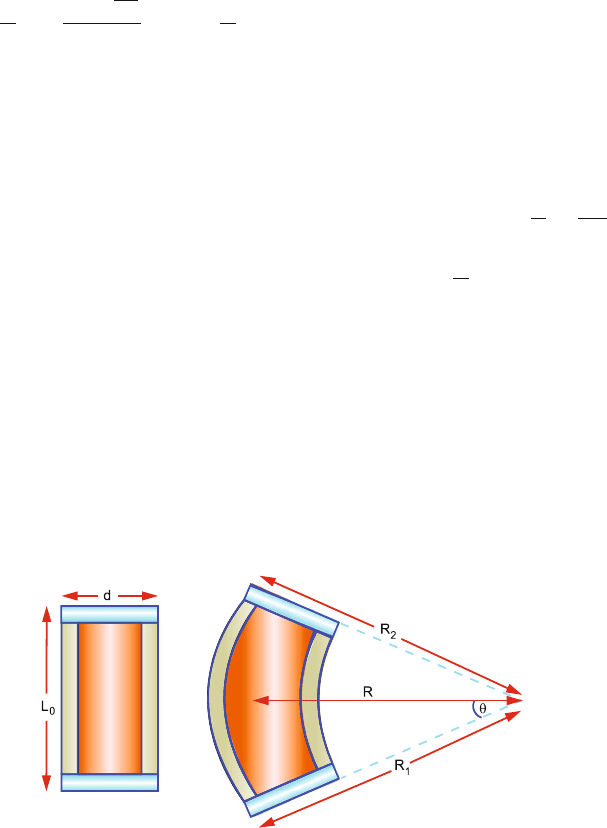
10.9 Two parallel bars of different material with linear coefficient of expansion
α
1
and α
2
, respectively, are riveted together at a distance d apart. An increase
in temperature T will cause them to bend into circular arcs with a common
centre subtending an angle θ at the centre (Fig. 10.2). Find the mean radius of
curvature.
Fig. 10.2 Expansion of a bimetal strip of bars
10.2 Problems 417
10.10 A 20 m long steel rail is firmly attached to the road bed only at its ends. The
sun raises the temperature of the rail by 30
◦
C, causing the rail to buckle.
Assuming that the buckled rail consists of two straight parts meeting in the
centre, calculate how much the centre of the rail rises? For steel α = 12 ×
10
−6
/
◦
C.
10.11 What should be the lengths of a steel and copper rod if the steel rod is 4 cm
longer than the copper rod at any temperature. α(steel) = 1.1 × 10
−5
/
◦
C;
α(copper ) = 1.7 × 10
−5
/
◦
C.
10.12 A 1 l glass flask contains some mercury. It is found that at different tem-
peratures the volume of air inside that flask remains the same. What is the
volume of mercury in this flask? Coefficient of linear expansion of glass =
9×10
−6
/
◦
C; coefficient of volume expansion of mercury = 1.8×10
−4
/
◦
C.
[Indian Institute of Technology 1973]
10.13 A steel wire of cross-sectional area 0.5 mm
2
is held between two fixed sup-
ports. If the tension in the wire is negligible and it is just taut at a temperature
of 20
◦
C, determine the tension when the temperature falls to 0
◦
C (assume
that the distance between the s upports remains the same). Young’s modulus
of steel = 2.1 × 10
11
dynes/cm
2
; α = 12 × 10
−6
/
◦
C.
[Indian Institute of Technology 1973]
10.14 A glass vessel just holds 50 g of toluene at 0
◦
C. What mass of toluene will it
hold at 80
◦
C if between 0 and 80
◦
C the expansion coefficients are constant.
The coefficient of linear expansion of glass is 8 × 10
−6
/
◦
C and the absolute
expansion of toluene is 11 × 10
−4
◦
C.
[University of Dublin]
Gas Laws
10.15 Determine the constant in the gas equation given that a gram molecule of a
gas occupies a volume of 22.4 l at NTP. [University of Durham]
10.16 A bubble of gas rises from the bottom of a lake 30 m deep. At what depth will
the volume be thrice as great as it was originally (atmospheric pressure =
0.76 m of mercury; specific gravity of mercury = 13.6)?
10.17 A balloon will carry a total load of 175 kg when the temperature and pressure
are normal. What load will the balloon carry on rising to a height at which
the barometric pressure is 50 cm of mercury and the temperature is −10
◦
C,
assuming the envelope maintains a constant volume?
[University of London]
10.18 Two glass bulbs of volume 500 and 100 cc are connected by a narrow tube
whose volume is negligible. When the apparatus is sealed off, the pressure
