Leroy C., Rancoita P.-G. Principles Of Radiation Interaction In Matter And Detection
Подождите немного. Документ загружается.

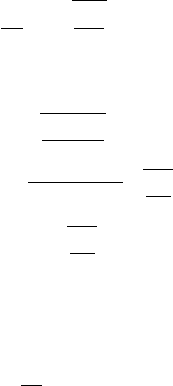
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
130 Principles of Radiation Interaction in Matter and Detection
2.2.3 Emission of Transition Radiation
Transition radiation is emitted when a fast charged particle passes through the
boundary between media with different indices of refraction (r
1
and r
2
), as shown
in Fig. (2.45) ([Ginzburg and Frank (1946)], see also [Goldsmith and Jelley (1959)]
for the first experimental observation of the transition radiation).
The reorganization of the field associated with the incoming particle occurs be-
cause a sudden change of the dielectric property of matter. The emitted radiation
comes from a coherent superposition of radiation fields generated by the molecular
polarization. The coherence is kept in a small volume surrounding the particle path,
whose length extension is referred to as the coherent length or formation zone. It can
result in an observable amount of energy in the X-ray region when a high enough
energy particle (i.e., when its Lorentz factor γ is À 1) traverses the boundary of
a macroscopically thick medium. Like
˘
Cerenkov emission, the process depends on
the particle velocity and is a collective response of the matter close to the particle
path. Like bremsstrahlung, it is sharply peaked in the forward direction [Garibyan
and Barsukov (1959)]. The intensity of the pro cess, i.e., the overall number of emit-
ted photons, can be enhanced by radiators consisting of several boundaries [Frank
(1964)]. The transition-radiation emission depends on a few parameters such as, for
instance, the radiator configuration and the Lorentz factor γ of the particle.
In the X-ray frequency region, a material behaves as an electron gas (see [Artru,
Yodh and Mennessier (1975)]), whose plasma frequency [Eq. (2.25)] is
ν
p
=
ω
p
2π
=
r
ne
2
πm
,
where n is the electron density and m is the electron mass. The corresponding
plasma photon energy is
hν
p
= ~ω
p
= h
r
ZρNe
2
πmA
=
p
4πNr
e
~
2
c
2
r
Zρ
A
≈ 28.8
r
Zρ
A
[eV]. (2.142)
The plasma photon energies are ≈ 0.7 eV for normal air, 0.27 eV for He, 20 eV
for polypropylene and styrene, 13.8 eV for Li and 24.4 eV for mylar. The dielectric
constant of the medium is given by
²(ω) = 1 −
³
ω
p
ω
´
2
= 1 − Υ
2
, (2.143)
where Υ is ¿ 1 in the X-ray region. The formation length D is of the order of
≈ (γc)/ω
p
and represents the largest value of the frequency dependent depth over
which the coherent superposition can occur [Jackson (1975)].
The complete expression for the energy radiated is rather complicated. But at
large γ, most of the energy is in the forward direction, i.e., for θ < π/2, where θ
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electromagnetic Interaction of Radiation in Matter 131
Fig. 2.45 Transition-radiation emission at the boundary between two media with different indices
of refraction (adapted and republished with permission from Artru, X., Yodh, G.B. and Mennessier,
G., Phys. Rev. D 12, 1289 (1975); Copyright (1975) by the American Physical Society).
is the angle between the direction of the emitted photon and the trajectory of the
incoming particle. For the forward direction (see Fig. 2.45), the energy radiated by
a particle of charge ze at the b oundary between two media, per unit of solid angle
and per unit of frequency interval can be approximated by
d
2
W
dν dΩ
' z
2
hα
π
2
θ
2
µ
1
γ
−2
+ θ
2
+ Υ
2
1
−
1
γ
−2
+ θ
2
+ Υ
2
2
¶
2
(2.144)
with Υ
1
=
ω
p,1
ω
, and Υ
2
=
ω
p,2
ω
; where ν
p,1
= ω
p,1
/(2π) and ν
p,2
= ω
p,2
/(2π) are the
plasma frequencies of the two media (see for instance [Artru, Yodh and Mennessier
(1975); Fayard (1988)] and references therein). Equation (2.144) can be rewritten
per unit of solid angle and unit of energy as:
d
2
W
d(~ω) dΩ
=
d
2
W
d(hν) dΩ
' z
2
α
π
2
θ
2
µ
1
γ
−2
+ θ
2
+ Υ
2
1
−
1
γ
−2
+ θ
2
+ Υ
2
2
¶
2
. (2.145)
The radiation is concentrated in a narrow forward cone, defined by θ
2
and of an
order ranging from
¡
γ
−2
+ Υ
2
1
¢
to
¡
γ
−2
+ Υ
2
2
¢
. Therefore, the angular distribution
is confined to a forward cone for which γθ is of the order of the unity. As long as
the cone is completely contained in the second medium, there is no dependence on
the particle incidence angle relative to the boundary. If ω
p,2
¿ ω
p,1
, as it is the case
for a dense material and a gas, the most probable emission angle θ
mp
and the root
mean square angle θ
rms
are given by [Fayard (1988)]:
θ
mp
≈
q
γ
−2
+ Υ
2
2
and
θ
rms
≈
q
γ
−2
+ Υ
2
1
.
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
132 Principles of Radiation Interaction in Matter and Detection
It has to be noted that the distributions given by Eqs. (2.144, 2.145) for an incoming
particle with a Lorentz factor γ and two media with ω
p,1
and ω
p,2
are the same as
for an incoming particle with a Lorentz factor γ
0
and, as media, the vacuum and a
material with plasma frequency ν
0
p,1
= ω
0
p,1
/(2π) where:
ω
0
p,1
=
q
ω
2
p,1
− ω
2
p,2
,
ω
0
p,2
= 0 (i.e., the vacuum, for which we have: Υ
0
2
= 0),
γ
0
=
1
p
γ
−2
+ Υ
2
2
.
We have always γ
0
< γ and ω
0
p,1
< ω
p,1
. Integrating Eq. (2.145) over Ω, we obtain:
dW
d(~ω)
' z
2
α
π
·µ
Υ
2
1
+ Υ
2
2
+ 2γ
−2
Υ
2
1
− Υ
2
2
¶
ln
µ
Υ
2
1
+ γ
−2
Υ
2
2
+ γ
−2
¶
− 2
¸
. (2.146)
In Eq. (2.146), dW /d(~ω) depends on γ/ω only. It can be rewritten introducing γ
0
and Υ
0
1
= ω
0
p,1
/ω, i.e.,
dW
d(~ω)
' z
2
α
π
½·
(ω
p,1
/ω)
2
+ (ω
p,2
/ω)
2
+ 2γ
−2
(ω
p,1
/ω)
2
− (ω
p,2
/ω)
2
¸
ln
·
(ω
p,1
/ω)
2
+ γ
−2
(ω
p,2
/ω)
2
+ γ
−2
¸
− 2
¾
= z
2
α
π
"
(ω
0
p,1
/ω)
2
+ 2γ
0−2
(ω
0
p,1
/ω)
2
#
ln
ω
2
p,1
−ω
2
p,2
ω
2
+
¡
ω
p,2
ω
¢
2
+ γ
−2
γ
0−2
− 2
= z
2
α
π
("
(ω
0
p,1
/ω)
2
+ 2γ
0−2
(ω
0
p,1
/ω)
2
#
ln
"
(ω
0
p,1
/ω)
2
+ γ
0−2
γ
0−2
#
− 2
)
= z
2
α
π
1 + 2
Ã
ω
γ
0
ω
0
p,1
!
2
ln
"
1 +
µ
ω
0
p,1
γ
0
ω
¶
2
#
− 2
= z
2
α
π
·µ
1 + 2
1
γ
02
Υ
02
1
¶
ln
¡
1 + γ
02
Υ
02
1
¢
− 2
¸
= z
2
α
π
G
µ
1
γ
0
Υ
1
¶
, (2.147)
where the function G
³
1
γ
0
Υ
1
´
is given by
G
µ
1
γ
0
Υ
1
¶
=
·µ
1 + 2
1
γ
02
Υ
02
1
¶
ln
¡
1 + γ
02
Υ
02
1
¢
− 2
¸
(2.148)
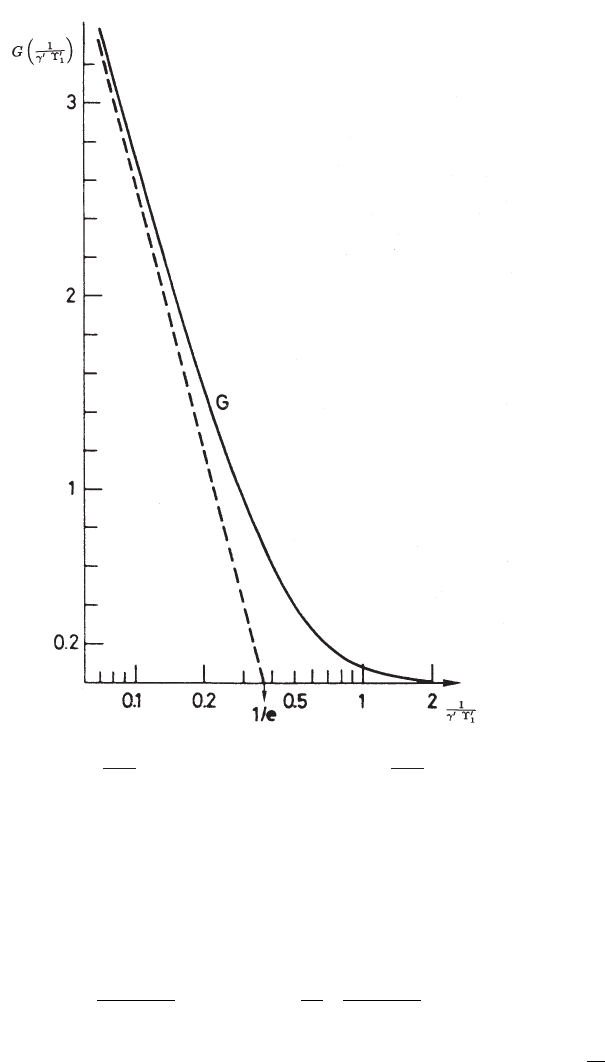
and it is shown in Fig. 2.46. For instance, in the case of ω
p,2
¿ ω
p,1
, we can
distinguish three regimes as a function of γ
(i) γ ¿ ω/ω
p,1
= 1/Υ
1
, i.e., a very low yield for which
dW
d(~ω)
' z
2
α
6π
(γΥ
1
)
4
= z
2
α
6π
³
γω
p,1
ω
´
4
and, in order to have enough yield, it must be noted that there is a frequency cutoff
given by ω ≤ γω
p,1
;
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electromagnetic Interaction of Radiation in Matter 133
(ii) 1/Υ
1
= ω/ω
p,1
¿ γ ¿ ω/ω
p,2
= 1/Υ
2
, where there is a logarithmic increase of
the yield with γ and we have
dW
d(~ω)
' 2z
2
α
π
[ln (γΥ
1
) − 1] = 2z
2
α
π
h
ln
³
γω
p,1
ω
´
− 1
i
;
(iii) γ À ω /ω
p,2
= 1/Υ
2
, in which the yield is almost constant (saturation).
The total energy emitted in forward direction by transition radiation per bound-
ary [Fayard (1988)] is calculated by integrating Eq. (2.146):
W =
Z
∞
0
dW
d(~ω)
d(~ω)
= z
2
γ
α~ω
p,1
3
³
1 −
ω
p,2
ω
p,1
´
2
³
1 +
ω
p,2
ω
p,1
´
= z
2
γ
α~
3
(ω
p,1
− ω
p,2
)
2
ω
p,1
+ ω
p,2
. (2.149)
It has to be noted that the energy emitted by transition radiation has a linear
dependence on the Lorentz factor γ of the incoming particle. This property can be
exploited for detector applications [Dolgoshein (1993)]. In the case of medium to
vacuum transition for which ω
p,1
= ω
p
and ω
p,2
= 0, Eq. (2.146) reduces to:
W = z
2
γα
~ω
p
3
.
Furthermore (see Section 27.7 in [PDB (2008)]), the average number of emitted
photons by transition radiation above a minimal threshold ~ω
0
is given by:
hN
γ
i
~ω>~ω
0
=
Z
∞
~ω
0
1
~ω
dW
d(~ω)
d(~ω)
= z
2
α
π
"
µ
ln
γ~ω
p
~ω
0
− 1
¶
2
+
π
2
12
#
. (2.150)
Thus, the overall number of photons grows as (ln γ)
2
, but it is constant above a fixed
fraction of γ~ω
p
. For instance, over the typical ionization cluster energy between
(2–3) keV in gas detectors, at a lithium to vacuum boundary the number of emitted
transition-radiation photons by an electron
∗
of 5 GeV is hN
γ
i ≈ 5.6 α ≈ 2.6 × 10
−2
with ~ω > 2 keV. The quantum yield is of the order α, whence the necessity of
having a large number of boundary crossings to increase it.
As seen before [i.e., Eq. (2.150)], the photon emission probability from a bound-
ary is quite low, even for low photon detectable energies. Usually, in order to increase
the probability of photon emission, many adjacent thin layers (foils) of material re-
ferred to as radiators are joined together. Thus, the number of surfaces can become
large enough to allow a detectable photon-emission. The limiting factor is the pho-
ton re-absorption inside the radiator itself. The foil thickness cannot be reduced
∗
The Lorentz factor is γ ≈ 10
4
for an electron of ≈ 5 GeV.
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
134 Principles of Radiation Interaction in Matter and Detection
Fig. 2.46 The function G
³
1
γ
0
Υ
0
1
´
from Eq. (2.148) as a function of
1
γ
0
Υ
0
1
(adapted and republished
with permission from Artru, X., Yodh, G.B. and Mennessier, G., Phys. Rev. D 12, 1289 (1975);
Copyright (1975) by the American Physical Society). The dashed line represents 2[ln(γ
0
Υ
0
1
) − 1].
beyond some minimal value, without compromising the creation of the formation
zone. It can be shown that [Artru, Yodh and Mennessier (1975); Fayard (1988)] the
energy emitted per unit of energy and unit of angle in a foil of thickness l
1
is
·
d
2
W
d(~ω) dΩ
¸
foil
= 4 sin
2
³
ϕ
1
2
´
d
2
W
d(~ω) dΩ
, (2.151)
where d
2
W /d(~ω)dΩ is given by Eq. (2.145) for the boundary crossing; 4 sin
2
¡
ϕ
1
2
¢
is the interference term and ϕ
1
= (γ
−2
+ θ
2
+ Υ
2
1
)ωl
1
/(2c). In the relevant region
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electromagnetic Interaction of Radiation in Matter 135
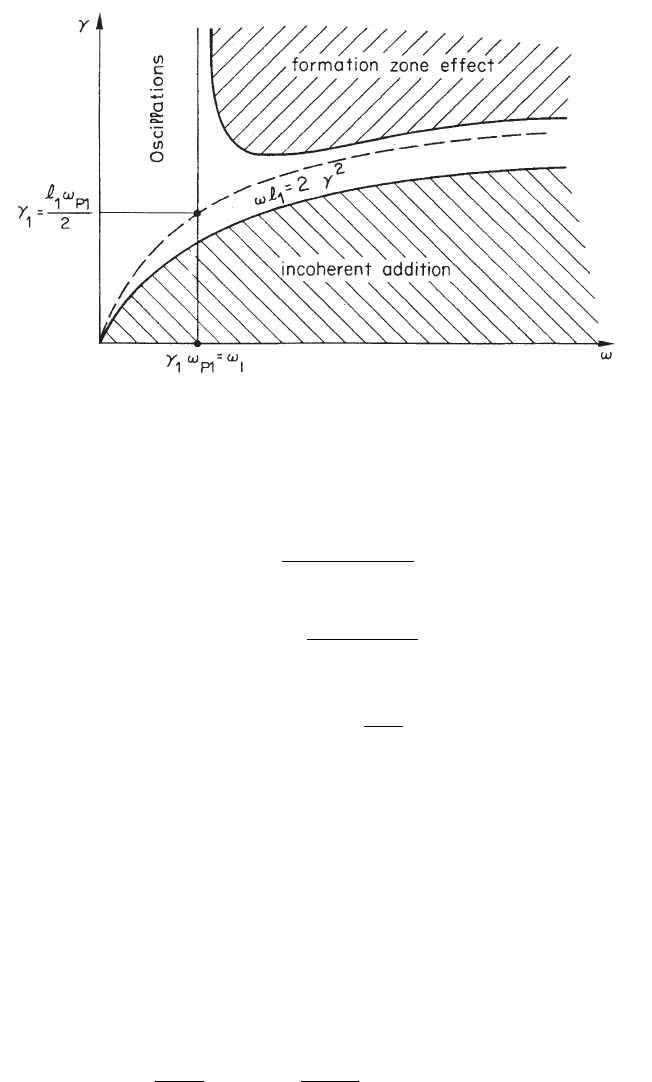
Fig. 2.47 Different regions of the ω–γ plane regarding the single foil yield, in the vacuum case
(adapted and republished with permission from Artru, X., Yodh, G.B. and Mennessier, G., Phys.
Rev. D 12, 1289 (1975); Copyright (1975) by the American Physical Society). In this figure units
are chosen such that c = ~ = 1.
of integration over θ, the values of ϕ
1
are
ϕ
1
≈
¡
γ
−2
+ Υ
2
1
¢
ωl
1
2c
.
Therefore, for thicknesses much lower than Z
1
given by
Z
1
(ω) =
2c
ω(γ
−2
+ Υ
2
1
)
, (2.152)
the yield is strongly reduced. For large ω’s, we have
l
1
≥ Z
1
(ω) ∼
2cγ
2
ω
,
where Z
1
(ω) is referred to as the formation zone and can be understood as the
minimal depth inside a foil required by the electromagnetic field carried by the
incoming charged-particle to reach a new equilibrium state inside the medium. The
ω–γ plane related to the formation zone is shown in Fig. 2.47 [Artru, Yodh and
Mennessier (1975)]. For Lorentz factors larger than γ
1
= ω
p,1
l
1
/(2 c), the frequency
cutoff is no longer γω
p,1
as in the single surface case, but it is rather determined by
the formation-zone effect. The condition to have enough yield becomes:
ω < min(γω
p,1
, ω
1
) (2.153)
where ω
1
= γ
1
ω
p,1
= (ω
2
p,1
l
1
)/(2c) [Artru, Yodh and Mennessier (1975)]. For practi-
cal calculations with l
1
in units of µm and the plasma photon energy hν
p,1
in units
of eV, we have:
γ
1
=
ω
p,1
l
1
2 c
× 10
−4
=
hν
p,1
l
1
2 c~
× 10
−4
≈ 2.5 hν
p,1
l
1
.

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
136 Principles of Radiation Interaction in Matter and Detection
Furthermore, from Eq. (2.142) assuming Z
1
/A
1
≈ 0.5 for a foil with density ρ
1
in
units of g/cm
3
and l
1
in units of µm, we have:
~ω
1
= γ
1
(~ω
p,1
) =
(~ω
p,1
)
2
l
1
2~c
× 10
−4
≈
³
ρ
1
28.8
2
2
´
l
1
4 × 10
−5
× 10
−4
≈ 10
3
× (ρ
1
l
1
) [eV].
Experimentally, the formation zone effect was observed by reducing the foil thickness
to the order of some µm’s.
2.3 Photon Interaction and Absorption in Matter
Ionizing processes embrace fields like nuclear, atomic, solid state, molecular
physics. They affect the kinetic energy of incoming particles. Usually, these par-
ticles are not removed from the incoming beam, except when their kinetic energy
is fully absorbed in matter or (as discussed later) a shower generation process is
initiated.
In sharp contrast with the behavior of charged particles, any beam of monochro-
matic photons traversing an absorber exhibits a characteristic exponential reduction
of the number of its own photons traveling along the original direction. The reason
is that, in processes of photon scattering or absorption, each photon is individually
removed from the incoming beam by the interaction.
Let us consider a monochromatic photon beam of initial intensity
††
I
0
. In ad-
dition, let σ
a,tot
be the total photon atomic cross section for either scattering or
absorbing photons with energy equal to the beam energy. In the passage through a
thickness dx
0
of a medium, the number of removed photons
‡‡
per unit of time −dI
is proportional to the photon beam intensity I
0
at depth x
0
and to the number of
target atoms per unit of volume n
A
of the traversed material, i.e.,
−dI = I
0
P
rem
,
where P
rem
= n
A
σ
a,tot
dx
0
is the probability for a photon removal in the thickness
dx
0
. In addition, we have
−dI = I
0
n
A
σ
a,tot
dx
0
= I
0
µ
att,l
dx
0
.
As a consequence, we obtain
dI
I
0
= −µ
att,l
dx
0
⇒
Z
I
I
0
dI
I
0
=
Z
x
0
−µ
att,l
dx
0
⇒ ln
I
I
0
= −µ
att,l
x
and, finally,
I = I
0
exp [−(µ
att,l
x)] . (2.154)
††
The intensity is given by the number of photons per unit of time impinging onto the absorber
surface.
‡‡
These photons can be fully or partially absorbed so that they have no longer their initial energy
and initial incoming direction.
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electromagnetic Interaction of Radiation in Matter 137
The coefficient
µ
att,l
= n
A
σ
a,tot
[cm
−1
] (2.155)
is the so-called linear attenuation coefficient with σ
a,tot
in cm
2
/atom. In Eq. (2.155),
the number of atoms per cm
3
(n
A
) of the traversed material is given by (ρN)/A
[Eq. (1.39)], where ρ is the material density in g/cm
3
, N is the Avogadro number (see
Appendix A.2), A is atomic weight (see page 14 and Sect. 1.4.1) of the material. In
this section as usual, Z indicates the atomic number (Sect. 3.1) of the material. By
introducing the absorber density ρ, we get:
µ
att,m
=
n
A
ρ
σ
a,tot
[g
−1
cm
2
], (2.156)
i.e., the so-called mass attenuation coefficient. If the absorber is a chemical com-
pound or a mixture, its mass attenuation coefficient µ
att,m
can be calculated from
the mass attenuation coefficients of its constituent elements µ
att,m,i
using the
weighted average
µ
att,m
=
X
i
w
i
µ
att,m,i
,
where w
i
is the proportion by weight of the ith constituent element [Hubbell (1969)].
As mentioned above, the photon interaction on atoms or atomic electrons in
matter results in a change of the incoming photon energy and/or of the scattered-
photon direction. Atomic electrons can be emitted following the full or partial ab-
sorption of the primary photon. Apart resonance effects at frequencies related to
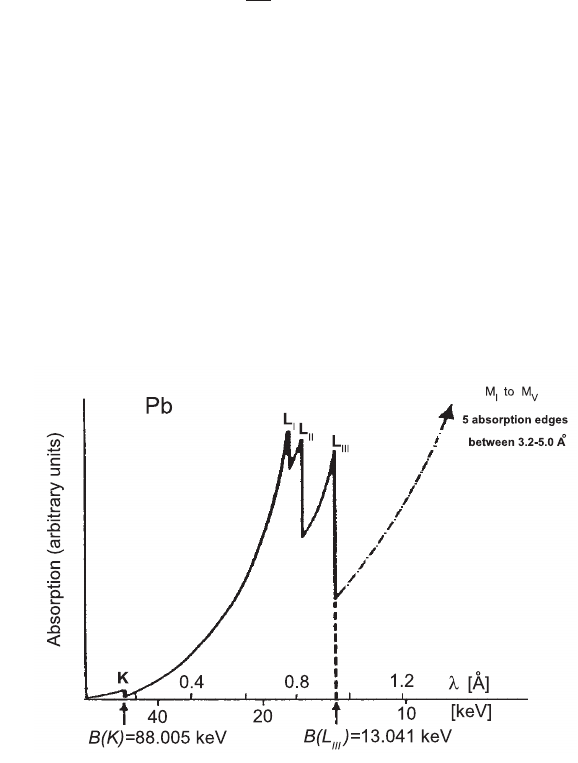
Fig. 2.48 Absorption curve for X-rays in Pb as a function of incident photon wavelength and
energy (see for instance [Marmier and Sheldon (1969)]), showing the characteristic absorption
edges (see Table 2.12).
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
138 Principles of Radiation Interaction in Matter and Detection
atomic or nuclear transitions, the main competing and energy dependent processes
contributing to the total cross section are:
• the photoelectric effect, in which the interaction occurs with the entire
atomic electron cloud and results in the complete absorption of the pri-
mary photon energy;
• Thomson and Compton scattering on atomic electrons at photon energies
so that the electron binding energies can be neglected and electrons can be
treated as quasi-free;
• pair production, in which the photon incoming energy is high enough to
allow the creation of an electron–positron pair in the Coulomb field of an
electron or a nucleus.
The photoelectric process dominates at low energies, i.e., below 50 keV for alu-
minum and 500 keV for lead absorb er. As the energy increases, between 0.05 and
15 MeV for aluminum and between 0.5 and 5 MeV for lead, the main contribution
to the attenuation coefficient comes from Compton scattering. At larger energies,
pair production becomes the dominant mechanism of photon interaction with mat-
ter. The photon can be scattered or absorbed by the nucleus. The photonuclear cross
section is a measurable effect. However, this kind of process is not easily treated
for systematic calculations due to a number of factors. Among these factors, we
have both A and Z, and sensitivity to the isotopic abundance. Reviews of the γ-
ray interaction processes and practical coefficients tables can be found in Chapter 2
of [Marmier and Sheldon (1969)], and [Hubbell (1969); Messel and Crawford (1970);
Hubbell and Seltzer (2004)] and references therein. At present, the tabulations of
mass attenuation coefficients are also available on the web (see Sect. 2.3.5).
2.3.1 The Photoelectric Effect
When the energy hν is larger than the binding energies (B
e
) of atomic electrons,
photons can be completely absorbed in the interaction with an atom, which, in
turn, emits an electron raised into a state of the continuous spectrum. This effect
is called photoelectric effect.
The interaction involves the entire electron cloud, rather than the individual
(corpuscular) electron. Furthermore, the atom as a whole takes up the quite small
recoil energy to preserve the momentum and energy conservation. Thus, the kinetic
energy K
e
of the electron after leaving the atom is determined by the equation:
K
e
= hν − B
e
. (2.157)
Since a free electron cannot absorb a photon, we should expect that the photoelectric
absorption probability is larger for more tightly bound electrons, i.e., for K-shell
electrons. In fact, for incoming photon energies larger than K-shell energies, more
than about 80% of the photoelectric absorption occurs involving the emission of
K-shell electrons (see for instance Chapter V, Section 21 in [Heitler (1954)]). If
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electromagnetic Interaction of Radiation in Matter 139
the photon energy is lower than the binding energy of a shell (see Tables 2.11 and
2.12), an electron cannot be emitted from that shell. Therefore, the absorption
curve exhibits the characteristic absorption edges, whenever the incoming photon
energy coincides with the ionization energy of electrons of K, L, M, . . . shells. In
addition (see Fig. 2.48), the electron shells (except the K-shell) have substructures
with slightly different binding energies, which result in close absorption edges (3 for
the L-shell, 5 for the M-shell, etc).
The binding energy depends on the atomic number Z and the electron shell: it
decreases, as proceeding towards the outer shells, according to these approximate
formulae for the K, L, M shells, respectively:
B
e
(K) ≈ Ry(Z − 1)
2
[eV],
B
e
(L) ≈
1
4
Ry(Z − 5)
2
[eV],
B
e
(M) ≈
1
9
Ry(Z − 13)
2
[eV],
where Ry = 13.61 eV is the Rydberg energy. These formulae are in agreement with
the values given in Tables 2.11 and 2.12 within ± (3–7)% for K-shell absorption
edges and within ±10% for L-shell absorption edges.
An exact theoretical calculation of the photo electric effect presents difficulties
and, thus, empirical formulae are used for computing the total (τ
ph
) and K-shell
cross sections per atom. However, an estimate of the K-shell photoelectric cross
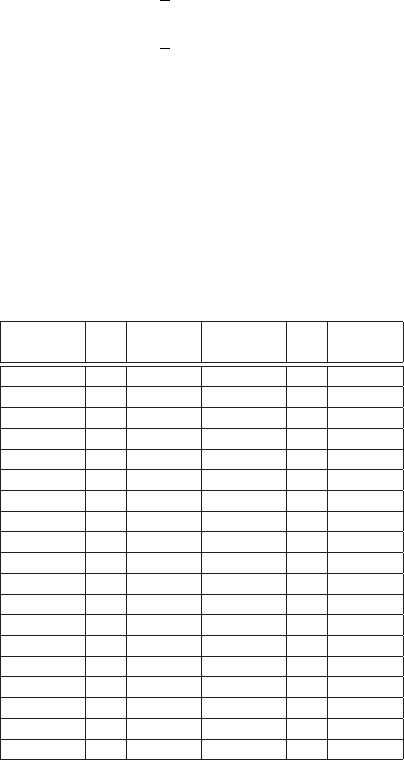
Table 2.11 Energies of the absorption edges above
10 keV for elements with Z up to 68 from [Hubbell
(1969)].
Element Z K-edge Element Z K-edge
keV keV
Ga 31 10.368 Sn 50 29.195
Ge 32 11.104 Sb 51 30.486
As 33 11.865 Te 52 31.811
Se 34 12.654 I 53 33.166
Br 35 13.470 Xe 54 34.590
Kr 36 14.324 Cs 55 35.987
Rb 37 15.202 Ba 56 37.452
Sr 38 16.107 La 57 38.934
Y 39 17.038 Ce 58 40.453
Zr 40 17.999 Pr 59 42.002
Nb 41 18.987 Nd 60 43.574
Mo 42 20.004 Pm 61 45.198
Tc 43 21.047 Sm 62 46.849
Ru 44 22.119 Eu 63 48.519
Rh 45 23.219 Gd 64 50.233
Pd 46 24.348 Tb 65 52.002
Ag 47 25.517 Dy 66 53.793
Cd 48 26.716 Ho 67 55.619
In 49 27.942 Er 68 57.487
