Lin S.D. Water and Wastewater Calculations Manual
Подождите немного. Документ загружается.

Estimation of the expected softener hardness. Since 40 mg/L NaOH
reduces 100 mg/L of the total hardness, the expected total hardness of
at the softener, TH
s
, can be estimated as:
TH
s
⫽ TH
r
– (NaOH
cal
– f ) ⫻ 100/40 (5.94e)
where TH
r
⫽ raw water total hardness, mg/L as CaCO
3
NaOH
cal
⫽ calculated average NaOH dose, mg/L
f ⫽ correction factor, 20 mg/L NaOH
40 ⫽ molecular weight of NaOH, g/mol
100 ⫽ molecular weight of CaCO
3
, g/mol
Example: Given: TH
r
⫽ 250 mg/L as CaCO
3
; NaOH
cal
⫽ 98 mg/L. Determine
the total hardness at the softener, TH
s
.
solution: Using Eq. (5.94e)
TH
s
⫽ TH
r
– (NaOH
cal
– f ) ⫻ 100/40
⫽ 250 mg/L – (98 – 20) mg/L ⫻ 2.5
⫽ 55 mg/L
Estimation of the expected treated water hardness. The expected treated
water hardness, TH
w
, can be estimated by the following formula:
TH
w
⫽ TH
r
– (NaOH
avg
– f ) ⫻100/40 (5.94f)
where NaOH
avg
⫽ calculated average NaOH dose for entire water
flow including bypass and filtration flow rate, mg/L
Others ⫽ as previously mentioned
Example: Given: TH
r
⫽ 250 mg/L as CaCO
3
; NaOH
avg
⫽ 88 mg/L. Determine
the expected total hardness in the treated water, TH
w
.
solution: Using Eq. (5.94f)
TH
w
⫽ TH
r
– (NaOH
avg
– f ) ⫻100/40
⫽ 250 mg/L – (88 – 20) mg/L ⫻ 2.5
⫽ 80 mg/L
NaOH dosing pumps.
The actual delivery rate of a NaOH dosing pump
is estimated as follows:
Pump rate ⫽ maximum pump capacity ⫻ efficiency
⫻ % stroke adjustment (5.94g)
Public Water Supply 415
Example: Given: maximum pump capacity ⫽ 720 L/h, speed effic-
iency ⫽ 0.85, and stroke adjustment ⫽ 50%. Estimate the actual delivery
rate of the NaOH dosing pump.
solution: Using Eq. (5.94g)
Actual pumping rate ⫽ 720 L/h ⫻ 0.85 ⫻ 0.50
⫽ 306 L/h
Sulfuric acid dose rate.
Sulfuric acid is dosed into the treated water to
neutralize the excess of NaOH. The dose rate is calculated as:
H
2
SO
4
dose rate ⫽ acid flow rate ⫻ concentration /
treated water flow rate (5.94h)
Example: Given: required H
2
SO
4
flow rate ⫽ 60 L/h; H
2
SO
4
(50%) concen-
tration ⫽ 690 g/L; and the treated water flow rate ⫽ 1900 m
3
/h. Compute the
dose rate in mg/L of H
2
SO
4
.
solution: Using Eq. (5.94h)
1900 m
3
/h ⫽ 1,900,000 L/h
H
2
SO
4
dose rate ⫽ 60 L/h ⫻ 690 g/L / 1,900,000 L/h
⫽ 0.0218 g/L
⫽ 21.8 mg/L
Seeding sand demand.
A lack of sand supply for crystallization has a
negative impact on the efficiency of the softening (then more NaOH is
needed to get the desired total hardness). This excess dosage of NaOH
to be neutralized triggers an additional sulfuric acid demand.
The seeding sand is added according to the demanded ratio between
effective sand size and pellet size. If the effective sand size or pellet size
is altered, the sand dosing rate also must be altered.
It is essential that very fine, fine, and smaller sand particles should
be removed from sand prior feeding to the reactors. If not, the fine par-
ticles would be flushed out from the reactor due to up to 100 m/h oper-
ation velocity there and enter the rapid filtration units, causing
additional permanent head losses. Those fine particles will not be
removed during the rapid filter backwash sequence from the filter, as
the filter backwash velocity is only 40 to 50 m/h. The separation of the
very fine, fine, and smaller sand particles from large particles is effec-
tive by washing the sand with a velocity of 120 m/h for a set time.
Sand can be fed (must be dry from a silo with free flow) to a reactor
batchwise at a time. The expected sand dosing rates range from 3 to 7 mg
416 Chapter 5
of sand per liter of water treated. Using the pellet-to-sand weight ratio,
the demanded dosage of seeding sand can be calculated as:
Required sand dosage, mg/L ⫽ (TH
r
– TH
w
)/pellet-
to-sand ratio (5.94i)
where TH
r
and TH
w
are stated previously.
Example: Determine the required sand dosage and sand butch per volume
under the following conditions: The total hardness of the raw water and
treated water are respective 250 and 88 mg/L as CaCO
3
; the pellet-to-sand
ratio is 25 to 1. The surface area of the sand washer is A ⫽ 0.2 m
2
. Specific
density of sand ␥ ⫽ 1.55 g/L.
solution: Using Eq. (5.94i)
Step 1. Determine the required sand dosage, using Eq. (5.94i)
Required sand dosage ⫽ (TH
r
⫺ TH
w
)/pellet-to-sand ratio
⫽ (250 mg/L ⫺ 88 mg/L)/25
⫽ 6.48 mg/L
Step 2. Determine sand butch per volume
Sand dosing is effected batchwise with fixed amount of sand per batch.
The height of sand after washing in the sand washer should be measured
frequently.
Washer (nozzle bottom) depth measured from the top ⫽ 235 cm
Sand level after washing measured from the top ⫽173 cm
Difference, D ⫽ 62 cm ⫽ 0.62 m
Volume of washed sand, V ⫽ A ⫻ D ⫽ 0.2 m
2
⫻ 0.62 m ⫽ 0.124 m
3
⫽ 124 L
Weight of sand, W ⫽ V ⫻␥⫽124 L ⫻ 1.55 kg/L ⫽192.2 kg
⫽ 192.2 ⫻ 10
6
mg
Step 3. Calculate the water can be treated with one batch of sand
Effected volume ⫽ W/sand dosage ⫽ 192.2 ⫻ 10
6
mg/6.48 mg/L
⫽ 29.66 ⫻ 10
6
L
⫽ 29,660 m
3
13 Ion Exchange
Ion exchange is a reversible process. Ions of a given species are dis-
placed from an insoluble solid substance (exchange medium) by ions of
another species dissolved in water. In practice, water is passed through
Public Water Supply 417
the exchange medium until the exchange capacity is exhausted and
then it is regenerated. The process can be used to remove color, hard-
ness (calcium and magnesium), iron and manganese, nitrate and other
inorganics, heavy metals, and organics.
Exchange media which exchange cations are called cationic or acid
exchangers, while materials which exchange anions are called anionic
or base exchangers.
Common cation exchangers used in water softening are zeolite, green-
sand, and polystyrene resins. However, most ion exchange media are
currently in use as synthetic materials.
Synthetic ion exchange resins include four general types used in water
treatment. They are strong- and weak-acid cation exchangers and
strong- and weak-base anion exchangers. Examples of exchange reac-
tions as shown below (Schroeder, 1977):
Strong acidic
2R—SO
3
H ⫹ Ca
2⫹
↔ (R—SO
3
)
2
Ca ⫹ 2H
⫹
(5.95)
2R—SO
3
Na ⫹ Ca
2⫹
↔ (R—SO
3
)
2
Ca ⫹ 2Na
⫹
(5.96)
Weak acidic
2R—COOH ⫹ Ca
2⫹
↔ (R—COO)
2
Ca ⫹ 2H
⫹
(5.97)
2R—COONa ⫹ Ca
2⫹
↔ (R—COO)
2
Ca ⫹ 2Na
⫹
(5.98)
Strong-basic
2R—X
3
NOH ⫹ ↔ (R—X
3
N)
2
SO
4
⫹ 2OH
–
(5.99)
2R—X
3
NCl ⫹ ↔ (R—X
3
N)
2
SO
4
⫹ 2Cl
–
(5.100)
Weak-basic
2R—NH
3
OH ⫹ ↔ (R—NH
3
)
2
SO
4
⫹ 2OH
–
(5.101)
2R—NH
3
Cl ⫹ ↔ (R—NH
3
)
2
SO
4
⫹ 2Cl
–
(5.102)
where in each reaction, R is a hydrocarbon polymer and X is a specific
group, such as CH
2
.
The exchange reaction for natural zeolites (Z ) can be written as
(5.103)Na
2
Z 1 •
Ca
21
Ca
21
Mg
21
4 Mg
21
Fe
21
Fe
21
¶Z 1 2Na
1
SO
22
4
SO
22
4
SO
22
4
SO
22
4
418 Chapter 5
In the cation-exchange water softening process, the hardness-causing
elements of calcium and magnesium are removed and replaced with
sodium by a strong-acid cation resion. Ion-exchange reactions for soft-
ening may be expressed as
(5.104)
where R represents the exchange resin. They indicate that when a hard
water containing calcium and magnesium is passed through an ion
exchanger, these metals are taken up by the resin, which simultaneously
gives up sodium in exchange. Sodium is dissolved in water. The normal
rate is 6 to 8 gpm/ft
2
(350 to 470 m/d) of medium.
After a period of operation, the exchanging capacity would be
exhausted. The unit is stopped from operation and regenerated by back-
washing with sodium chloride solution and rinsed. The void volume for
backwash is usually 35% to 45% of the total bed volume.
The exchange capacity of typical resins are in the range of 2 to 10 eq/kg.
Zeolite cation exchangers have the exchange capacity of 0.05 to 0.1 eq/kg
(Tchobanoglous and Schroeder, 1985). During regeneration, the reaction
can be expressed as:
(5.105)
The spherical diameter in commercially available ion exchange resins
is of the order of 0.04 to 1.0 mm. The most common size ranges used in
large treatment plant are 20 to 50 mesh (0.85 to 0.3 mm) and 50 to 100
mesh (0.3 to 0.15 mm) (James M. Montgomery Consulting Engineering,
1985). Details on the particle size and size range, effective size, and
uniform coefficient are generally provided by the manufacturers.
The affinity of exchanges is related to charge and size. The higher the
valence, the greater affinity and the smaller the effective size the greater
the affinity. For a given sense of similar ions, there is a general order of
affinity for the exchanger. For synthetic resin exchangers, relative affini-
ties of common ions increase as shown in Table 5.6.
The design of ion exchange units is based upon ion exchange equilibria.
The generalized reaction equation for the exchange of ions A and on a
cation exchange resin can be expressed as
nR
⫺
A
⫹
⫹ B
n⫹
↔ Rn
⫺
B
n⫹
nA
⫹
(5.106)
where R
⫺
⫽ an anionic group attached to exchange resin
A
⫹
, B
n⫹
⫽ ions in solution
Ca
Mg
fR 1 2NaCl
S
Na
2
R 1
Ca
Mg
fCl
2
Na
2
R 1
Ca
Mg
f
sHCO
3
d
2
SO
4
Cl
2
S
Ca
Mg
fR 1 •
2 NaHCO
3
Na
2
SO
4
2NaCl
Public Water Supply 419

The equilibrium expression for this reaction is
(5.107)
where K
A → B
⫽⫽selectivity coefficient, a function of
ionic strength and is not a true constant
[R
–
A
⫹
], [R
–
B
n⫹
] ⫽ mole fraction of A
⫹
and B
⫹
exchange resin,
respectively, overbars represent the resin
phase, or expressed as and
[A
⫹
], [B
n⫹
] ⫽ concentration of A
⫹
and B
⫹
in solution,
respectively, mol/L
q
A
, q
B
⫽ concentration of A and B on resin site,
respectively, eg/L
C
A
, C
B
⫽ concentration of A and B in solution,
respectively, mg/L
[B][A],
K
B
A
K
A
S
B
5
[R
2
n
B
n1
][A
1
]
n
[R
2
A
1
]
n
[B
n1
]
5
q
B
C
A
q
A
C
B
420 Chapter 5
TABLE 5.6 Selectivity Scale for Cations on Eight Percent Cross-
Linked Strong-Acid Resin and for the Anions on Strong-Base Resins
Cation Selectivity Anion Selectivity
Li
⫹
1.0 HPO
2⫺
4
0.01
H
⫹
1.3 CO
2⫺
3
0.03
Na
⫹
2.0 OH
⫺
(type I) 0.06
UO
2⫹
2.5 F
⫺
0.1
2.6 0.15
K
⫹
2.9 CH
3
COO
⫺
0.2
Rb
⫹
3.2 0.4
Cs
⫹
3.3 OH
⫺
(type II) 0.65
Mg
2⫹
3.3 1.0
Zn
2⫹
3.5 Cl
⫺
1.0
Co
2⫹
3.7 CN
⫺
1.3
Cu
2⫹
3.8 1.3
3.9 1.6
Ni
2⫹
3.9 Br
⫺
3
Be
2⫹
4.0 4
Mn
2⫹
4.1 I
⫺
8
Pb
2⫹
5.0 9.1
Ca
2⫹
5.2 17
Sr
2⫹
6.5 100
Ag
⫹
8.5
Pb
2⫹
9.9
Ba
2⫹
11.5
Ra
2⫹
13.0
SOURCES: James M. Montgomery Consulting Engineering (1985),
Clifford (1990)
CrO
22
4
SeO
22
4
SO
22
4
NO
2
3
HSO
2
4
Cd
21
NO
2
2
BrO
2
3
HCO
2
3
SO
22
4
NH
1
4

The selectivity constant depends upon the valence, nature, and con-
centration of the ion in solution. It is generally determined in labora-
tory of specific conditions measured.
For monovalent/monovalent ion exchange process such as
The equilibrium expression is (by Eq. (5.107))
(5.108)
and for divalent/divalent ion exchange processes such as
then using Eq. (5.107)
(5.109)
Anderson (1975) rearranged Eq. (5.107) using a monovalent/monovalent
exchange reaction with concentration units to equivalent fraction as
follows:
1. In the solution phase: Let C ⫽ total ionic concentration of the solu-
tion, eq/L. The equivalent ionic fraction of ions A
⫹
and B
⫹
in solu-
tion will be
(5.110)
(5.111)
then
or (5.112)
2. In the resin phase: Let ⫽ total exchange capacity of the resin per
unit volume, eq/L. Then we get
(5.113)
⫽ equivalent fraction of the A
⫹
ion in the resin
and
(5.114)X
B
1
5 [R
2
B
1
]/C or [R
2
B
1
] 5 C X
B
1
X
A
1
5 [R
2
A
1
]/C or [R
2
A
1
] 5 C X
A
1
C
[A
1
] 5 1 2 [B
1
]
[A
1
] 1 [B
1
] 5 1
X
B
1
5 [B
1
]>C or [B
1
] 5 CX
B
1
X
A
1
5 [A
1
]>C or [A
1
] 5 CX
A
1
K
NaR
S
CaR
5
[Ca
21
R][Na
1
]
2
[Na
1
R]
2
[Ca
21
]
5
q
Ca
C
2
Na
q
2
Na
C
Ca
2sNa
1
R
2
d 1 Ca
21
4
Ca
21
R
22
1 2Na
1
K
HR
S
NaR
5
[Na
1
R
2
][H
1
]
[H
1
R
2
][Na
1
]
5
q
Na
C
H
q
H
C
Na
H
1
R
2
1 Na
1
4 Na
1
R
2
1 H
1
Public Water Supply 421

also
or
(5.115)
Substitute Eqs. (5.110), (5.111), (5.113), and (5.114) into Eq. (5.107)
which yields
or
(5.116)
Substituting for Eqs. (5.112) and (5.115) in Eq. (5.116) gives
(5.117)
If the valence is n, Eq. (5.117) will become
(5.118)
Example 1: Determine the meq/L of Ca
2⫹
if Ca
2⫹
concentration is 88 mg/L
in water.
solution:
Step 1. Determine equivalent weight (EW)
EW ⫽ molecular weight/electrical charge
⫽ 40/2
⫽ 20 g/equivalent weight (or mg/meq)
Step 2. Compute meq/L
meq/L ⫽ (mg/L)/EW
⫽ (88 mg/L)/(20 mg/meq)
⫽ 4.4
Example 2: A strong-base anion exchange resin is used to remove nitrate ions
from well water which contain high chloride concentration. Normally bicar-
bonate and sulfate is presented in water (assume they are negligible). The total
X
B
n1
s1 2 X
B
n1
d
n
5 K
B
A
a
C
C
b
n21
X
B
n1
s1 2 X
B
n1
d
n
X
B
1
1 2 X
B
1
5 K
B
A
X
B
1
1 2 X
B
1
X
B
1
X
A
1
5 K
B
A
X
B
1
X
A
1
K
B
A
5
sCX
B
1
dsCX
A
1
d
sCX
A
1
dsCX
B
1
d
K
B
A
5
[CX
B
1
][CX
A
1
]
[CX
A
1
][CX
B
1
]
X
A
1
5 1 2 X
B
1
X
A
1
1 X
B
1
5 1
422 Chapter 5
resin capacity is 1.5 eq/L. Find the maximum volume of water that can be
treated per liter of resin. The water has the following composition in meq/L:
Ca
2⫹
⫽ 1.4 CI
⫺
⫽ 3.0
Mg
2⫹
⫽ 0.8 ⫽ 0.0
Na
⫹
⫽ 2.6 ⫽ 1.8
Total cations ⫽ 4.8 Total anions ⫽ 4.8
solution:
Step 1. Determine the equivalent fraction of nitrate in solution
Step 2. Determine selective coefficient for sodium over chloride from Table 5.5
Step 3. Compute the theoretical resin available for nitrate ion by Eq. (5.117)
It means that 71% of resin sites will be used
Step 4. Compute the maximum useful capacity Y
Step 5. Compute the volume of water (V ) that can be treated per cycle
Example 3: A strong-acid cation exchanger is employed to remove calcium
hardness from water. Its wet-volume capacity is 2.0 eq/L in the sodium form.
If calcium concentrations in the influent and effluent are 44 mg/L (2.2 meq/L)
and 0.44 MG/l, respectively, find the equivalent weight (meq/L) of the com-
ponent in the water if given the following:
5 4429 gal of water/ft
3
of resin
5 592
L
L
3
1 gal
3.785 L
3
28.32 L
1 ft
3
5 592 L of water/L of resin
V 5
1065 meq/L of resin
1.8 meq/L of water
Y 5 1.5 eq/L 3 0.71 5 1.065 eq/L 5 1065 meq/L
X
NO
2
3
5 0.71
X
NO
2
3
1 2 X
NO
2
3
5 4 3
0.38
1 2 0.38
5 2.45
K
NO
3
Cl
5 4/1 5 4
X
NO
2
3
5 1.8/4.8 5 0.38
NO
2
3
SO
22
4
Public Water Supply 423
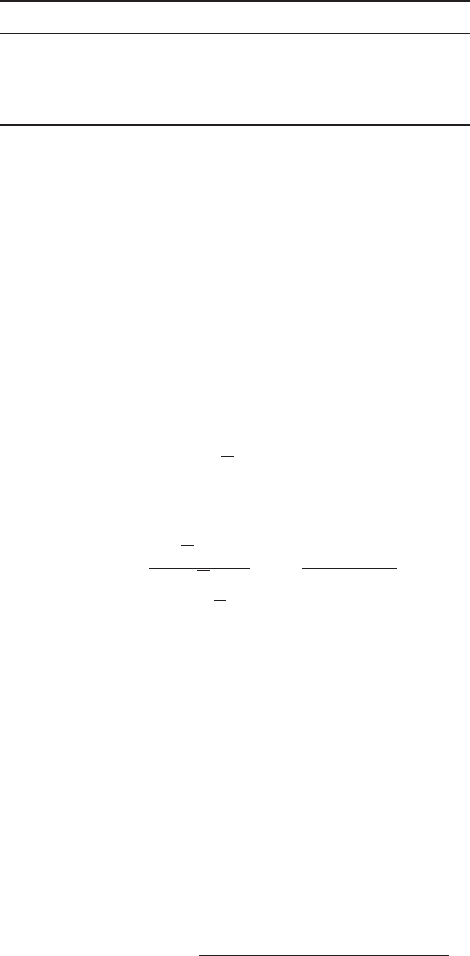
Cations Anions
Ca
2⫹
2.2 2.9
Mg
2⫹
1.0 Cl
–
3.1
Na
⫹
3.0 0.2
Total cations 6.2 Total anions 6.2
solution:
Step 1. Determine the equivalent fraction of Ca
2⫹
Step 2. Find the selectivity coefficient K for calcium over sodium from
Table 5.5
Step 3. Compute the theoretical resin composition with respect to the cal-
cium ion
Using Eq. (5.118), n ⫽ 2
This means that a maximum of 51% of the resin sites can be used with cal-
cium ions from the given water. At this point, the water and resin are at
equilibrium with each other.
Step 4. Compute the limiting useful capacity of the resin (Y )
Step 5. Compute the maximum volume (V ) of water that can be treated
per cycle
5 3472 gal of water/ft
3
of resin
5 464 L of water/L of resin
V 5
1020 meq/L of resin sfor Cad
2.2 meq/L of calcium in water
5 1.02 eq/L sor 1020 meq/Ld
Y 5 2.0 eq/L 3 0.51
X
Ca
21
5 0.51
X
Ca
21
s1 2 X
Ca
21
d
2
5 2.6
0.35
s1 2 0.35d
2
5 2.15
C
/C 5 6.2/6.2 5 1.0
K
Ca
Na
5 5.2/2.0 5 2.6
X
Ca
21
5 2.2/6.2 5 0.35
SO
2
4
HCO
2
3
424 Chapter 5
