Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

ZINC-CARBON BATTERIES 8.39
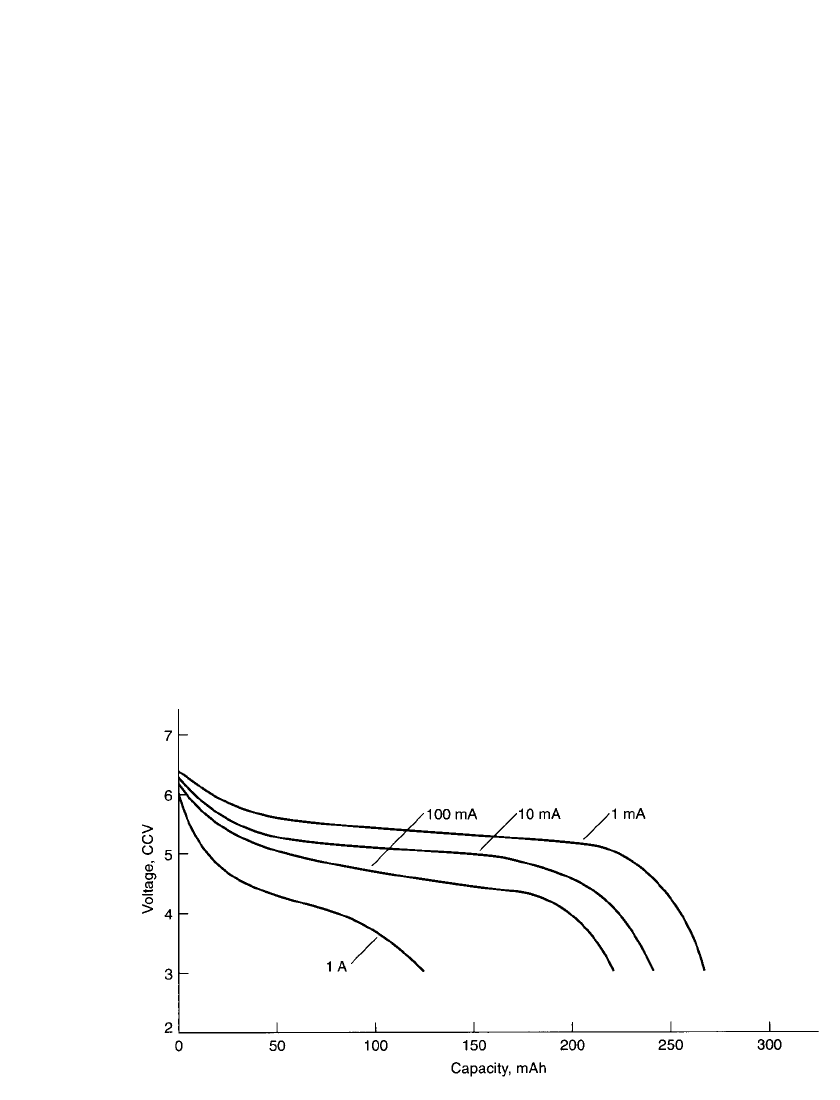
FIGURE 8.33 Polaroid P-80; battery voltage profile at various discharge loads.
zinc particle size and amount of hydrogen present. The polarization effect occurs when
the load is held for some time period, as in the case of charging the camera strobe
circuit. Total resistance is then a summation of the two resistances, that is, the internal
resistance of the battery and the resistance due to polarization effect, the latter being very
time sensitive. To minimize polarization effect resistance, the pulse period for
⌬V mea-
surements was minimized.
The 56-day point is of interest, as that is the normal age when the battery is released for
assembly into film packs. At that time, every battery is measured for electrical characteristics
and defective ones are screened.
The total internal resistance is expressed by the following:
R
⫽ R ⫹ R
tip
where: R
i
⫽ battery internal resistance, ⍀
R
p
⫽ polarization resistance effect, ⍀
For the P-80 battery, R
i
was 0.50 ⍀ and the R
p
was 0.12 ⍀.
•
Capacity: The capacity simulator mimics the energy used to charge the camera strobe. The
pulse consists of an open circuit voltage at rest, followed by a pulse at a 2-A load to result
in a 50 watt-second (50 Ws) pulse. The 50 Ws cycle test is maintained until the final CCV
reaches the 3.7 cut-off voltage, where the number of cycles is determined.
During the time while the 50 Ws load is maintained, the polarization drop occurs. The
time to produce the 50 Ws increases with each cycle as the battery is ‘‘consumed.’’ A 30-s
rest between cycles is used. Initially, the voltage drop is fairly constant; however, near the
end of the test, the resistance increases. The test is maintained to 3.7 V to indicate the cut-
off point of the camera.
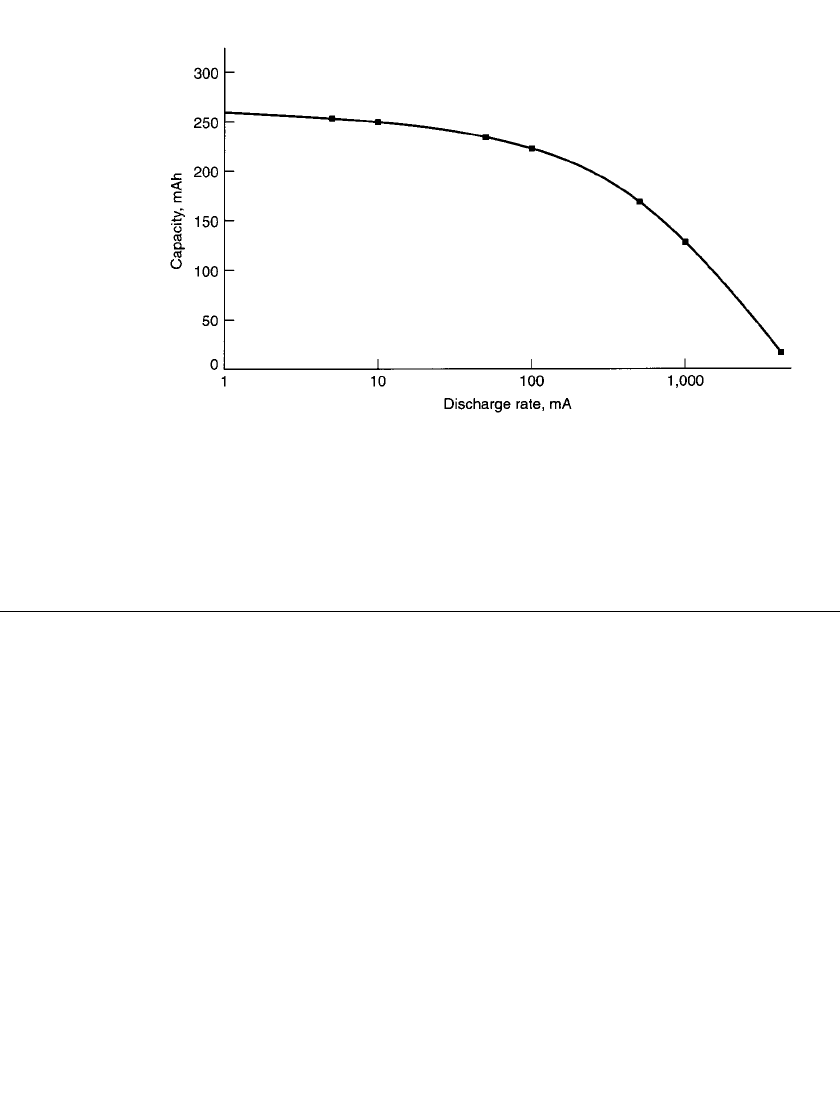
Figure 8.33 illustrates the voltage profile at different discharge loads, and Figure 8.34
shows rate sensitivity of the batteries described above versus capacity.
8.40 CHAPTER EIGHT
FIGURE 8.34 Polaroid P-80 battery; rate sensitivity vs capacity (to 3.0-V cutoff).
8.8 TYPES AND SIZES OF AVAILABLE CELLS AND BATTERIES
Zinc-carbon batteries are made in a number of different sizes with different formulations to
meet a variety of applications. The single-cell and multicell batteries are classified by elec-
trochemical system, either Leclanche´ or zinc chloride, and by grade; general purpose, heavy
duty, extra heavy duty, photoflash, and so on. These grades are assigned according to their
output performance under specific discharge conditions.
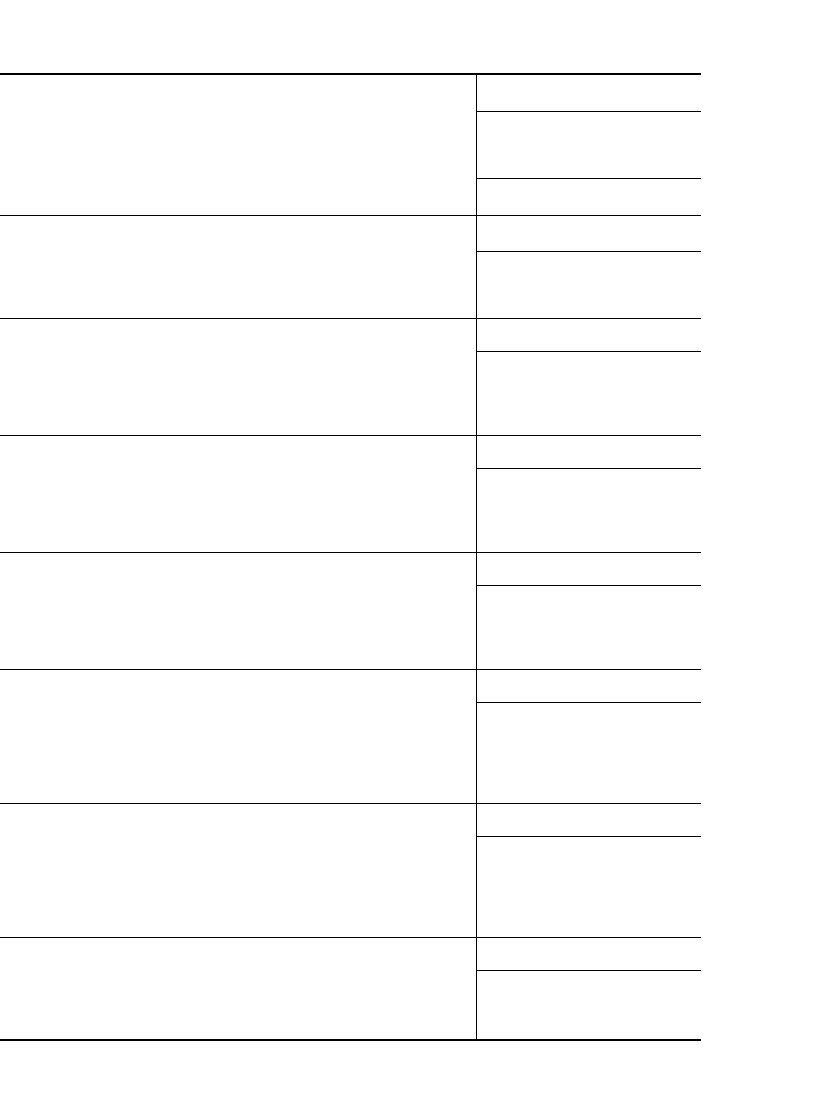
Table 8.9 lists the more popular battery sizes with typical performance at various loads
under a two-hour per day intermittent discharge, except for the continuous toy battery test.
The performance of these batteries, under several intermittent discharge conditions, is given
in Table 8.10.
The AA-size battery is becoming the predominant one and is used in penlights, photoflash
and electronic applications. The smaller AAA-size is used in remote control devices and
other small electronic applications. The C and D-size batteries are used mainly in flashlight
applications and the F-size is usually assembled into multicell batteries for lanterns and other
applications requiring these large batteries. Flat cell are used in battery assemblies, in par-
ticular, the 9-volt battery used in smoke detectors and electronic applications.
Table 8.11 lists some of the major multicell zinc-carbon batteries that are available com-
mercially. The performance of these batteries can be estimated by using the IEC designation
to determine the cell compliment (e.g. NEDA 6, IEC 4R25 battery consists of four F-size
cells connected in series). Table 8.12 gives cross-references to the zinc-carbon batteries and
manufacturer’s designations. The most recent manufacturer’s catalogs should be consulted
for specific performance data to determine the suitability of their product for a particular
application.

ZINC-CARBON BATTERIES 8.41
TABLE 8.9 Characteristics of Zinc-Carbon Batteries
Size IEC
ANSI,
NEDA
Weight
g
Maximum
dimensions,
mm
Diameter Height
Typical service, 2 h/ d*
Leclanche´
Drain
mA
Service
h
Zinc-chloride
Drain
mA
Service
h
N R1 910 6.2 12 30.2 1 480
10
15
45
20
AAA R03 24 8.5 10.5 44.5 1
10
20
—
—
—
1
10
20
520
55
26
AA R6 15 15 14.5 50.5 1
10
100
300
950
80
4
0.6
1
10
100
300
1200
110
8
1
C R14 14 41 26.2 50 5
25
100
300
380
75
6
1.7
5
20
100
300
800
150
20
5.5
D R20 13 90 34.2 61.5 10
50
100
500
400
70
25
3
10
50
100
500
700
135
55
6
F R25 60 160 34† 92† 25
100
500
300
60
5.5
25
100
500
400
85
9
G R26 — 180 32† 105† —
No. 6 R40 905 900 67 170.7 5
50
100
500
8000
700
350
70
*Typical values of service to 0.9-V cutoff.
†Typical values.
8.42 CHAPTER EIGHT
TABLE 8.10 ANSI Standards for Zinc-Carbon and Alkaline-Manganese Dioxide Batteries
Size Use Ohms Schedule
Cutoff
voltage
Specification requirements
Zinc-carbon
batteries
Initial*
Alkaline-
manganese
dioxide batteries
Initial*
N 910D 910A
Portable lighting
Pager
then
5.1
(10.0
3000.0
5 min/d
5 sec/hr,
3595 sec /h)
0.9
0.9
NA
NA
100 min
888 hr
AAA 24D 24A
Pulse test
Portable lighting
Recorder
Radio
3.6
5.1
10.0
75.0
15 sec /min 24 hr/ d
4 min/hr 8 hr/d
1 hr/d
4 hr/d
0.9
0.9
0.9
0.9
150 pulses
48.0 min
1.5 hr
24.0 hr
450 pulses
130.0 min
5.5 hr
48.0 hr
AA 15D 15A
Pulse test
Motor/ toy
Recorder
Radio
1.8
3.9
10.0
43.0
15 sec /min 24 hr/ d
1 hr/d
1 hr/d
4 hr/d
0.9
0.8
0.9
0.9
100 pulses
1.2 hr
5.0 hr
27.0 hr
450 pulses
5hr
13.5 hr
60 hr
C 14D 14A
Portable lighting
Toy
Recorder
Radio
3.9
3.9
6.8
20.0
4 min/hr 8 hr/d
1 hr/d
1 hr/d
4 hr/d
0.9
0.8
0.9
0.9
350 min
5.5 hr
10.0 hr
30 hr
830 min
14.5 hr
24.0 hr
60.0 hr
D 13D 13A
Portable lighting
Portable lighting
Motor/ toy
Recorder
Radio
1.5
2.2
2.2
3.9
10.0
4 min/15 min 8 hr/d
4 min/hr 8 hr/d
1 hr/d
1 hr/d
4 hr/d
0.9
0.9
0.8
0.9
0.9
150 min
120 min
5.5 hr
10 hr
33 hr
540 min
950 min
17.5 hr
26.0 hr
90.0 hr
9 Volt 1604D 1604A
Calculator
Toy
Radio
Electronic
180
270
620
1300
30 min /d
1 hr/d
2 hr/d
24 hr /d
4.8
5.4
5.4
6.0
380 min
7hr
23 hr
NA
630 min
14 hr
38 hr
NA
Smoke detector Currently under consideration.
6 Volt 908D 908A
Portable lighting
Portable lighting
Barricade
3.9
3.9
6.8
4 min/hr 8 hr/d
1 hr/d
1 hr/d
3.6
3.6
3.6
5hr
50 hr
165 hr
21 hr
80 hr
300 hr
* Performance after 12 month storage
zinc-carbon batteries: 80% of initial requirement
alkaline-manganese dioxide batteries: 90% of initial requirement
Source: ANSI C18.1M-1999.
11

ZINC-CARBON BATTERIES 8.43
TABLE 8.11 ANSI / NEDA Dimensions of Zinc-Carbon Batteries*
ANSI IEC
Diameter,
mm
Max Min
Overall height,
mm
Max Min
Length,
mm
Max Min
Width,
mm
Max Min
13C R20S 34.2 32.3 61.5 59.5
13CD R20C 34.2 32.3 61.5 59.5
13D R20C 34.2 32.3 61.5 59.5
13F R20S 34.2 32.3 61.5 59.5
14C R14S 26.2 24.9 50.0 48.5
14CD R14C 26.2 24.9 50.0 48.5
14D R14C 26.2 24.9 50.0 48.5
14F R14S 26.2 24.9 50.0 48.5
15C R6S 14.5 13.5 50.5 49.2
15CD R6C 14.5 13.5 50.5 49.2
15D R6C 14.5 13.5 50.5 49.2
15F R6S 14.5 13.5 50.5 49.2
24D R03 10.5 9.5 44.5 43.3
903 — 163.5 158.8 185.7 181.0 103.2 100.0
904 — 163.5 158.8 217.9 214.7 103.2 100.0
908 4R25X 115.0 107.0 68.2 65.0 68.2 65.0
908C 4R25X 115.0 107.0 68.2 65.0 68.2 65.0
908CD 4R25X 115.0 107.0 68.2 65.0 68.2 65.0
908D 4R25X 115.0 107.0 68.2 65.0 68.2 65.0
915 4R25Y 112.0 107.0 68.2 65.0 68.2 65.0
915C 4R25Y 112.0 107.0 68.2 65.0 68.2 65.0
915D 4R25Y 112.0 107.0 68.2 65.0 68.2 65.0
918 4R25-2 127.0 — 136.5 132.5 73.0 69.0
918D 4R25-2 127.0 — 136.5 132.5 73.0 69.0
926 — 125.4 122.2 136.5 132.5 73.0 69.0
1604 6F22 48.5 46.5 26.5 24.5 17.5 15.5
1604C 6F22 48.5 46.5 26.5 24.5 17.5 15.5
1604CD 6F22 48.5 46.5 26.5 24.5 17.5 15.5
1604D 6F22 48.5 46.5 26.5 24.5 17.5 15.5
Source: ANSI C18.1M-1999.
11

8.44 CHAPTER EIGHT
TABLE 8.12 Cross-Reference of Zinc-Carbon Batteries
ANSI IEC Duracell Eveready Ray-O-Vac Panasonic Toshiba Varta Military
13C R20 M13SHD EV50 GP-D — — — —
13CD R20 M13SHD EV150 HD-D UM1D — — —
13D R20 M13SHD 1250 6D UMIN R20U 3020 —
13F R20 — 950 2D UM1 R20S 2020 BA-30/ U
14C R14 M14SHD EV35 GP-C — — — —
14CD R14 M14SHD EV135 HD-C UM2D — — —
14D R14 — 1235 4C UM2N R14U 3014 —
14F R14 — 935 1C UM2 R14S 2014 BA-42 / U
15C R6 M15SHD EV15 GP-AA — — — —
15CD R6 M15SHD EV115 HD-AA UM3D — — —
15D R6 M15SHD 1215 5AA UM3N R6U 3006 —
15F R6 — 1015 7AA UM3 R6S 2006 BA-58 / U
24D R03 — 1212 — UM4N — — —
24F R03 — — — — — — —
210 20F20 — 413 — — — — BA-305 / U
215 15F20 — 412 — 15 — V72PX BA-261 / U
220 10F15 — 504 — W10E — V74PX BA-332 / U
221 15F15 — 505 — MV15E — — —
900 R25-4 — 735 900 — — — —
903 5R25-4 — 715 903 — — — BA-804/ U
904 6R25-4 — 716 904 — — — BA-207/ U
905 R40 — EV6 — — — — BA-23
906 R40 — EV6 — — — — BA-23
907 4R25-4 — 1461 641 — — — BA-429 / U
908 4R25 M908 509 941 4F — — BA-200/ U
908C 4R25 M908SHD EV90 GP-6V — — 430 —
908CD 4R25 M908SHD EV90HP — — — 431 —
908D 4R25 M908SHD 1209 944 — — 430 —
915 4R25 M915 510S 942 — — — BA-803/ U
915C 4R25 M915SHD EV10S — — — — —
915D 4R25 M915SHD — 945 — — — —
918 4R25-2 — 731 918 — — — —
918C 4R25-2 — EV31 — — — — —
918D 4R25-2 — 1231 928 — — — —
922 — — 1463 922 — — — —
926 8R25-2 — 732 926 — — — —
1604 6F22 — 216 1604 006P — 2022 BA-90/ U
1604C 6F22 M9VSHD EV22 GP-9V — — — —
1604CD 6F22 M9VSHD EV122 HD-9V 006PD — — —
1604D 6F22 M9VSHD 1222 D1604 006PN 6F22U 3022 —
Source: Manufacturers’ catalogs.

ZINC-CARBON BATTERIES 8.45
REFERENCES
1. Frost and Sullivan Inc., U.S. Battery Market, New York, N.Y. 1997.
2. The Freedonia Group, Inc., Industry Study #1193, Primary and Secondary Batteries, Cleveland,
Ohio, December 1999.
3. Samuel Rubin, The Evolution of Electric Batteries in Response to Industrial Needs, Dorrance, Phil-
adelphia, 1978, chap. 5.
4. George Vinal, Primary Batteries, Wiley, New York, 1950.
5. N. C. Cahoon, in N. C. Cahoon and G. W. Heise (eds.), The Primary Battery, vol. 2, chap. 1, Wiley,
New York, 1976.
6. Richard Huber, in K. V. Kordesh (ed), Batteries, vol. 1, chap. 1, Decker, New York, 1974.
7. A. Kozawa and R. A. Powers, ‘‘Electrochemical Reactions in Batteries,’’ J. Chem. Ed. 49:587 (1972).
8. R. J. Brodd, A. Kozawa, and K. V. Kordesh ‘‘Primary Batteries 1951–1976,’’ J. Electrochem. Soc.
125(7) (1978).
9. M. Bregazzi, Electrochem. Technol., 5:507 (1967).
10. C. L. Mantell, Batteries and Energy Systems, 2d ed., McGraw-Hill, New York, 1983.
11. ‘‘American National Standards Specification for Dry Cells and Batteries.’’ ANSI C18.1M-1999,
American National Standards Institute, Inc., January 1999.
12. Eveready Battery Engineering Data; Information is available via the Internet at, www.Energizer.com;
Technical information website. This data is frequently updated and current.
13. M. Dentch and A. Hillier, Polaroid Corp., Progress in Batteries and Solar Cells, vol. 9 (1990).

9.1
TABLE 9.1 Major Advantages and Disadvantages of Magnesium Batteries
Advantages Disadvantages
Good capacity retention, even under
high-temperature storage
Twice the capacity of corresponding
Leclanche´ batteries
Higher battery voltage than zinc-carbon
batteries
Competitive cost
Delayed action (voltage delay)
Evolution of hydrogen during
discharge
Heat generated during use
Poor storage after partial dis-
charge
CHAPTER 9
MAGNESIUM AND ALUMINUM
BATTERIES
Patrick J. Spellman, Duane M. Larsen, Ron J. Ekern,
and James E. Oxley
9.1 GENERAL CHARACTERISTICS
Magnesium and aluminum are attractive candidates for use as anode materials in primary
batteries.* As shown in Table 1.1, Chap. 1, they have a high standard potential. Their low
atomic weight and multivalence change result in a high electrochemical equivalence on both
a gravimetric and a volumetric basis. Further, they are both abundant and relatively inex-
pensive.
Magnesium has been used successfully in a magnesium /manganese dioxide (Mg /MnO
2
)
battery. This battery has two main advantages over the zinc-carbon battery, namely, twice
the service life or capacity of the zinc battery of equivalent size and the ability to retain this
capacity, during storage, even at elevated temperatures (Table 9.1). This excellent storability
is due to a protective film that forms on the surface of the magnesium anode.
*The use of magnesium and aluminum in reserve and mechanically rechargeable batteries
is covered in Chaps. 17 and 38.
9.2 CHAPTER NINE
Several disadvantages of the magnesium battery are its ‘‘voltage delay’’ and the parasitic
corrosion of magnesium that occurs during the discharge once the protective film has been
removed, generating hydrogen and heat. The magnesium battery also loses its excellent
storability after being partially discharged and, hence, is unsatisfactory for long-term inter-
mittent use. For these reasons, the active (nonreserve) magnesium battery, while used suc-
cessfully in military applications, such as radio transceivers and emergency or standby equip-
ment, has not found wide commercial acceptance.
Furthermore the use of this magnesium battery is declining significantly, as the present
trend is towards the use of lithium primary and lithium-ion rechargeable batteries.
Aluminum has not been used successfully in an active primary battery despite its potential
advantages. Like magnesium, a protective film forms on the aluminum, which is detrimental
to battery performance, resulting in a battery voltage that is considerably below theoretical
and causing a voltage delay that can be significant for partially discharged batteries or those
that have been stored. While the protective oxide film can be removed by using suitable
electrolytes or by amalgamation, gains by such means are accompanied by accelerated cor-
rosion and poor shelf life. Aluminum, however, has been more successfully used as an anode
in aluminum /air batteries. (See chapter 38)
9.2 CHEMISTRY
The magnesium primary battery uses a magnesium alloy for the anode, manganese dioxide
as the active cathode material but mixed with acetylene black to provide conductivity, and
an aqueous electrolyte consisting of magnesium perchlorate, with barium and lithium chro-
mate as corrosion inhibitors and magnesium hydroxide as a buffering agent to improve
storability (pH of about 8.5). The amount of water is critical as water participates in the
anode reaction and is consumed during the discharge.
1
The discharge reactions of the magnesium/manganese dioxide battery are
⫺
Anode
Mg
⫹ 2OH ⫽ Mg(OH) ⫹ 2e
2
⫺
Cathode
2MnO
⫹ HO⫹ 2e ⫽ Mn O ⫹ 2OH
22 23
Overall
Mg
⫹ 2MnO ⫹ HO⫽ Mn O ⫹ Mg(OH)
22 23 2
The theoretical potential of the battery is greater than 2.8 V, but this voltage is not realized
in practice. The observed values are decreased by about 1.1 V, giving an open-circuit voltage
of 1.9–2.0 V, still higher than for the zinc-carbon battery.
The rest potential of magnesium in neutral and alkaline electrolytes is a mixed potential,
determined by the anodic oxidation of magnesium and the cathodic evolution of hydrogen.
The kinetics of both of these reactions are strongly modified by the properties of the passive
film, its history of formation, prior anodic (and to a limited extent cathodic) reactions, the
electrolyte environment, and magnesium alloying additions. The key to a full appreciation
of the magnesium electrode lies in an understanding of the predominantly Mg(OH)
2
film,
2
the factors which govern its formation and dissolution, as well as the physical and chemical
properties of the film.
The corrosion of magnesium under storage conditions is slight. A film of Mg(OH)
2
that
forms on the magnesium provides good protection, and treatment with chromate inhibitors
increases this protection. As a result of the formation of this tightly adherent and passivating
oxide or hydroxide film on the electrode surface, magnesium is one of the most electropos-
itive metals to find use in aqueous primary batteries. However, when the protective film is
broken or removed during discharge, corrosion occurs with the generation of hydrogen,
Mg
⫹ 2H O → Mg(OH) ⫹ H
222
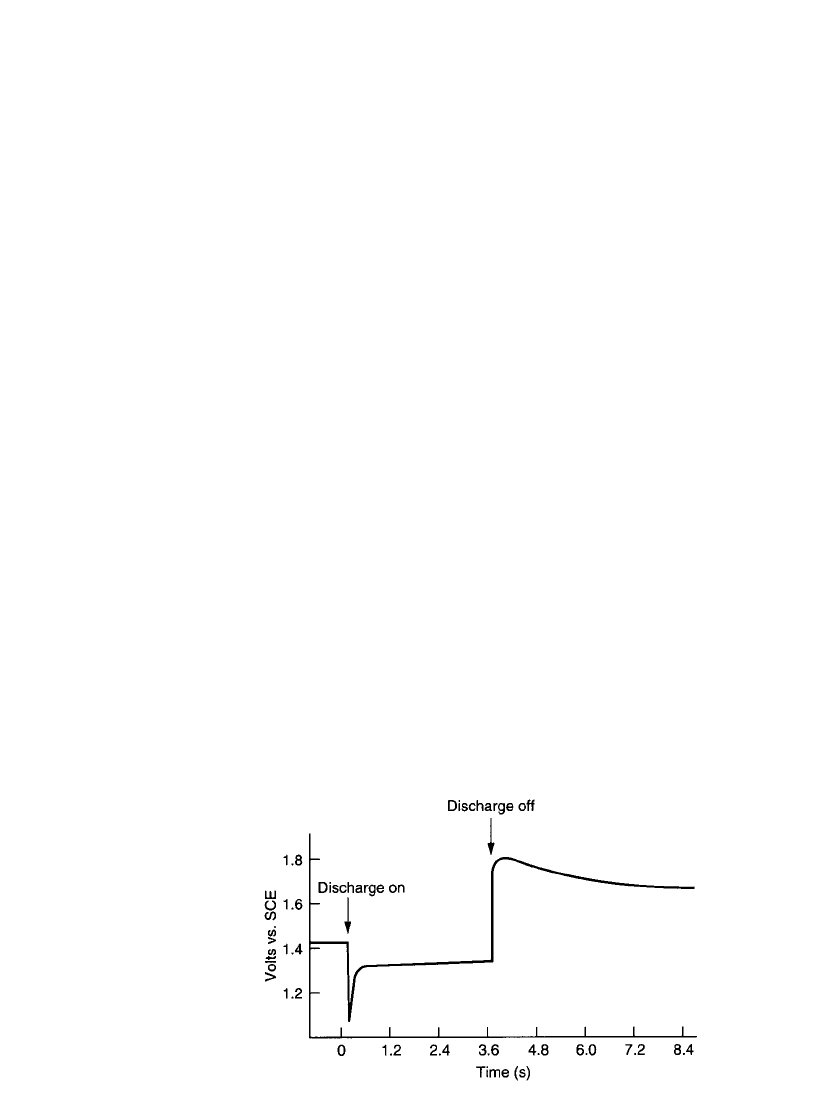
MAGNESIUM AND ALUMINUM BATTERIES 9.3
FIGURE 9.1 Voltage profile of magnesium primary battery at
20⬚C.
During the anodic oxidation of magnesium, the rate of hydrogen evolution increases with
increasing current density due to destruction of the passive film, which exposes more (ca-
thodic) sites on the bared magnesium surface. This phenomenon has often been referred to
as the ‘‘negative difference effect.’’ An appreciable rate of anodic oxidation of magnesium
can only take place on the bare metal surface. Magnesium salts generally exhibit low levels
of anion conductivity, and one could theoretically invoke a mechanism wherein OH
⫺
ions
migrate through the film to form reaction product Mg(OH)
2
at the magnesium-film interface.
In practice this does not occur at a sufficiently rapid rate and instead the film becomes
disrupted, in all likelihood mechanically, as the result of anodic current flow.
3
A theoretical
model for the breakdown of the passive film has been proposed.
4–7
This model involves,
successively, metal dissolution at the metal-film interface, film dilatation, and film break-
down. This wasteful reaction is a problem, not only because of the need to vent the hydrogen
from the battery and to prevent it from accumulating, but also because it uses water which
is critical to the battery operation, produces heat, and reduces the efficiency of the anode.
The efficiency of the magnesium anode is about 60 to 70% during a typical continuous
discharge and is influenced by such factors as the composition of the magnesium alloy,
battery components, discharge rate, and temperature. On low drains and intermittent service,
the anode efficiency can drop to 40 to 50% or less. The anode efficiency also is reduced
with decreasing temperature.
Considerable heat is generated during the discharge of a magnesium battery, particularly
at high discharge rates, due to the exothermic corrosion reaction (about 82 kcal per gram-
mole of magnesium) and the losses resulting from the difference between the theoretical and
operating voltage. Proper battery design must allow for the dissipation of this heat to prevent
overheating and shortened life. On the other hand, this heat can be used to advantage at low
ambient temperatures to maintain the battery at higher and more efficient operating temper-
atures.
A consequence of the passive film on these metals is the occurrence of a voltage delay—
a delay in the battery’s ability to deliver full output voltage after it has been placed under
load—which occurs while the protective film on the surface of the metal becomes disrupted
by the flow of current, exposing bare metal to the electrolyte (see Fig. 9.1). When the current
is interrupted, the passive film does indeed reform, but never to the original degree of pas-
sivity. Thus both the magnesium and the aluminum batteries are at a significant disadvantage
in very low or intermittent service applications.
3
This delay, as shown in Fig. 9.2, is usually
less than 1 s, but can be longer (up to a minute or more) for discharges at low temperatures
and after prolonged storage at high temperatures.
