Lyons W.C. (ed.). Standard handbook of petroleum and natural gas engineering.2001- Volume 1
Подождите немного. Документ загружается.

r.
I
I1
cc
c
-
Strength
of
Materials
205
Table
2-1
5
(continued)
Typical mechanical
properties
No.
Yield
shengh
Nominal
Form
and
(0.2%
offset),
Material
composition
condition 1000 Ib/sq
in.
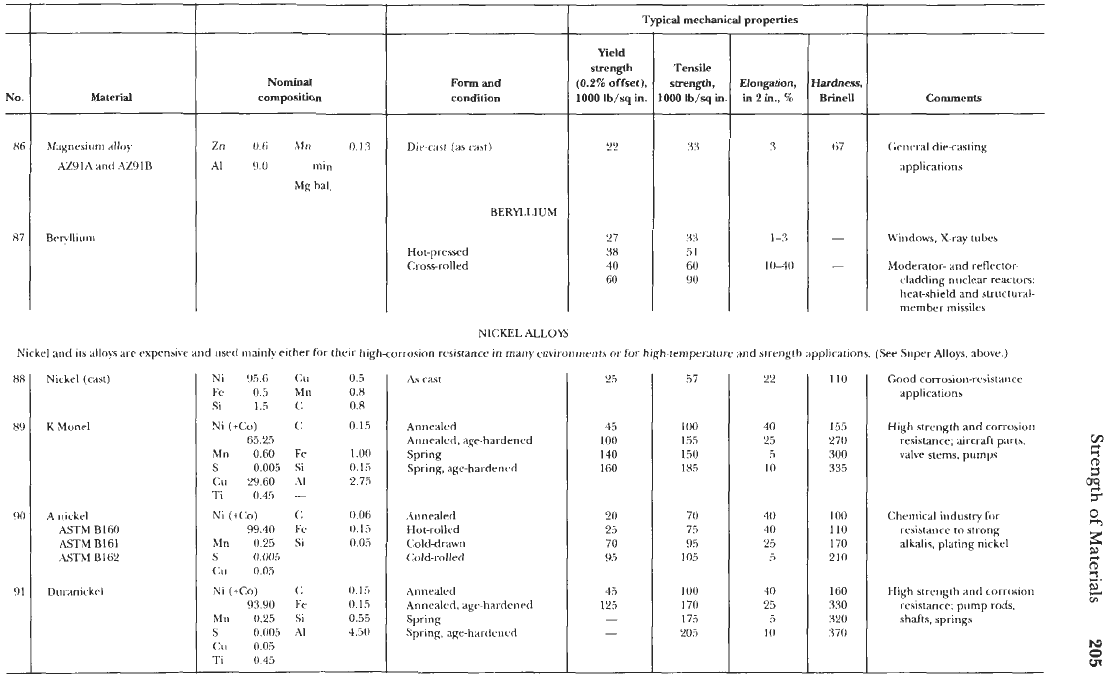
92
1
Cupronirkrl55-45
(Constantan)
45
20
2
30
-
-
-
270-350
30
50
65
8C-115
Cu
55.0
Ni
45.0
Ni
80.0
CT
20.0
NI
M).O
Cu
290
Fe
2.50
Mn
1.5
9
4.0
AI
0.5
max
max
max
Tensile
shength,
1000
lb/sq
in
Annealed
(:"Iddraw"
Cold-rolled
Sandimting
60
65
85
97
98
11@145
Titanium
alloy
Ti
4
AI
4Mn
Ti
Mnalloy
Fe
0.5
Ti bal.
Sheet
ASTM
Be65
5RT 7 Mn
7.0
8.0
ELongation,
Hardness,
inzin.,
%
1
Brinell
1
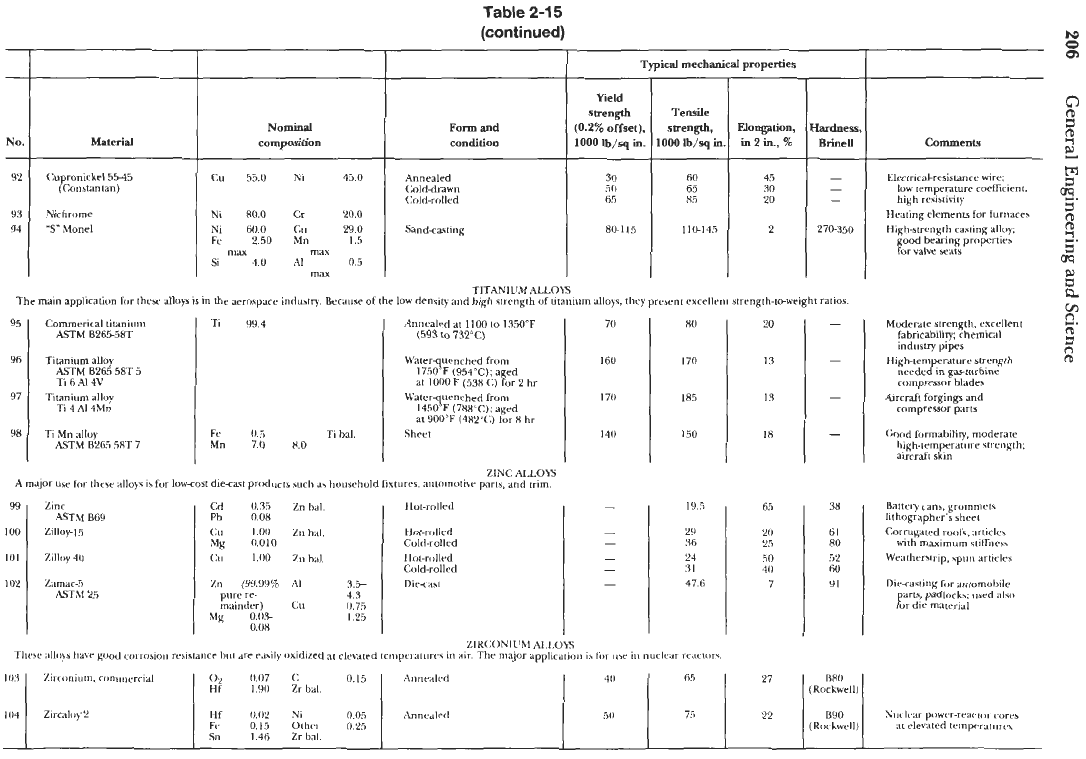
TITANIUM
ALLOYS
The
main
applicatiun for thew
alloys
IS
in
the
aerospace
industly
Because
of the
low
density
and
high Wcngrh
of
utanium alloys.
they
prebmt
e~~ellcni
strength-toweight ratios
ASTM B265-5XT
ASTM
B265
58T
5
Ti
6
AI
4V
Annealedat
1100
to
1350°F
1
(593
to
732°C)
Waier
uenched from
[
1753, (954°C);
aged
at
1000
F
15%
C)
fur
2
hr
Waterguenched from
1450
F
(7XX"C).
aged
at
900°F (48'2°C)
fur
8
hr
99
I00
101
102
Zinc
ASTM B69
zlll"y-15
ziiiOy
40
Zamac-5
ASTM
25
Cd
0.35
Zn
ha1
Pb
0.08
Cu
I
00
Zn
hal.
(:I,
1.00
7nhal.
M~
n.010
Zn
(YY.99%
AI
3.5-
pure
re-
4.3
mainder) Cu
11.75
Mg
0.0%
125
0.ox
I
lot-rolled
Hot-rolled
Cold-rolled
Hot-rc,llrd
Cold-rolled
Dir-cnst
XI)
I70
185
150
19.5
29
36
24
'II
47
6
Comments
20
13
13
1X
65
20
25
50
40
7
27
22
I
Electrical-resisuncr
wire:
low
temperature coefficient,
high
resistivity
Heating
elements
for
turnares
Highstrength casting
ally;
good bearing properlira
for
valw
sea5
Moderate strength,
excellent
fabricabdity:
chemical
indusiry
pipes
High-tern
erdturr
aucngth
needelw
gasturbme
compressor hladcs
Aircraft
forgings
and
compressor parts
Good
formability,
moderate
high-irmprrature
Wength.
aircraft
skin
Baitcry
cans,
grommets
lithographer's shee1
Corrugated
mots,
at
ticlca
with
maximum
sttNnrs*
Wrathentrip,
~prm
drti~lrs
Dirqasting
for
automobile
am,
padlock,;
rracd
also
!or
die
material
Strength
of
Materials
207
Table
2-16
Typical Properties of Glass-Fiber-Reinforced Resins
[lo]
Property
Polyester
Excellent
I
70
to
"LO
n
n
to
no02
0.5
tO
5.0
x
ttl
20
in
10
40
250
IO
2000
1
35
to
2.1
25
to
50
I5
1"
311
2
to
10
Mi0
LO
MI20
2
IO
x
lo-;
1
Y
1014
'350
to
.WJ
3.8
to
6.0
4.0
IO
6.11
0
01
tu
0.04
0.01
tO
0.05
I101
L"
1.0
Slight
FU'*
hod
Phenolic
Melamine
(;",,d
2x0
(0
340
0.001
to
0
004
3
10
IO
24
1
i
to
25
'%,"ti
1
i
x
Ill-.'
VXl0"
I70
to
AOII
Y7t<,ll.l
moo
to
noon
I
H
to
2.0
zn
to
15
I1
14
10
ll.2:i
0.09
10
I1
21
Slight
tioud
ver)
guodi
(text contznued
from
page
194)
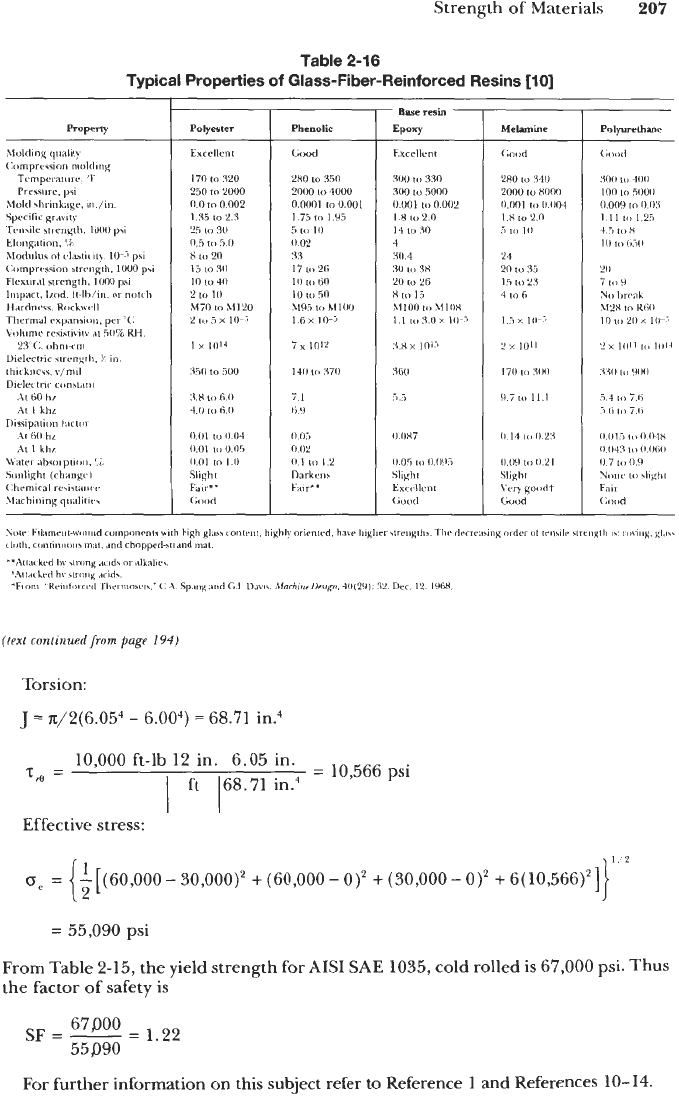
Torsion:
J
n/2(6.054
-
6.004)
=
68.71
in.4
10,000
ft-lb
12
in.
6.05
in.
I
ft
168.71
in.4
=
psi
T83
=
Effective stress:
30,000)2
+
(60,000-
0)'
+
(30,000-
0)'
+
6(10,566)']
=
55,090
psi
From Table
2-15,
the yield strength for AIS1 SAE
1035,
cold rolled is
67,000
psi. Thus
the factor of safety
is
67800
55890
SF
=
-
=
1.22
For
further information on this subject refer
to
Reference
1
and References
10-14.
208
General Engineering and Science
2x4
2
x
6
and wider
2x4
2
x
6
and wider
2x4
4x4
2x4
4x4
2x4
2
x
6
and
wder
2x4
2
x
6
and wder
2x4
2
x
6
and wider
2x4
2
x
6
and wider
2"
and
4"
"Comtmction"
Species
and
grades
(virunl
grading)'
Idaho white pine
W
W
w
S
S
W
W
W
W
W
R
Ponderosa pine
Lodgepole pine
650
7011
no0
500
-
Southern pine
Douglas
fir
Western hemlock
220
950
220
1
.no0
295
1,150
295
1,150
305
1,190
Western sprucc
Western cedar
Redwood
(unseaoned)
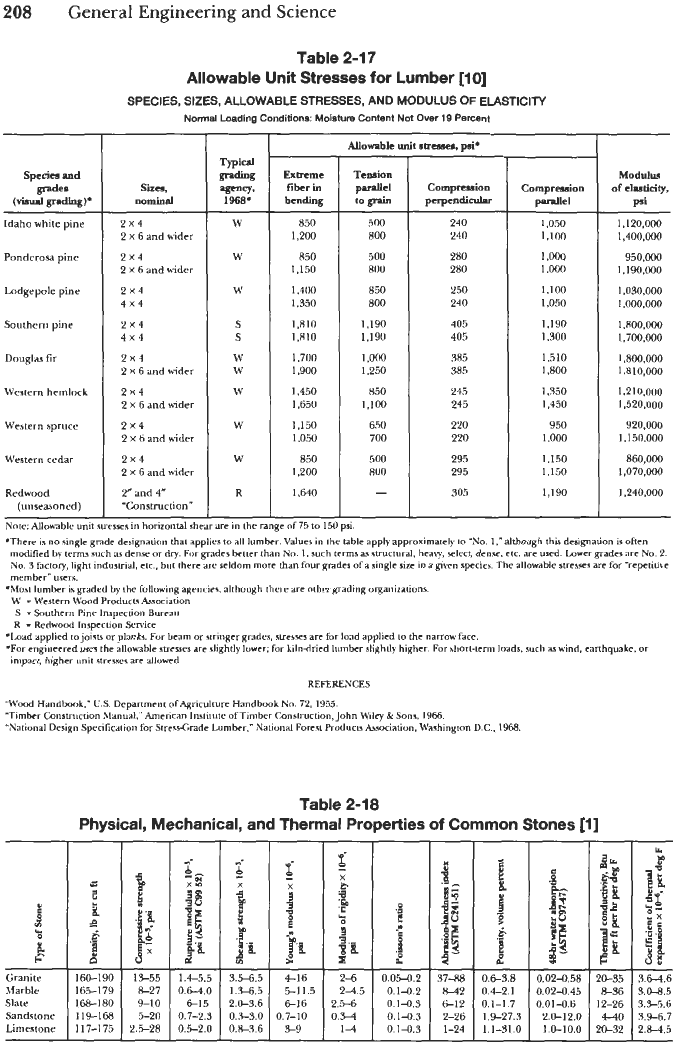
Table 2-17
Allowable Unit Stresses for Lumber
[lo]
SPECIES, SIZES, ALLOWABLE STRESSES, AND MODULUS
OF
ELASTICITY
Normal
Loading Conditions:
Moirlhlre
Content Not
Over
19 Percent
Exbeme
fiber in
bending
1,200
850
8511
1,150
1,400
1,350
1,RIO
1,NIO
1.900
1.450
1,650
1,150
1,700
1,050
1,200
1,640
850
~
Note:
Allowable
"nil
stresses
!n
horizontal shear
are
in the
range
of
75
to
15
AUowpble
unit
stresses,
psi'
Teasion
5no
240
800
I
240
500
280
1
,000
800
1
280
1
1,000
850
i.19n
800
1,190
1
,ooo
1,250
1,100
x5n
250
240
405
405
385
385
245
245
1,1nn
1,050
1,190
1.300
1,510
1.8nn
1.m
1,450
Modulus
of
elasticity,
psi
1.1
20,000
950,000
1.4on,wo
i,iw,oon
1,030,ooo
I
.onn,ooo
1,800,000
1,700,000
1,800,oon
i,8in,ooo
i,2in,ooo
1,520,000
920.000
860,000
1,070,000
1,150.000
I
,240,ono
'There is
no
ingle
grade
designation
that applies
to
all
lumber.
Values
in
the
table apply approximately
lo
"No
1
..although this
designarion
II
often
modified
by
terms such
as
denre
or
dry.
For
grades better than No.
I,
such
terms
as
~Vuciural.
heavy.
select.
dcnr,
etc.
arc
used.
hwcr
gradcr
arc
No
2.
No.
3
factory,
light industrial,
ec
,
but
iherc
are seldom
more
than
four
grader
of
a
single
size in
a given
species.
The
allowable
stress
arc
for
"rcpetiuve
member"
USCIS.
W
=
Weirern
Wood
ProducU
Arsociation
S
=Southern Pine
Inspection
Bureau
R
=Redwood
lnrpcruon
Sewice
*Most
lumber is graded
by
the
following agcncicr,
alrhough there
are
other
grading
organizarions.
"Inad
applied
tojoists
or
planks.
For
beam
or
~iringer
grades,
SL~CJYI
are
for
load
applied
IO
the
narrow
face.
'For
engineered
uses
rhc
allowable
IUCII~S
dm
slightly
lower;
for
kilndried
lumber
slightly higher
For
,horl-tem
loads,
such
ai(
wind,
earthquake,
or
impact. higher unit
~trrsies
are
allowed
REFERENCES
"Wwd Handbook,'C.S
Departmcnt
ofApculturc
Handboak
No
72.
1955
"Timber
Con~truruon
Manual,"Amencan
Institute
of
Timber
Conslructton,
John
Wilq
&Sons.
1966.
"National Design Specification
for
SrrcwGradc
Lumber,"
National
Forest
Producu Aswciarion. Washington D
C
,
1968
Table 2-18
Physical, Mechanical, and Thermal Properties of Common Stones [l]
Granite
Marble
Slate
Sandstone
Limestone
165-179
1%-180
i
.t,
i
4
-
3.56.5
1..%6.5
2.0-3.6
0.3-3.0
0.&3
6
0.63.8
I
0.024.58
0.42.1
0.024.45
1.9-27.3 2.0-12.0
0.1-1.7
0.01-0.6
1.1-31
n
10-10.0
2W35
3.6-4.6
12-26
3.S5.6
4-40
3.9-6.7
20-32
2.8-4.5
a36
3.0-8.5
Thermodynamics
209
Figure
2-30.
Diagram
for
Example
2-21
THERMODY
NAMlCS
Thermodynamics centers around the concept of “energy in transit,” but is con-
siderably more encompassing in its applications. The science
of
thermodynamics
deals very broadly with the concepts of how things work, why some things cannot
work, and why some things do not work as intended. Three “laws”
of
thermodynamics
have been formulated, which can be summarized as follows:
1.
The
First
Law
of
Thermodynamics:
2.
The Second Law
of
Thermodynamics:
A
statement
of
the principle
of
conservation of energy.
Deals with the concept of entropy, which
serves
as
a means of determining
whether or not a process is possible.
Defines the zero entropy state for any
substance in a single, pure quantum
state as the absolute zero
of
temperature.
3.
The Third Law
of
Thermodynamics:
Units
of
Energy
Measurements
of
energy are made in terms
of
absolute joules, but engineering
practice has persistently retained the thermochemical calorie as the unit
of
energy.
The two are related by the definition:
1
calorie
=
4.1840
absolute joules
(2-101)
which is often referred to as the mechanical equivalent
of
heat.
units and the calorie:
The following table expresses the relationship between several other useful energy
1
Btu
=
252.16
cal
1
kWhr
=
8.6056
x
lo9
cal
1
hp-hr
=
6.4162
x
10
cal
1
ft(lb,)
=
0.324
cal
1
batm
=
24.218
cal
A
=
work function (Helmholtz free energy), Btu/lb,,, or Btu
C
=
heat capacity, Btu/lbnloR
C,
=
heat capacity at constant pressure
C,
=
heat capacity at constant volume
F
=
(Gibbs) free energy,
Btu/lb,”
or Btu
g
=
acceleration due
to
gravity
=
32.174
ft/s‘
g,
=
conversion factor between force and mass
=
32.174
(lbr,,)(ft/s‘)/lb,
h,
H
=
enthalpy or heat content, Btu/lb,,, or Btu
K
=
ratio
CJC,
Mw
=
molecular weight, lbJlbm-mole
m,
M
=
mass
of
fluid, lbm
m,
M
=
mass flow rate, lb,/s

210
General Engineering and Science
P
=
absolute pressure, Ib,/ft2
Pq
=
entropy production rate, Btu/”R*s
Q=
heat transferred
to
system across a system boundary, Btu/lb, or Btu
Q
=
rate of heat transfer, Btu/s
R
=
universal gas constant, lb,-ftg/mole*”R
T
=
absolute temperature,
“R
V
=
volume, ft’/lb, or ft’
v
=
flow velocity, ft/s
W
=
work done by a system against its surroundings, Btu/lb, or Btu
Z
=
height from center
of
gravity
of
a fluid mass to
a
fixed base level, ft
s,
S
=
entropy, Btu/lbmoR
or
Btu/”R
u,
U
=
internal energy, Btu/lb, or Btu
The First
Law of
Thermodynamics
The differrential form of the first law as applied to
a
closed
system, for which there
is no exchange
of
matter between the system and its surroundings, is given by
dU
=
SQ
-
6W
(2-102)
where
dU
represents an infinitesimal increase in the internal energy
of
the system,
6Q
is the heat absorbed by the system from its surroundings, and
6W
is the work
done by the system on its surroundings. The state
of
a system is defined by its
temperature, pressure, specific volume, and chemical composition. The change in
internal energy expressed by Equation
2-102
depends only upon the difference between
the final and initial states and not upon the process or processes that occurred during
the change. The heat and work terms, on the other hand, are dependent upon the
process path. For a change from a state
A
to a state
B,
the first law becomes
AU=U,-
U,=Q-
W
(2-103)
Work interchange between a system and its surroundings can take on any of a variety
of
forms including mechanical shaft work, electrical work, magnetic work, surface tension,
etc. For many applications, the only work involved is that
of
compression or expansion
against the surroundings, in which case the work term in Equation
2-102
becomes
6W
=
PdV
or
W
=
I’”
P
dV
”a
(2-104)
where
V,
is the final volume and
V,
the initial volume of the system, and
P
is the
system pressure. Thus, for a
constant pressure
process:
W
=
PAV
=
P(V,
-
V,)
(constant pressure process)
(2-105)
or,
combining Equations
2-103
and
2-104:
AU
=U,
-
U,
=
Q-
PV,
+
PV,
or
Q
(U,
+
PV,)
-
(U,
+
PV,)
(2-
106)
(2-107)
The combination
of
properties
(U
+
PV)
occurs
so
frequently in thermodynamics
that it is given a special symbol,
H,
and termed the “enthalpy” or “heat content” of
the system. Thus Equation
2-107
can be written as
Thermodynamics
2
1
1
Q
=
A(U
+
PV)
=
H,
-
HA
=
AH
[constant pressure process]
(2-
108)
Enthalpy is a
property
of the system independent of the path selected. Processes can
be conveniently represented graphically. For example, a
P-V
diagram can be used to
illustrate the work done when a system undergoes a change in state (see Figure 2-3
1).
In each of the cases depicted in Figure
2-31,
the work
is
equal to the shaded area
under the P-V curve as shown.
Since the mass is fixed for a closed system, the equations in this discussion
will
be
valid for the entire mass
(M)
or on a unit mass basis.
The First Law
of
Thermodynamics Applied to Open Systems
An open system is one which exchanges mass with its surroundings in addition to
exchanging energy. For open systems, the first law is formulated from
a
consideration
of the conservation of energy principle which can be stated as follows:
Net increase Stored energy Stored energy Net energy
[of stored energy)
=
[
of mass
1
-
[
7:s;
1
+
[
~~~~~~
z;,
of
system entering
forms of work
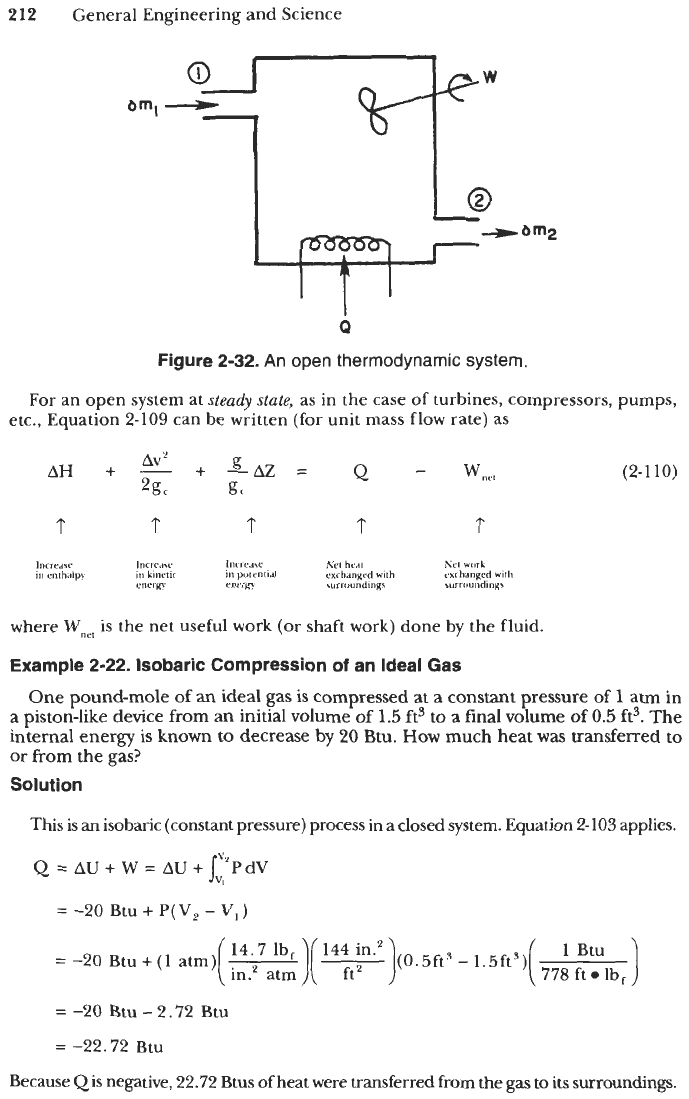
Consider the arbitrary open thermodynamic system illustrated in Figure 2-32. The
foregoing statement of the first law for this open system can be written as
m2
+
Q
-
W
(2-109)
where 6m refers to a differential mass
of
fluid, and the subscripts
f
and
i
refer to the
entire system in its final state and initial state, respectively. Clearly, for a closed system
defined as one which exchanges no mass with its surroundings, Equation
2-109
reduces
to
Equation 2-103.
path (isobaric)
path (isothermal)
P
P
V
(0)
V
(bl
Figure
2-31.
P-V
process diagrams: (a) isothermal expansion;
(b)
isobaric
compression.
212
General Engineering and Science
0
W
1
.L
Q
Figure
2-32.
An open thermodynamic system.
For
an open system at
steady
state,
as in the case of turbines, compressors, pumps,
etc., Equation 2-109 can be written (for unit mass flow rate) as
(2-110)
where
W,,,
is
the net useful work
(or
shaft work) done by the
fluid.
Example
2-22.
Isobaric Compression of an Ideal Gas
One
pound-mole
of
an
ideal gas is compressed at
a
constant pressure
of
1
atm in
a piston-like device from an initial volume of
1.5
ft3 to a final volume of
0.5
ft3. The
internal
energy
is known
to
decrease
by
20
Btu.
How
much heat
was
transferred to
or
from
the
gas?
Solution
This is
an
isobaric (constant pressure) process in a closed system. Equation 2-103 applies.
Q
=
AU
+
W
=
AU
+j>PdV
I
=
-20 Btu
+
P(V,
-VI)
778
ft lb,
=
-20 Btu+(1 atm)
=
-20 Btu
-
2.72 Btu
=
-22.72
BtU
Because
Qis
negative, 22.72 Btus of heat were transferred from the
gas
to its surroundings.
Thermodynamics
2
13
Example
2-23.
Hydroelectric Power System
A hydroelectric power plant proposes
to
use 1,500 ft’/s of river water to generate
electricity. The water enters the system at
1
atm and 50°F and is discharged at 1 atm
and
50.4”F
after passing through a turbine generator. The discharge point
is
600
ft
below the inlet. The increase in enthalpy of the water is known to be 0.36 Btu/lb,,,.
Assuming 70% efficiency for the conversion, what power output can be expected
from the power plant?
Solution
The following assumptions pertain to this
open
system:
1.
Steady-state flow.
2.
No
heat transferred between system and surroundings.
3. Change in kinetic energy of the flow streams is negligible.
With these assumptions, we take as a reference point the discharge level of the water,
and apply Equation 2-110. Thus
Z,
=
0,
Z,
=
600
ft
and the energy balance becomes
AH+-AZ
g
=-W,,,
g<
1,500 ft‘ 62.4 lb, 3,600
s
whereAH =[0.36E)(y)(
fti
j(1j
=
1.213~ 10’ Btu/hr
=
35,553 kW
ft
32.2,
32.2
~
1,500
ft’
62.4 lb,
and
-AZ
g
=I
ft lb, ](0-600ft)[7)(
ft3
)(%)
gc
Ib,
s2
=
-2.599~
lo8
Btu/hr
=
-76,163 kW
Therefore,
W,,eL
-35,553 kW
+
76,163 kW
=
40,610 kW
At 70% efficiency, this
would
yield
=
(0.70)(40,610)
=
28,427 kW
=
28.427 mega-Watts.
Entropy and the Second Law
The second law
of
thermodynamics provides a basis for determining whether or
not a process is possible. It is concerned with availability of the energy of a given

214
General Engineering and Science
system for doing work. All natural systems proceed towards a state of equilibrium
and, during any change process, useful work can be extracted from the system. The
property called
entropy,
and given the symbol
S
or
s,
serves as a quantitative measure
of the extent to which the energy of a system is “degraded” or rendered unavailable
for doing useful work.
For any
reversible process,
the sum of the changes in entropy for the system and
its surroundings is zero. All natural or
real
processes are
irreversible
and are
accompanied by a net
increase
in entropy.
Several useful statements have been formulated concerning the second law that
are helpful in analyzing thermodynamic systems, such as:
No
thermodynamic cycle can be more efficient than a reversible cycle operating
between the same temperature limits.
The efficiency of all reversible cycles absorbing heat from a single-constant higher
temperature and rejecting heat at a single-constant lower temperature must be
the same.
Every real system tends naturally towards a state of maximum probability.
For any actual process, it is impossible to devise a means
of
restoring to its
original state every system participating in the process.
For any reversible process, the increase in entropy of any participating system is
equal to the heat absorbed by that system divided by the absolute temperature
at which the transfer occurred. That is, for a system,
i,
SQi
dSi
=-
T~
(reversible processes)
(2-111)
Alternatively, for an ideal reversible process, the sum of all the changes in entropy
must be zero or
reversible processes)
xdSi=x-=O
SQ
(
Ti
(2-112)
Because all
real
processes are
irreversible
as a result of friction, electrical resistance,
etc., any processes involving real systems experience an
increase in entropy.
For
such systems
x
dS,
>
0
(irreversible processes)
(2-113)
The entropy change of a system during any process depends only upon its initial
and final states and not upon the path of the process by which it proceeds from its
initial to its final state. Thus one can devise a reversible idealized process to restore a
system
to
its initial state following a change and thereby determine
AS
=
Sfin=,
-
Sinitia,.
This is one of the most useful aspects of the concepts of a reversible process.
Entropy Production:
Flow
Systems
In general, for all real processes, there is a net production
of
entropy and Equation
2-1
13
applies. Since many practical engineering processes involve open systems, it is
useful to develop a generalized expression of the second law applied to such systems.
