Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

latent manufacturing defects and long-term wearout [119]. These two causative
mechanisms are very different directly correlate to the two primary reasons for
performing accelerated aging noted in the first paragraph of this section. The
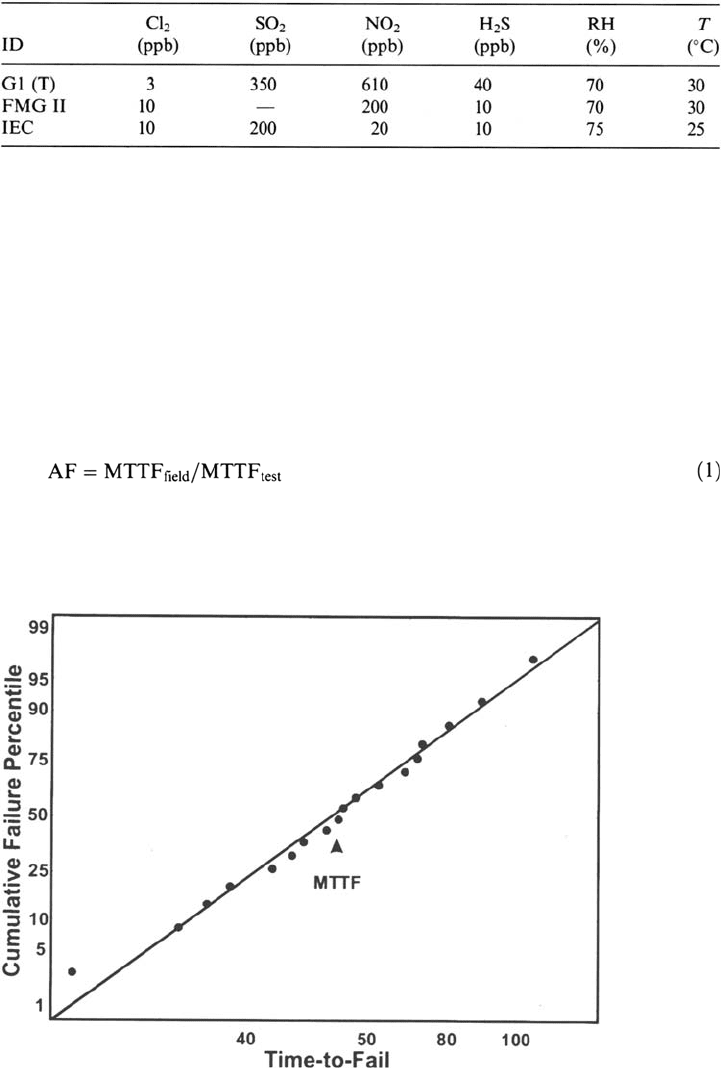
elapsed time to fail and cumulative failure percentile are often plotted using a
lognormal distribution (Fig. 7). Two statistical measures result: the mean time to
failure (MTTF, the time for 50% of the population to fail) and the population standard
deviation (calculated from the slope) [8,119]. Because the goal of accelerated
aging is to establish a relationship between the environmental parameters and
service life under operating conditions, the MTTF metric can be used to calculate
an important parameter known as the acceleration factor (AF). In terms of MTTF,
664 Frankel and Braithwaite
Table 1 Specifications for Three Standardized Atmospheric Corrosion Test Environments
The acceleration factors are experimentally measured as a function of the
environmental stresses using the techniques described in the previous subsection.
Figure 7 Example of a lognormal distribution of failure times and the definition of the
mean-time-to-failure (MTTF) metric.
Copyright © 2002 Marcel Dekker, Inc.
Using triple-track test devices, the surface conductance, G, can be directly
measured. This parameter is a more fundamental indicator of the susceptibility to
electrolytic degradation mechanisms compared with MTTF because, as noted earlier,
electrolytic corrosion will occur only if the ohmic resistance between lines does
not consume the applied voltage difference and leakage current flows [111,120].
G is calculated by multiplying the leakage current by the area of insulator and
dividing by the applied voltage. The resistance change of the lines can also be used
as a measure of degradation (either electrolytic or open circuit). The important
assumption inherent in these types of tests is that the specific metric being measured
(e.g., surface conductance or wirebond resistance) is directly related (proportional
or inversely proportional) to failure rate and thus to MTTF [66,111,120].
The cumulative values for MTTF determined by any of these techniques for a
range of conditions permit the formulation of general relationships that describe the
dependence of failure rate on the stressing parameters. The effect of temperature, T,on
reaction or failure rate, R, can usually be described by an Arrhenius-type relationship:
Corrosion of Data Storage Devices 665
where E
a
is activation energy, k is Boltzmann’s constant, and A is an empirical constant.
A range of activation energies for failure of ICs has been reported, from 0.4 to 1.2 eV.
The acceleration factor associated with temperature becomes
Because of the concern that higher voltage levels will increase the internal
temperature (ohmic heating) and dry the device, most investigators use the normally
applied operating voltage during testing. However, for the corrosion mechanisms that
occur due to electrical bias, the corrosion rate is proportional to the current between
the conductors and therefore scales with applied voltage. As such, the MTTF
decreases as bias increases and an inverse relationship may exist [7,60,121,122]. For
the limited cases in which a higher voltage level is used, Shirley [123] has proposed
a general linear acceleration model where a and b are constants:
Unfortunately, the influences of RH and impurities are most poorly established
than the effects of temperature and bias. Many investigators have measured MTTF
or G for actual devices or test structures in high-temperature and -humidity
environments and developed empirical relationships by fitting the data to various
equations. Several commonly referenced variants are presented in Table 2. Each
expression in this table has a parameter A that represents a proportionality constant.
These equations can be used to determine an acceleration factor by considering the
ratio of field to test MTTF as was done in Eq. (3). Using this technique, the
proportionality constants cancel. Then by using Eq. (1), the service life under
operational conditions may be predicted after measuring lifetime under stress
conditions. The first four expressions in Table 2 are “Eyring” models because
the influences of T and RH are considered separately and then multiplied
together to get a combined equation. The last expression does not assume that the
Copyright © 2002 Marcel Dekker, Inc.
effects of temperature and humidity are independent and has an activation energy
that is dependent on RH [124]. These models have been used in a number of
investigations to fit experimental data, and different values for the constants have
been determined for various systems and degradation modes [96,98,99,102–
105,119,121,125]. An example of how such models are applied is shown in
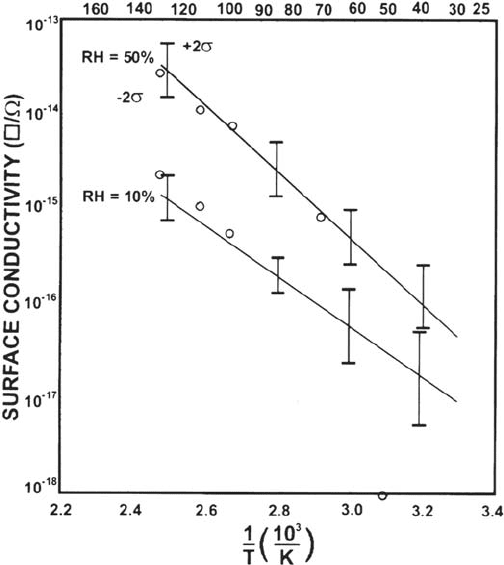
Figure 8, which is based on surface conductivity measurements that were made
with an Al-Cu triple-track structure in a HAST chamber [103]. The temperature
and humidity combinations were fit to various equations and those shown in
Figure 8 are for the last expression in Table 2, which contains an RH-dependent
activation energy. The correlation coefficient for this expression was found to be
higher than those for other models [103].
As will be discussed in the next subsection, to be truly effective, predictive
capabilities must be based on fundamental physical understanding. The models
presented in Table 2 are simply empirical correlations. Limited progress has been
made to date to improve this situation. Comizzoli [46] has provided a mechanistic
explanation and associated mathematical expression for the exponential dependence
of surface conductance (and MTTF) on relative humidity that is contained in some of
the models listed above. Pecht and Ko [83] developed a comprehensive model for
predicting absolute time to failure when microelectronic die metallization is corroded
by an electrolytic process. Their phenomenological underpinning involves ion
transfer based on Ohm’s and Faraday’s laws. The other key feature is their treatment
of the critical process involving moisture ingress that permits this model to be useful
for both PEM and CHP devices. A final reference that is relevant to this subject is
the work being performed by Graedel and co-workers [128]. This team is developing
and exercising a physically based atmospheric corrosion model referred to as
GILDES that conceptually can include all microelectronic corrosion processes (e.g.,
gas transport, adsorbed water layer, corrosion product layer, electrochemistry).
666 Frankel and Braithwaite
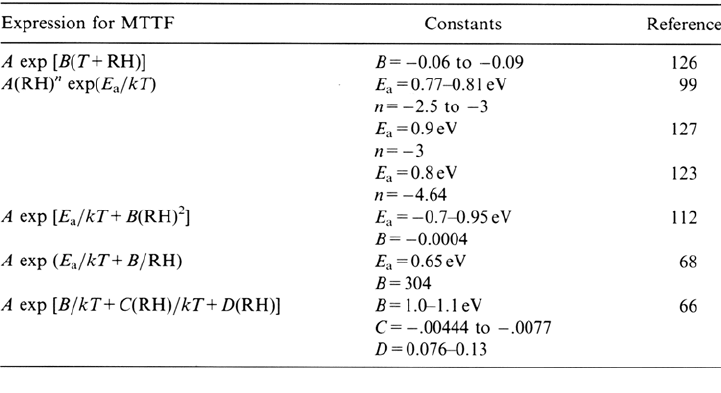
Table 2 Empirically Derived Models Describing the Influence of Temperature, T, and
Relative Humidity, RH, on Mean Time to Failure, MTTF
Copyright © 2002 Marcel Dekker, Inc.
Concerns and Limitations
In general, the reliability engineer cannot ignore failures observed during testing.
The expense of accelerated testing and the often costly effort required to address the
problems highlighted by such tests are rationalized by the assumption that failures
have a finite probability of occurring during service if they are observed at all during
testing. In other words, the accepted belief is that improved test yields correlate with
longer service life. A device may actually be redesigned without any knowledge of
whether the failure mechanism occurs under operating conditions at a rate that can be
extrapolated from stress conditions or if it is just an artifact of the test. The prediction
of device lifetime in the field by extrapolation of data obtained under high-stress
conditions is extremely sensitive to the model chosen. The basic issue here goes back
to the use of empirical equations—one cannot reliably extrapolate outside the range
of testing conditions. Thus, the goal has to be to obtain data on the behavior in the
mildest conditions possible [97]. The authors of this chapter do not know of a sole
expert in this field who believes at this time that actual service life can be accurately
predicted and, as such, in practice, accelerated aging is presently useful only for
product qualification and identification of latent manufacturing defects and
Corrosion of Data Storage Devices 667
Figure 8 Surface conductivity as a function of temperature. RH is the relative humidity
and the lines are fits of the last equation shown in Table 2. (From Ref. 103. Copyright 1980
by IEEE.)
Copyright © 2002 Marcel Dekker, Inc.
design deficiencies. The remainder of this subsection provides support for these
statements.
Changing Failure Mechanisms as a Function of Environmental Stress This
classical issue is relevant to all applications of accelerated aging [97]. The
complexity inherent in microelectronic devices results in a multitude of coupled
processes that must be properly identified and accounted for in accelerated aging
models. Every process that affects a failure mechanism could have a different
sensitivity to the environmental parameters. Lall [129] has documented some of
the problems associated with the common use of temperature as a stress agent for
microelectronics. The wide variability in activation energy mentioned before is
indicative of different or changing mechanisms. Three relevant examples further
illustrate the issue of changing mechanisms. The first involves the formation of
conductive anodic filaments (CAFs) on PCBs described earlier. Work performed
subsequent to the discovery of CAF formation found that a threshold in the
temperature/humidity phase space may exist below which CAF growth does not take
place [130]. This threshold is apparently not encountered in typical use environments
and thus the phenomenon of CAF may be only an artifact of accelerated testing.
The second example is from an investigation of conduction in printed circuit
boards by Takahashi [130]. He studied the various conduction paths in boards using
an AC impedance technique during humidified exposures without bias and identified
both ohmic and diffusion-controlled processes. He then made the point that
because multiple conduction paths and mechanisms exist at a single temperature
and humidity condition, it is possible for conduction processes determined in
stress tests to have no bearing on failure mechanisms under field conditions. The
final example concerns the observation that the glass-transition temperature of the
encapsulating plastic in PEM devices cannot be exceeded in testing because
a large difference in water permeability results. Nevertheless, sometimes this
requirement is satisfied. A few studies have shown that data obtained from THB
and HAST/pressure cooker testing can be fit to a single mathematical relationship,
indicating that the failure mechanisms may be the same [68,99,104].
Evolving and Improving Technology The continuing rapid evolution of
microelectronic technology leads to several related difficulties. The first is that the
dominant failure mechanisms change and thus long-term field failure information
is not necessarily relevant to state-of-the art devices. Historically, during accelerated
aging, two types of moisture-related phenomena have been observed: distributed Al
track corrosion and Au wire/Al bondpad interfacial degradation. The root cause of
track corrosion was probably moisture penetration through defects in the protective
passivation layer and the presence of contamination. Modern best commercial
practice has effectively eliminated passivation defects and thus track corrosion as a
significant failure mechanism. Now, the exposed wirebonds are the prime
susceptibility. Lall [129] documents another important factor: field failure
information is becoming more limited because the reliability of state-of-the art
devices has now improved to the point that it no longer limits useful system lifetime.
The final factor is that posttest analyses of aged devices have often been limited to
simply confirming a failure, not determining root cause. Thus, the ability to correlate
the results with true service life or identify actual failure mechanisms has not been
developed to any significant extent.
668 Frankel and Braithwaite
Copyright © 2002 Marcel Dekker, Inc.

Effect of Humidity and Device Type A specific disconcerting aspect of the
existing T/H models that is easy to identify is their wild divergence at low RH
values. Figure 9 shows humidity acceleration factors relative to 85% RH calculated
for each of the expressions in Table 2. In these calculations, temperature was
normalized out by setting its value to 25°C. There is relatively good corres-
pondence of the models in the high-RH range where the experimental data exist.
However, at low RH, the models differ by many orders of magnitude. One possible
explanation is that none of the mathematical relationships proposed have a
phenomenological basis. For example, water adsorption on the metallization
may be the key process and, if so, the rate response should be sigmoidal (which
none of these equations simulate). Osenbach [17] describes another physical
basis that involves the observation that surface leakage current is minimal below
about 50% RH and has an exponential dependence above this level. Few exper-
imenters examine the RH range less than 50% because of the long times needed
to obtain failure. Unfortunately, this RH range is where most operating conditions
exist, especially considering the local heating associated with power dissipation.
Furthermore, there is no reason to expect different systems to exhibit similar
temperature or, for this topic, different humidity relationships. For instance, silicone-
encapsulated devices fail at much lower rates in a given environment than
devices encapsulated in epoxy [60,96,112]. This occurs despite the faster transport
of moisture in silicones and could result from low impurity content and
improved adhesion to the die surface [75,112]. Silicone-encapsulated parts may
therefore display a different humidity acceleration factor than unencapsulated or
epoxy-encapsulated parts [66].
Corrosion of Data Storage Devices 669
Figure 9 Humidity acceleration factor as a function of RH relative to 85% for the various
models shown in Table 2.
Copyright © 2002 Marcel Dekker, Inc.
Effect of Contamination As discussed previously, contamination at
metallization surfaces is a critical corrosion factor. For example, the level of
contamination affects the ionic strength in the adsorbed water layer that, in turn,
influences surface conductance. Contamination also affects the critical relative
humidity, which, for some salts, can be quite low. Finally, the type of contamination
often determines the corrosion mechanism (e.g., whether electrolytic anodic or
cathodic corrosion of Al predominates). In many accelerated aging approaches, this
dominant factor is uncontrolled and often uncharacterized. In fact, T/H testing may
really be a measure of the cleanliness of the part prior to testing. This observation
is an example of why Osenbach’s second assumption noted earlier (testing of
representative devices) is probably not, in general, valid. To address this problem,
many investigators have either purposely contaminated the part prior to exposure or
added controlled contamination to the stressing environment [42,65,74,106,107,122,
131–133]. These approaches are useful for making comparative assessments of
various structures. However, it is a difficult task to generate a model that predicts
behavior under conditions of lower contamination levels. Pitting potentials of various
metals have been found to decrease linearly with a logarithmic increase in chloride
concentration [134]. Predicting part lifetime for a given surface contamination
concentration from such information, however, remains quite challenging.
CORROSION OF MAGNETIC DATA-STORAGE COMPONENTS
The critical metallic components of advanced magnetic and magneto-optic (MO)
storage devices—thin-film metal disks, inductive or magnetoresistive heads, and
MO layers—are all susceptible to corrosion and each has been a subject of
considerable study. Several review articles covering corrosion of magnetic-storage
media may be found in the literature [135,136].
Thin-Film Magnetic Disks
As described in the technology overview section, the carbon overcoat layer on
thin-film disks typically does not fully cover the underlying layers as a result of
intentional roughening of the disk. The lack of coverage has two implications for the
corrosion behavior. First, the Co-based magnetic layer and perhaps even the NiP
substrate are exposed at small regions and can corrode. Furthermore, the overcoat
layer, which is often sputter-deposited carbon, can be somewhat conductive and quite
noble in comparison with the exposed areas of magnetic alloy. The unfavorable
anode-to-cathode area ratio can therefore result in aggressive galvanic corrosion.
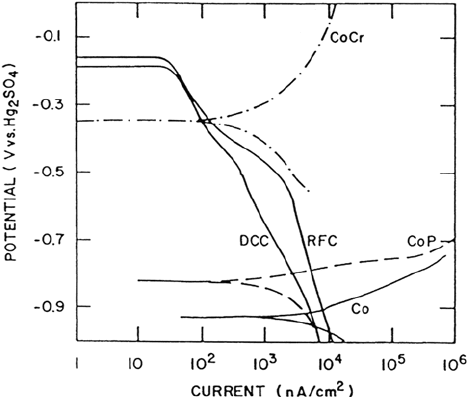
Figure 10 shows potentiodynamic polarization curves measured in DI water
[135,137]. The plated Co-8%P material was considered for use as the magnetic
alloy in thin-film disks when they were first developed. Like pure Co, it is not very
corrosion resistant and does not readily passivate. The corrosion potential of two
different sputter-deposited carbon thin films is seen to be about 600 mV higher than
that of plated CoP. Although the nature of C thin films can change drastically as a
function of deposition conditions, the two C films sustain reasonably large cathodic
670 Frankel and Braithwaite
Copyright © 2002 Marcel Dekker, Inc.
currents. In fact, galvanically induced corrosion of the small active areas of the
magnetic alloy by the C overcoat is the primary mechanism of corrosion of thin-film
disks. When an uncoated CoP film is exposed to an aggressive gaseous environment
at 25°C containing 70% RH and 10 ppb Cl
2
gas, a 30-Å-thick uniform corrosion
product forms [135,137]. However, a C-coated CoP sample forms a high density
of localized corrosion product particles that are several μm in diameter. This
dimension is 100 times larger than the head-disk separation in advanced disk files.
Corrosion can thus be minimized by decreasing the galvanic mismatch between
the magnetic layer and the overcoat. As shown in Figure 10, a sputter-deposited
Co-17%Cr film has a corrosion potential much closer to C and is spontaneously
passive in the DI water droplet. In deaerated Na
2
SO
4
, the corrosion current was
found to decrease and the corrosion potential increased as the Cr content in CoCr
alloys increased from 0 to 20% [136,138]. Nonconducting overcoats can also
reduce corrosion but do not eliminate it if they are not totally covering, a requirement
that is quite difficult given the thickness limitations of the overcoat and the roughness
of most disks.
The trend in magnetic recording is, of course, to continually higher densities.
As with microelectronic devices, higher density is achieved by shrinking the
dimensions, including the separation of the magnetic medium and the sensor in the
head, which has implications relative to corrosion. In order to bring the head and
disk closer, the head must fly closer to the disk, and the carbon overcoat thickness
must decrease. The decrease in fly height means that less corrosion product
accumulated on the disk surface will cause detrimental interactions with the head.
Adecrease in carbon thickness is also potentially deleterious to corrosion resistance
Corrosion of Data Storage Devices 671
Figure 10 Potentiodynamic polarization curves of thin-film disk materials in a droplet
of DI water. Parameter definitions: DCC DC sputtered C; RFC RF sputtered C; CoCr, Co +
l7%Cr; CoP, CO + 8%P; Co, pure Co. (From Ref. 135.)
Copyright © 2002 Marcel Dekker, Inc.
because it is harder to cover the magnetic layer with a very thin carbon overcoat. On
the other hand, in order to fly closer, the disk roughness must also decrease. The trend
toward smoother disks is beneficial to corrosion resistance because the magnetic
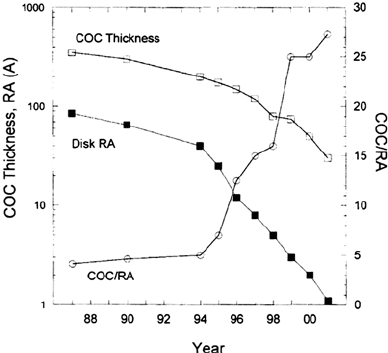
layer can then be more easily covered by the carbon overcoat. Figure 11 shows the
time trends for carbon overcoat thickness and disk roughness average (RA), as well
as the ratio of the two. The average roughness of magnetic disks has decreased faster
than the carbon overcoat thickness. Therefore, the ratio of COC thickness to disk
roughness increased significantly during the 1990s, indicating that the COC should
now provide a better covering despite being much thinner. In essence, the disk
structure now tends to be more like Figure 3a than 3b. As a result, disk corrosion
should be less of a problem than in the past, and this trend will apparently continue
for the next few years.
Figure 12 presents polarization behavior in a droplet of water for more recent
disk materials and structures than those shown in Figure 10 [139]. The disk had low
roughness and a thin carbon overcoat. First note the two curves from full disk
structures. One is from a lubricated disk, the other from an unlubed disk. The lube
decreases the current only slightly, perhaps as a result of water displacement. The
anodic current flowing at a given potential from the unlubed disk is about 100 times
less than that of the blanket magnetic layer on glass. It is clear that the magnetic layer
is rather well covered by carbon. In the past, it was assumed that the anodic
current measured from a disk originated largely from spots not covered by COC.
However, for the disks studied here, the anodic signal is not coming only from the
small amount of uncovered magnetic layer because a blanket layer of C (unlubed) on
glass exhibits an electrochemical signal that is a large fraction (about half as large) of
the signal measured on the unlubed disk. Carbon is not really an extremely noble
material. More accurately, the oxidation of C is kinetically hindered. Carbon can, in
fact, oxidize to form various species, such as carbonyl, carboxyl, phenol, quinone, or
672 Frankel and Braithwaite
Figure 11 Trend of carbon overcoat (COC) thickness and disk texture or average roughness
(RA) with time. Also shown, with right axis, is the ratio COC/RA. (Data from HMT
Technology, Inc.)
Copyright © 2002 Marcel Dekker, Inc.
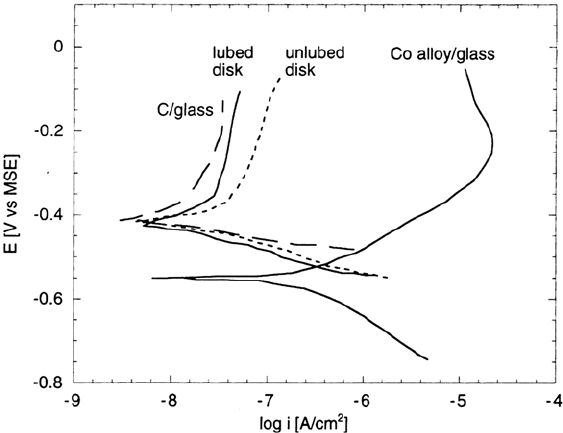
lactone [140]. The polarization curve for the carbon film is similar to that of a
spontaneously passive metal. It supports a reasonable amount of cathodic current,
which is the cause of galvanic corrosion. At anodic potentials, there is some steady-
state oxidation current, which is probably associated with a low rate of C oxidation.
From the polarization curves, it could be suggested that the difference in current
between the unlubed disk and the C blanket is the corrosion current of the magnetic
layer. The finding that carbon oxidation is now a significant fraction of the total
current flowing to a thin-film disk is reasonable given the trend of the increasing ratio
of carbon thickness to disk roughness. However, despite the good coverage, very
small areas of magnetic layer are still exposed in the disk structure. Therefore,
corrosion will still occur if the disks are exposed to aggressive environments (such as
chloride) or if problems in disk texture control occur.
An electrochemical study of the protectiveness of carbon overcoats as thin as
5 nm has been reported [141]. Polarization resistance of disk structures in
0.5 M sulfuric acid was measured. Disks coated with hydrogen-containing ion
beam deposited carbon were found to have much lower corrosion rates than those
coated with standard sputtered C of equivalent thickness. The composition of the
sputtered carbons was varied by changing the sputtering gas. The corrosion rate
depended on the type of carbon: ion beam C-H < sputtered C-H, < sputtered
C-N < sputtered C. The different corrosion rates may be a result of differences in
both coverage and catalytic activity of the carbon layers.
Novotny and colleagues [136,142] have described methods for estimating
lifetimes of disks in drives using accelerated T/H tests. In the first approach, the
extent of corrosion of a disk following T/H exposure was determined by a measurement
of the surface Co concentration on the disk surface using Auger electron spectroscopy
Corrosion of Data Storage Devices 673
Figure 12 Polarization curves in a water droplet for a lubed and unlubed disk as well as
a blanket magnetic layer and carbon layer on glass. (From Ref. 141.)
Copyright © 2002 Marcel Dekker, Inc.
