Martienssen W., Warlimont H. (Eds.). Handbook of Condensed Matter and Materials Data
Подождите немного. Документ загружается.

922 Part 4
070
140
120
100
80
60
40
20
0
30 40 50 60
T (K)
σ
r
(A m
2
kg
–1
)
H
c
=20×10
6
Am
–1
[110]
[110]
23
22
21
20
19
18
17
16
15
14
1.0
0.5
0
0 50 100 150 200 250 300
T (K)
'''
κκ
33
33
'
κ
33
33
''
κ
,
19.93
19.66
55
'
33
κ
60 65
100 kHz
T (K)
'
33
κ
21.60
21.15
1 MHz
T (K)
55 60 65
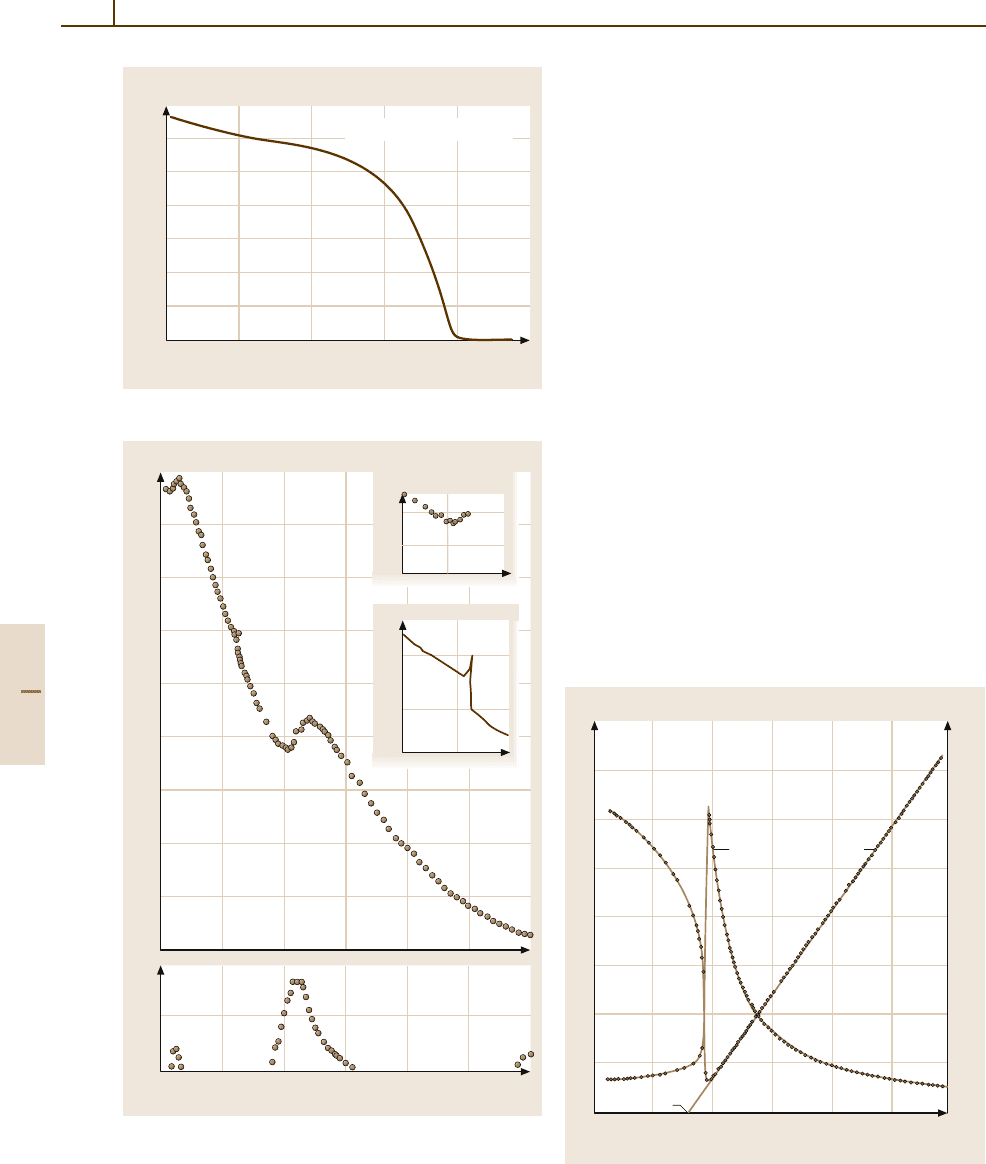
Fig. 4.5-46 Ni
3
B
7
O
13
I. κ
33
and κ
33
versus T . f =
100 kHz. The insets show details of κ
33
versus T at 100 kHz
and 1 MHz around Θ
f
Fig. 4.5-45 Ni
3
B
7
O
13
I. σ
r
versus T. σ
r
is the remanent
magnetization. The sample was cooled down to 4.2K in
a magnetic field of 1.6×10
6
A/m parallel to [100] prior to
the measurement
take place simultaneously. There is a magnetoelec-
tric effect, where the magnetic polarization is reversed
by reversal of the electric polarization and vice
versa.
4.5.4.2 Inorganic Crystals
Other Than Oxides [5.3]
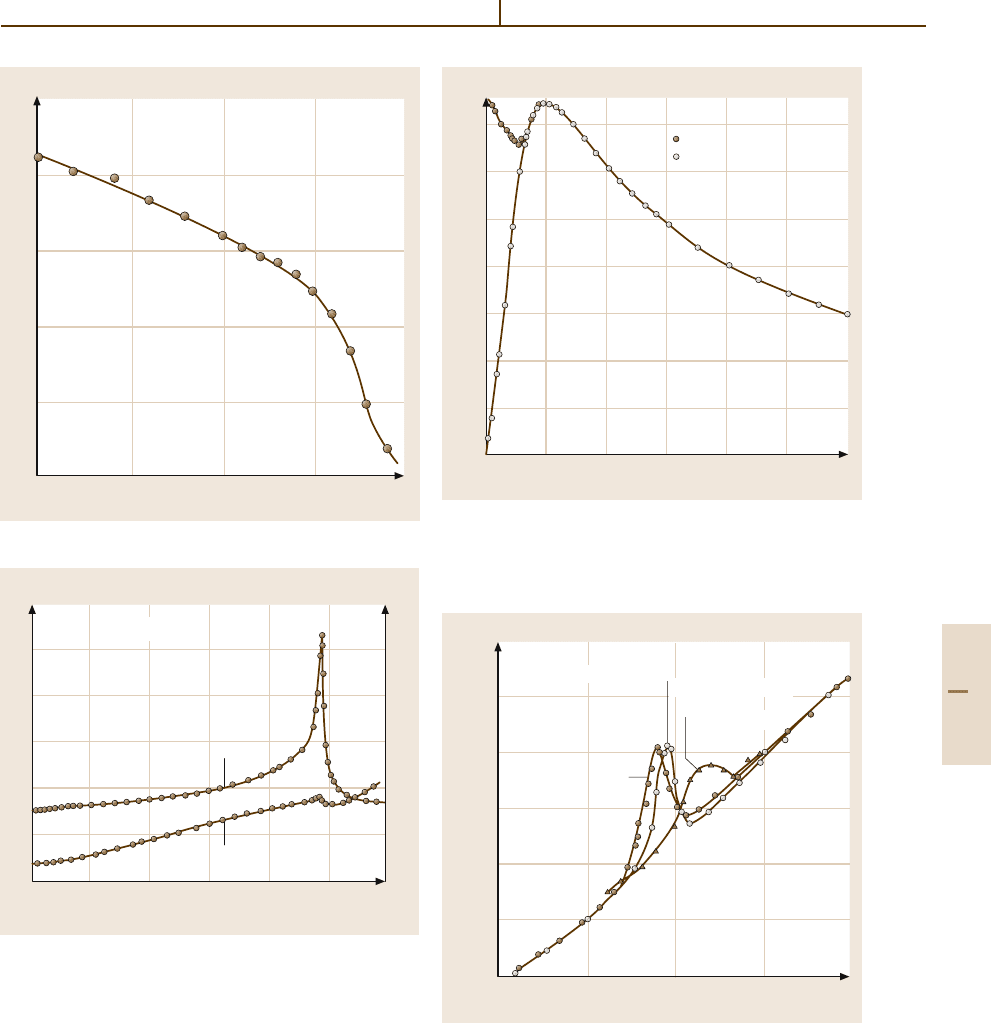
SbSI Family
SbSI (LB Number 20A-7). SbSI is ferroelectric be-
low 20
◦
C. The phase transition is of the displacive
type, a relatively rare characteristic in nonoxide ma-
terials. The crystal is photoconductive (Figs. 4.5-47 and
4.5-48).
BaMnF
4
Family
BaMnF
4
(LB Number 26A-2). This crystal exhibits a di-
electric anomaly at about 242 K (Fig. 4.5-49). The
coercive field is very large. The crystal is antiferromag-
netic below 25 K (Fig. 4.5-50). The dielectric constant
varies depending upon the magnetic field at low tem-
peratures (Fig. 4.5-51).
0
8
7
6
5
4
3
2
1
0
10 20 30 40 50 60
Θ
p
T (°C)
κ
c
f = 1 kHz
2.0
1.5
1.0
0.5
0
(10
4
))(10
–4
)
κ
c
(1/
κ
c
κ
c
1/
Fig. 4.5-47 SbSI. κ
c
and 1/κ
c
versus T
Part 4 5.4
Ferroelectrics and Antiferroelectrics 5.4 Physical Properties of 43 Representative Ferroelectrics 923
0
25
20
15
10
5
0
5101520
T (°C)
P
s
(10
–2
C m
–2
)
Fig. 4.5-48 SbSI. P
s
versus T
a
0
300
21
19
17
15
13
11
9
8.8
8.6
8.4
8.2
50 100 150 200 250
κ
a
κ
c
κ
c
κ
T (K)
f = 100 kHz
Fig. 4.5-49 BaMnF
4
. κ
a
and κ
c
versus T
0
350
300
250
200
150
100
50
0
50 100 150 200 250 300
T (K)
(10
–9
m
3
/mol)
magn m
χ
χ
χ
⊥
H
b
H
b
⊥
Fig. 4.5-50 BaMnF
4
. χ
magn m
versus T . χ
magn m
is the
magnetic susceptibility, and χ
⊥
and χ
are the magnetic
susceptibilities measured perpendicular and parallel, re-
spectively, to the b axis
0
12.095
12.090
12.085
12.080
12.075
12.070
12.065
20 22 24 26
T (K)
κ
a
f = 9.75 kHz
H = 8.36 ×10
4
A/m
2.51 ×10
5
A/m
H = 0
Fig. 4.5-51 BaMnF
4
. κ
a
versus T . Parameter: magnetic
field H. f = 9.75 kHz
Part 4 5.4
924 Part 4
HCl Family
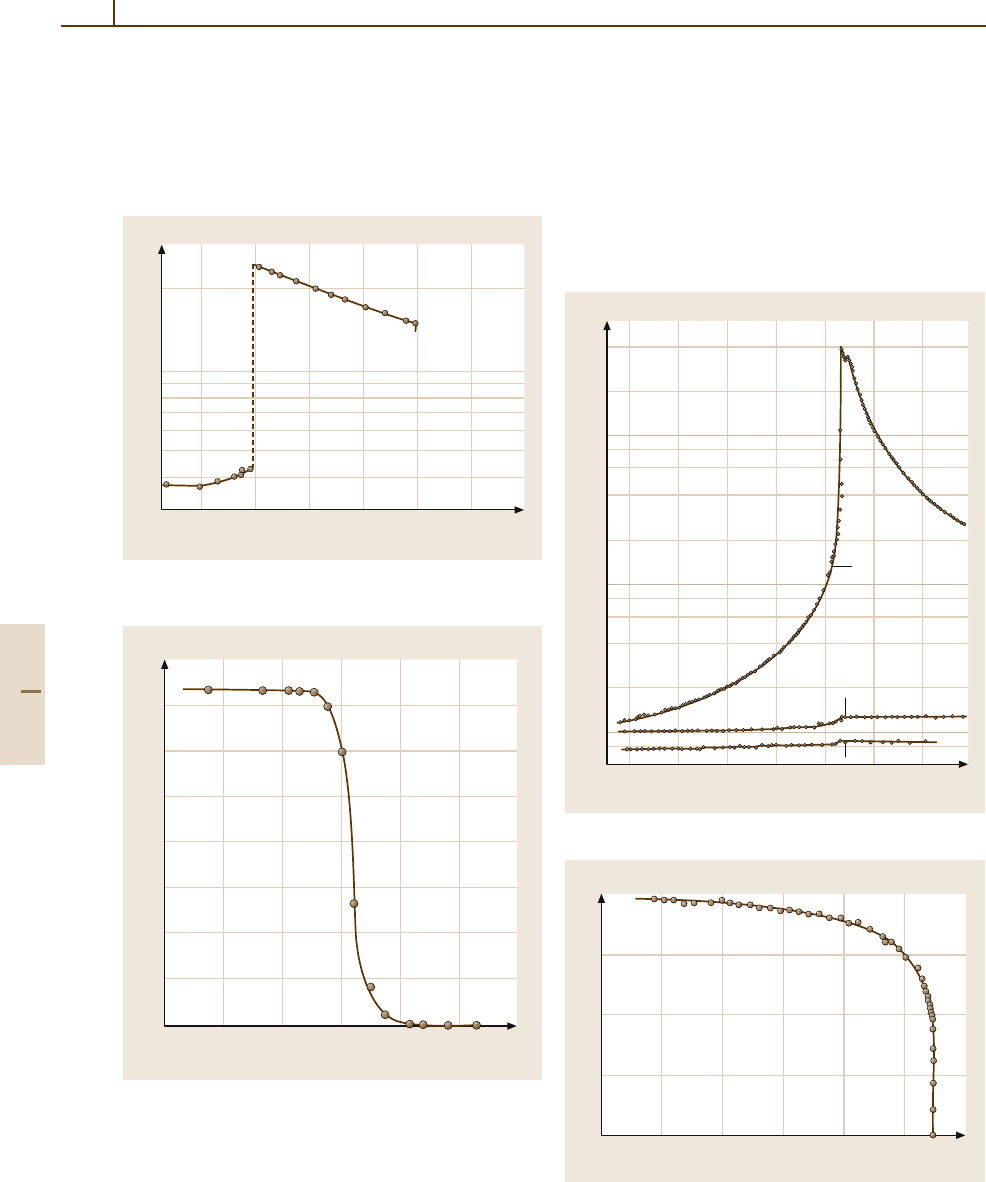
HCl (LB Number 27A-1). This crystal is ferroelectric be-
low 98 K. The chemical formula is the simplest one
among all known ferroelectrics. The coercive field is
large (Figs. 4.5-52 and 4.5-53).
0
30
20
10
8
6
5
4
3
80 100 120 140 160 180 200
κ
0
T (K)
T
melt
Fig. 4.5-52 HCl (polycrystalline). κ
0
versus T . κ
0
is the
static dielectric constant
4.0
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0
0.97 0.98 0.99 1.00 1.01 1.02 1.03
T/
f
Θ
P
s
(10
–2
C m
–2
)
Fig. 4.5-53 HCl. P
s
versus T/Θ
f
obtained from
pyroelectric-current measurement. Θ
f
= 98 K
Fig. 4.5-55 NaNO
2
. P
s
versus T , determined by
pyroelectric-charge measurement
NaNO
2
Family
NaNO
2
(LB Number 28A-1). This crystal is ferroelectric
below 163.9
◦
C (Figs. 4.5-54 and 4.5-55). The sponta-
neous polarization results from orientational order of
the NO
−
2
ions. Between 163.9 and 165.2
◦
C, the crystal
structure is incommensurately modulated with a wave
vector δa
∗
, where δ varies from 0.097 to 0.120 with
increasing temperature.
120 130 140 150 160
170 180 190
κ
10
3
10
2
10
f = 10 kHz
T (°C)
κ
b
κ
c
κ
a
Fig. 4.5-54 NaNO
2
. κ
a
,κ
b
,andκ
c
versus T
0
180
30 60 90 120 150
P
s
(10
–2
C m
–2
)
12
9
6
3
0
T (°C)
Part 4 5.4
Ferroelectrics and Antiferroelectrics 5.4 Physical Properties of 43 Representative Ferroelectrics 925
0 20025 50 75 100 125 150 175
II III I
70
60
50
40
30
20
10
0
κ
c
f = 100 kHz
T (°C)
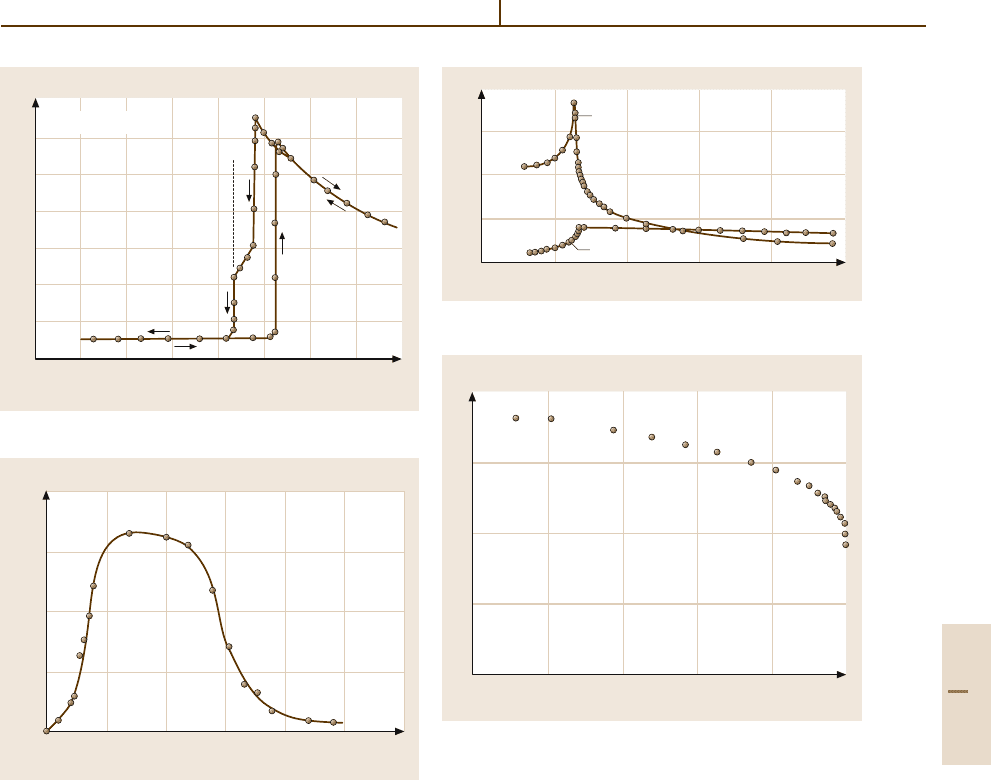
Fig. 4.5-56 KNO
3
. κ
c
versus T
10.0
7.5
5.0
2.5
0
115.0 117.5 120.0 122.5 125.0 127.5 130.0
P
s
(10
–2
C m
–2
)
T (°C)
Fig. 4.5-57 KNO
3
. P
s
versus T
KNO
3
Family
KNO
3
(LB Number 30A-2). This crystal is ferroelectric
between about 115 and 125
◦
C in a metastable phase III
which appears on cooling. Hydrostatic pressure stabi-
lizes this phase (Figs. 4.5-56 and 4.5-57).
KDP (KH
2
PO
4
) Family
KH
2
PO
4
(KDP) (LB Number 33A-1). KH
2
PO
4
is a clas-
sical and extensively studied ferroelectric crystal. It is
ferroelectric below 123 K (Figs. 4.5-58 and 4.5-59). The
transition is a typical ferroelectric phase transition, re-
lated to a configuration change in a three-dimensional
hydrogen-bond network. Figures 4.5-61 and 4.5-60
demonstrate changes in the proton configuration asso-
ciated with the phase transition. The transitions related
10
5
10
4
10
3
10
2
10
50 100 150 200 250 300
κ
T (K)
κ
c
κ
a
Fig. 4.5-58 KH
2
PO
4
. κ
a
and κ
c
versus T . f = 800 Hz
1.25
4
3
2
1
0
1.00 0.75 0.50 0.25 0
–T (K)
f
Θ
P
s
(10
–2
C m
–2
)
Fig. 4.5-59 KH
2
PO
4
. P
s
versus (Θ
f
−T). Θ
f
= 123 K
to hydrogen atom rearrangement in the hydrogen-bond
network are characterized by sensitivity to deuter-
ation and hydrostatic pressure, as demonstrated in
Figs. 4.5-62 and 4.5-63, respectively. For theoretical
studies of the phase transition, readers should refer
to [5.7]. The crystal is useful in nonlinear optical
devices.
CsH
2
PO
4
(LB Number 33A-3). This crystal is ferroelec-
tric below about 151.5 K. The crystal system of its
paraelectric phase (monoclinic) is different from that
of KH
2
PO
4
(tetragonal). The temperature dependence
of the dielectric constant above the Curie point deviates
considerably from the Curie–Weiss law, suggesting that
the transition is related to one-dimensional ordering of
hydrogen atoms in a hydrogen-bond network. Deuter-
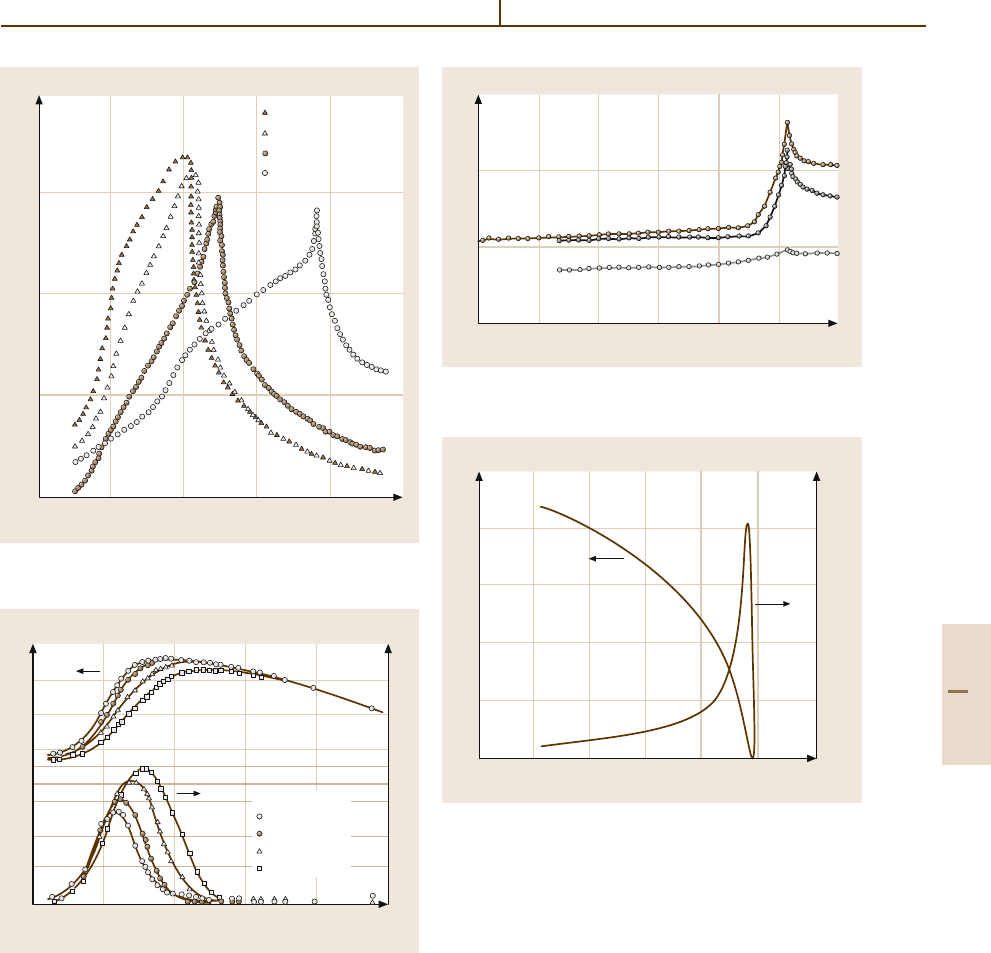
ation changes the transition temperature from 151.5to
264.7 K (Fig. 4.5-64).
Part 4 5.4
926 Part 4
K,P
O(1)
O(2)
O(1)
O(1)
K,P
O(2)
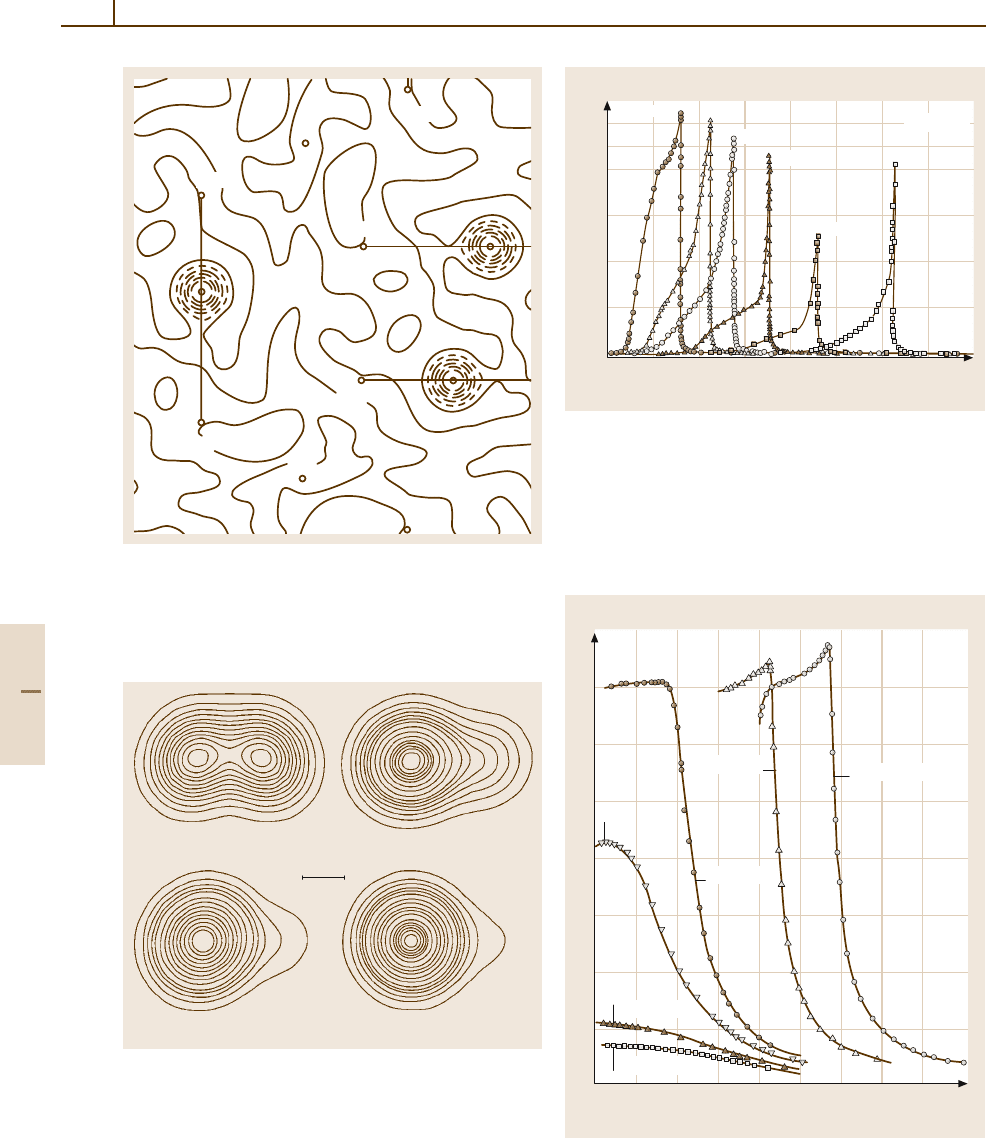
Fig. 4.5-60 KH
2
PO
4
. Fourier map of the projection of
the proton distribution on (001) in the ferroelectric phase
(77 K), determined by neutron diffraction. The proton dis-
tribution lies approximately on a line joining two oxygen
atoms O(1) and O(2) and closer to O(1)
0.2 Å
a)
b)
c) d)
f
Θ
+ 2K
f
Θ
– 1.3 K
f
Θ
– 20 K
f
Θ
– 10 K
Fig. 4.5-61a–d KH
2
PO
4
. Change of proton distribution
above and below the Curie point Θ
f
, determined by neutron
diffraction. Contours are all equally spaced.
(a) Θ
f
+2K;
(b) Θ
f
−1.3K;(c) Θ
f
−10 K; (d) Θ
f
−20 K
90
100
80
60
40
30
20
10
0
110110 130 150 170 190 210 230 250
T (K)
c
(10
3
)
κ
x =0
0.08
0.17
0.29
0.46
0.78
f = 3 kHz
Fig. 4.5-62 KH
2(1−x)
D
2x
PO
4
. κ
c
versus T . Parameter: x
RbH
2
PO
4
-NH
4
H
2
PO
4
(LB Number 33B-5). The mixed
crystals Rb
1−x
(NH
4
)
x
H
2
PO
4
, where 0.2 < x < 0.8,
show a characteristic temperature dependence of the di-
electric constants, suggesting that at low temperatures
0 18020 40 60 80 100 120 140 160
T (K)
κ
(10
2
)
c
p=1.8×10
8
Pa
8.4 ×10
8
15.4 ×10
8
16.9 ×10
8
19.3 ×10
8
21.0 ×10
8
Pa
8
7
6
5
4
3
2
1
0
Fig. 4.5-63 KH
2
PO
4
. κ
c
versus T . Parameter: hydrostatic
pressure p
Part 4 5.4
Ferroelectrics and Antiferroelectrics 5.4 Physical Properties of 43 Representative Ferroelectrics 927
0
10
6
10
5
10
4
10
3
10
2
100
150 200 250 300
T (K)
κ
b
x = 0
x = 0.03
x = 0.15
x = 0.57
Fig. 4.5-64 CsH
2
PO
4
and CsH
2(1−x)
D
2x
PO
4
. κ
b
versus
T . Parameter: x
0 100
60
40
20
0
8
6
4
2
0
20
40
60
80
c
κ
'''
c
κ
T (K)
f =
6.0 MHz
31.6 MHz
218.8 MHz
1000.0 MHz
Fig. 4.5-65 Rb
0.65
(NH
4
)
0.35
H
2
PO
4
. κ
c
and κ
c
versus T .
Parameter: f
a local order becomes predominant in a configuration of
hydrogen atoms without definite long-range order, i. e.
a dipole glass state develops (Fig. 4.5-65).
PbHPO
4
Family
PbHPO
4
(LB Number 34A-1). This crystal is ferroelec-
tric below 37
◦
C (Figs. 4.5-66 and 4.5-67). It exhibits
κ
T(K)
0 360
10
3
10
2
10
1
60 120 180 240 300
f = 1592 Hz
κ
b
c
(100)
κ
κ
Fig. 4.5-66 PbHPO
4
. κ
(100)
,κ
b
,andκ
c
versus T . κ
(100)
is
the dielectric constant perpendicular to the (100) plane
P
s
(10
–2
C m
–2
)
T (°C)
–60 60
2.0
1.6
1.2
0.8
0.4
0
0.15
0.12
0.09
0.06
0.03
0
–40 –20 0 20 40
p (10
–2
C/K m
2
)
Fig. 4.5-67 PbHPO
4
. P
s
and p versus T , measured on
(100) planar specimen. p =−dP
s
/dT is the pyroelectric
coefficient
characteristic critical phenomena, suggesting that the
spontaneous polarization results from an ordered ar-
rangement of hydrogen atoms in a one-dimensional
array of hydrogen bonds.
(NH
4
)
2
SO
4
Family
(NH
4
)
2
SO
4
(LB Number 39A-1). This crystal is fer-
roelectric below −49.5
◦
C. The dielectric constant
is practically independent of temperature above the
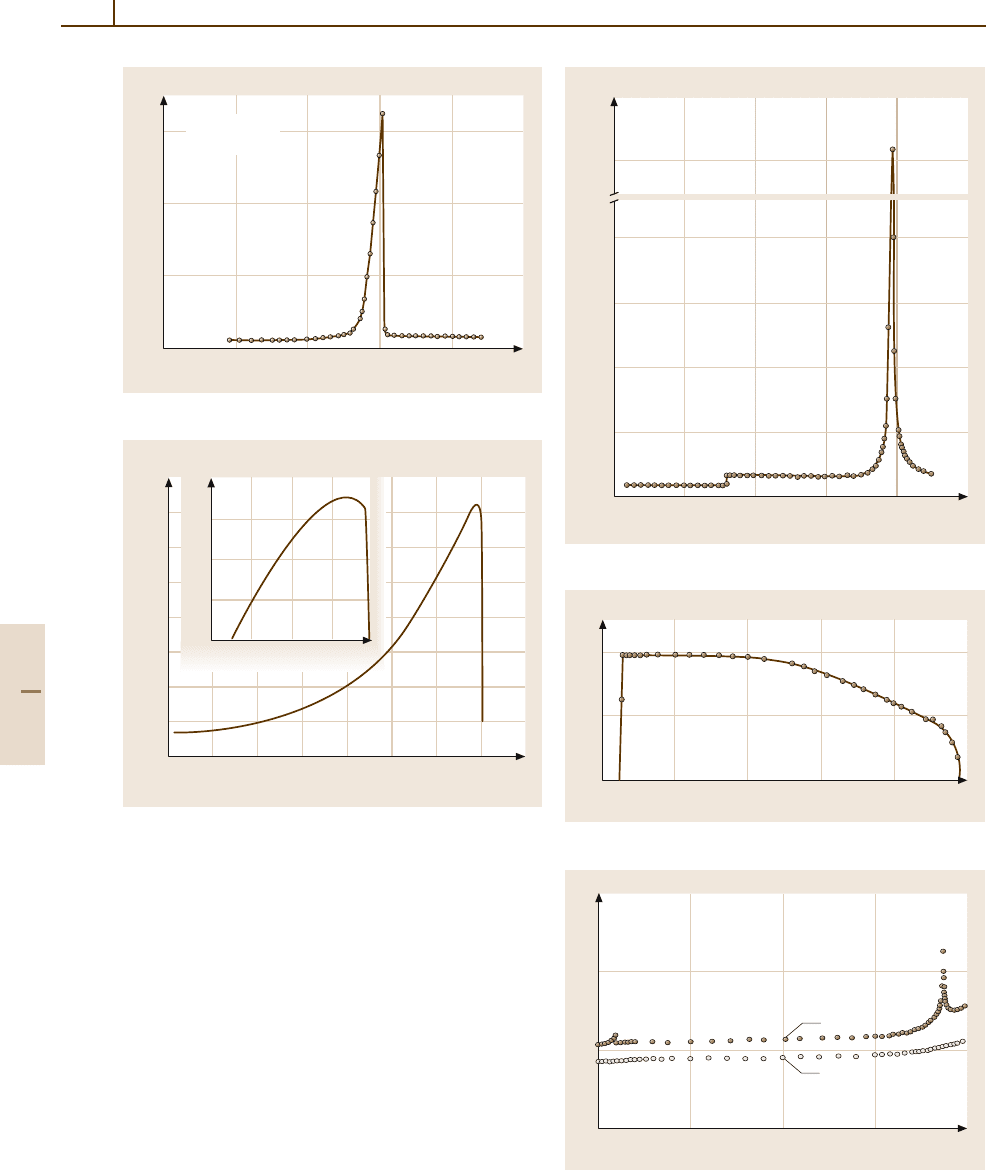
Curie point (Fig. 4.5-68). The spontaneous polarization
changes its sign at about −190
◦
C (Fig. 4.5-69), sug-
gesting a ferrielectric mechanism for the spontaneous
polarization.
Part 4 5.4
928 Part 4
T (°C)
–200
50
0
50
100
150
–150 –100 –50 0
5 ×10
2
V m
–1
f = 10 kHz
c
κ
Fig. 4.5-68 (NH
4
)
2
SO
4
. κ
c
versus T
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
–0.1
–260 –230 –200 –170 –140 –110 –80 –50 –20
T (°C)
P
s
(10
–2
Cm
–2
)
0.62
0.61
0.60
0.59
0.58
–60 –55
T (°C)
–50
Fig. 4.5-69 (NH
4
)
2
SO
4
. P
s
versus T
(NH
4
)HSO
4
Family
(NH
4
)HSO
4
(LB Number 40A-5). This crystal is ferroelec-
tric in the temperature range between −119 and −3
◦
C
(Figs. 4.5-70 and 4.5-71).
(NH
4
)LiSO
4
Family
(NH
4
)LiSO
4
(LB Number 41A-5). This crystal is ferroelec-
tric in the temperature range between 10 and 186.5
◦
C
(Figs. 4.5-72 and 4.5-73).
(NH
4
)
3
H(SO
4
)
2
Family
(NH
4
)
3
H(SO
4
)
2
(LB Number 42A-1). This crystal is fer-
roelectric in its phase VII below −211
◦
C. Another
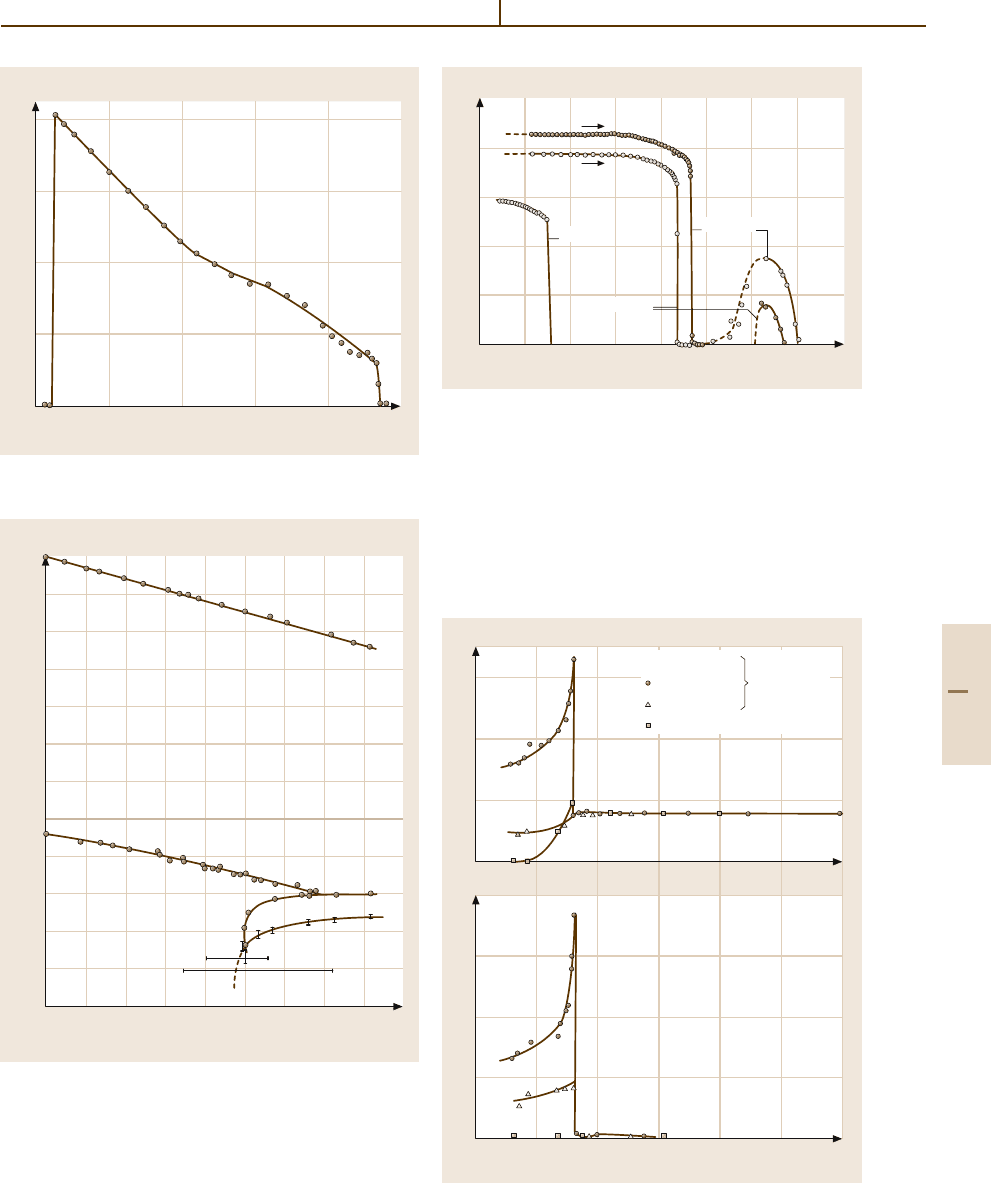
Fig. 4.5-72 NH
4
LiSO
4
. κ
a
and κ
b
versus T . f = 3kHz
–200 50
1450
–150
–100 –50 0
1400
200
150
100
50
0
T (°C)
c
κ
Fig. 4.5-70 NH
4
HSO
4
. κ
c
versus T . f = 10 kHz
–125
0.8
0.4
0
–100 –75 –50 –25 0
T (°C)
P
s
(10
–2
Cm
–2
)
Fig. 4.5-71 NH
4
HSO
4
. P
s
versus T
0 200
30
20
10
0
50 100 150
κ
a
κ
b
κ
T (°C)
Part 4 5.4
Ferroelectrics and Antiferroelectrics 5.4 Physical Properties of 43 Representative Ferroelectrics 929
0
0.8
0.6
0.4
0.2
0
40 80 120 160 200
T (°C)
P
s
(10
–2
Cm
–2
)
Fig. 4.5-73 NH
4
LiSO
4
. P
s
versus T
0912345678
(°C)
T
140
120
100
80
60
40
20
0
–20
–40
–60
–80
–100
p (10
8
Pa)
I
II
III
VI
VII
Fig. 4.5-74 (NH
4
)
3
H(SO
4
)
2
. T versus p phase diagram.
p is the hydrostatic pressure. Phases VI and VII are ferro-
electric
Fig. 4.5-76 (NH
4
)
2
Cd
2
(SO
4
)
3
. κ
[100]
and κ
[100]
versus T
–200 0
2.5
2.0
1.5
1.0
0.5
0
–175 –150 –125 –100 –75 –50 –25
T (°C)
P
s
(10
–2
C m
–2
)
x = 0.40
x = 0.93
x = 0.90
VI
VII
Fig. 4.5-75 ((NH
4
)
3
H)
1−x
((ND
4
)
3
D)
x
(SO
4
)
2
. P
s
versus
T . Parameter: x. Gray circles (for Phase VI), determined by
pyroelectric measurements. Brown circles (for phase VII),
determined by hysteresis loop measurements
ferroelectric phase, VI, is induced by hydrostatic pres-
sure (Fig. 4.5-74). When H is substituted by D, phase VI
appears at atmospheric pressure and the temperature of
the transition to phase VII becomes higher. Figure 4.5-75
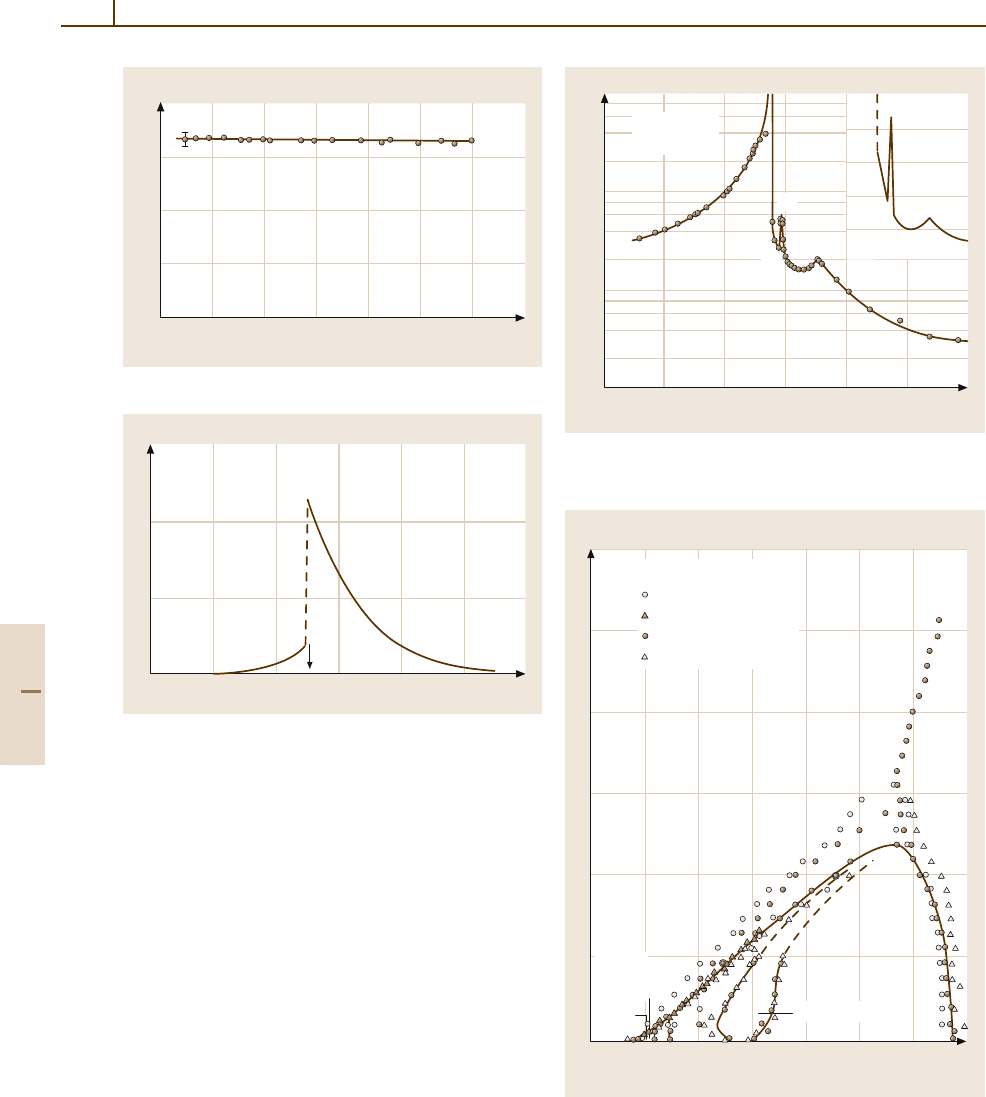
–200 140
12
11
10
9
–190 –180 –170 –160 –150
0.4
0.3
0.2
0.1
0
T (°C)
κ
'
[100]
E
bias
=
0
2000 kV m
–1
3.3 GHz
at 10 kHz
(600 V m
–1
)
κ
''
[100]
Part 4 5.4
930 Part 4
80 94
0.8
0.6
0.4
0.2
0
82 84 86 88
90 92
P
s
(10
–2
Cm
–2
)
T (K)
Fig. 4.5-77 (NH
4
)
2
Cd
2
(SO
4
)
3
. P
s
versus T
–186
3
2
1
0
–184 –182 –180 –178 –176 –174
f
T (°C)
Q
12
(10
3
m
4
C
–2
)
Θ
Fig. 4.5-78 (NH
4
)
2
Cd
2
(SO
4
)
3
. Q
12
versus T. Q
12
is the
electrostrictive constant
shows temperature dependence of P
s
for three values
of x in ((NH
4
)
3
H)
1−x
((ND
4
)
3
D)
x
(SO
4
)
2
.
Langbeinite-Type Family
(NH
4
)
2
Cd
2
(SO
4
)
3
(LB Number 43A-13). This crystal is fer-
roelectric below about −184
◦
C. The dielectric constants
are insensitive to temperature above the transition point
(Fig. 4.5-76), and the spontaneous polarization does not
depend upon temperature (Fig. 4.5-77). The electrostric-
tive constant Q
12
, however, exhibits an anomaly at the
transition point (Fig. 4.5-78).
4.5.4.3 Organic Crystals, Liquid Crystals,
and Polymers [5.4]
SC(NH
2
)
2
Family
SC(NH
2
)
2
(LB Number 50A-1). This crystal exhibits at
least five phases, I, II, III, IV, and V (Figs. 4.5-79
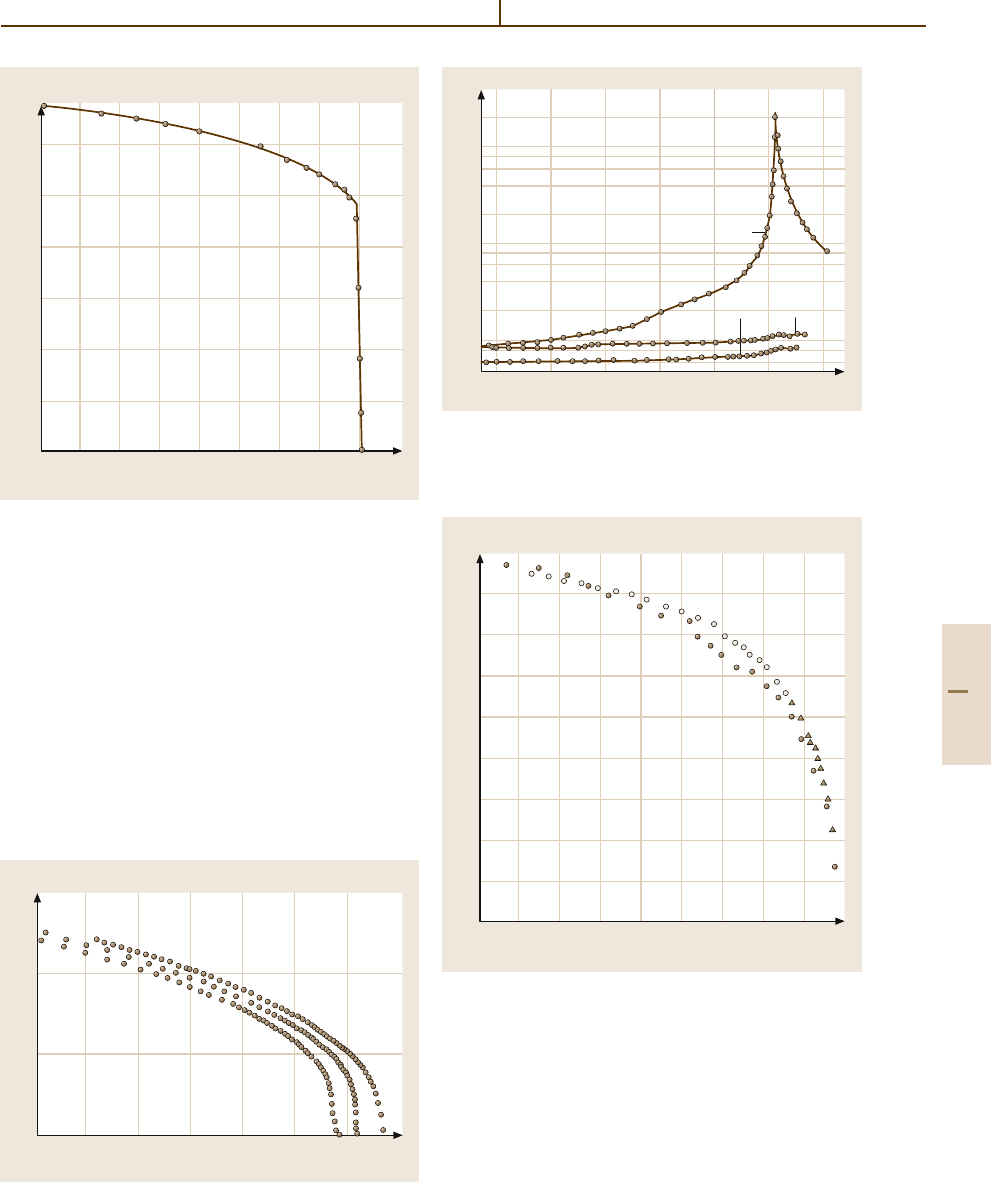
0 300
10
4
10
3
10
2
10
100 140 180 220 220
T (K)
κ
b
I
II
III
IV
V
f = 1 kHz
E = 10 V m
–1
Fig. 4.5-79 SC(NH
2
)
2
. κ
b
versus T
165
6
5
4
3
2
1
0
170 175 180 185 190 195 200
E
bias
(10
6
V/m)
T (K)
V
(Ferro-
electric)
Inc.
I
(Para-
elec-
tric)
δ =
1
–
9
III (δ=
1
–
8
)
Method
Pyroelectric response
Hysteresis loops
Dielectric constant
Polarization
Inc.
Fig. 4.5-80 SC(NH
2
)
2
. E
bias
versus T phase diagram.
The value of δ means that the phase is commensurately
modulated with a vector of wavenumber δc
∗
. Inc., incom-
mensurate phase
Part 4 5.4
Ferroelectrics and Antiferroelectrics 5.4 Physical Properties of 43 Representative Ferroelectrics 931
90
3.0
2.5
2.0
1.5
1.0
0.5
0
110 130 150 170
T (K)
P
s
(10
–2
Cm
–2
)
Fig. 4.5-81 SC(NH
2
)
2
. P
s
versus T for phase V
and 4.5-80). The crystal is ferroelectric in phase V
(Fig. 4.5-81), and slightly ferroelectric with a very
small spontaneous polarization in phase III. The crystal
structure is modulated commensurately or incommen-
surately except for phases I and V, as indicated in
Fig. 4.5-80.
DSP (Ca
2
Sr(CH
3
CH
2
COO)
6
) Family
Ca
2
Sr(CH
3
CH
2
COO)
6
(DSP) (LB Number 58A-1). This crys-
tal is ferroelectric below about 4
◦
C (Fig. 4.5-82). The
Curie–Weiss constant is small (60 K). Critical slowing-
down (see Sect. 4.5.3) takes place (Fig. 4.5-8).
–25 10
0.3
0.2
0.1
0
–20 –15 –10 –5 0 5
T (°C)
P
s
(10
–2
Cm
–2
)
1
2
3
–160
10
3
10
2
10
–120 –80 –40 0 40 80
f = 10 kHz
κ
b
κ
T (°C)
κ
c
κ
a
'
'
Fig. 4.5-83 (NH
2
CH
2
COOH)
3
· H
2
SO
4
. κ
a
,κ
b
,andκ
c
versus T . These quantities are referred to the unit cell
vectors: a
= a +c, b
=−b,andc
=−c
100
4.5
4.0
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0
125 150 175 200 225 250 275 300 325
T (K)
P
s
(10
–2
Cm
–2
)
Fig. 4.5-84 (NH
2
CH
2
COOH)
3
· H
2
SO
4
. P
s
versus T . P
s
is parallel to the b axis. Gray circles: values determined by
pyroelectric measurements. Triangles and brown circles:
determined from hysteresis loop by different authors
Fig. 4.5-82 Ca
2
Sr(CH
3
CH
2
COO)
6
. P
s
versus T .The
three curves show the effect of annealing: 1, unannealed; 2,
annealed at 330
◦
C for 60 h; 3, annealed at 330
◦
C for 60 h
and at 390
◦
Cfor5h
Part 4 5.4
