N?lting B. Methods in Modern Biophysics
Подождите немного. Документ загружается.


10.5 Detection of biological agents 195
bution of biological agents offers little information about the precise nature of the
agent. That is why biological agents are pyrolyzed prior to analysis in the IMS
(Fig. 10.27). Pyrolysis (see also Sect. 3.2) decomposes and vaporizes biological
agents and can be applied on bacteria and viruses (Fig. 10.28). The low vapor
pressure of most biological compounds requires an operation of the IMS at a
sufficiently high temperature (Fig. 10.29). The set-up virtual impactor / pyrolyzer
/ GC / IMS (Fig. 10.27) is capable to detect a few bacterial spores in a volume of
several 1000 liters.
11
Φ
-Value analysis
In Chap. 1 some inter-residue contact maps of protein transition states were
presented. Here, the method of
Φ
-value analysis underlying such maps and some
of its high-resolution applications are presented in more detail: the correlation of
inter-residue contacts with
Φ
-values (see Fig. 1.9) is the currently available
method with the highest resolution for protein folding transition states (Nölting,
1998, 1999a, b; Nölting and Andert, 2000).
The transition state corresponds to the state with the highest free energy in the
course of the reaction. Since it is only extremely short-living, at present its
structural resolution can not be carried out with NMR or X-ray crystallographic
analysis.
Φ
-Value analysis uses mutants as structural reporters and a combination
of equilibrium thermodynamics and kinetics methods (Nölting, 2005).
11.1 The method
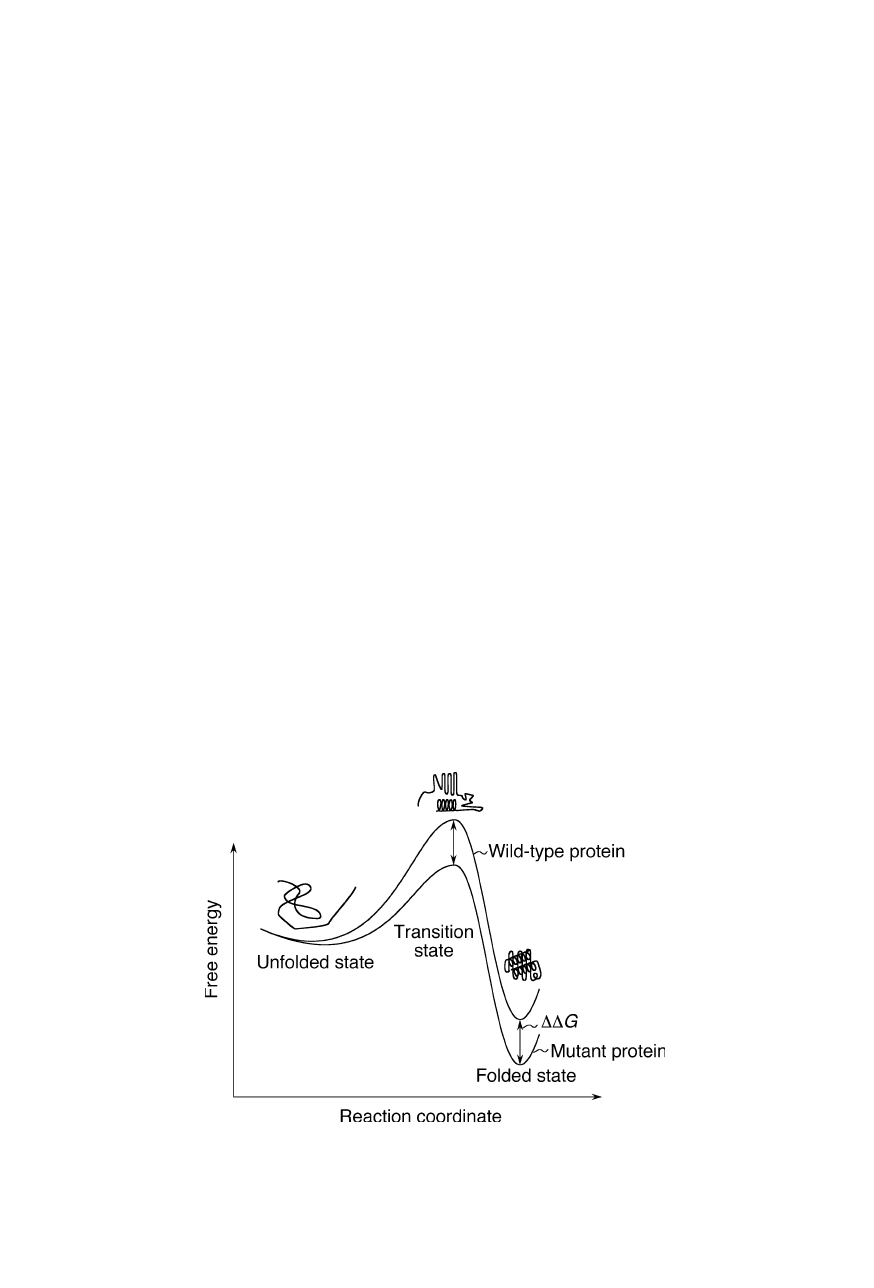
Fig. 11.1 shows the free energy changes in the folding reaction for a wild-type
protein and a mutant of this protein. One can see that in the course of the reaction
xxxxxxx
Fig. 11.1
Energy landscape along the reaction coordinate for the folding reaction of a
wild-type protein (top curve) and a mutant of this protein (bottom curve)
198 11
Φ
-Value analysis
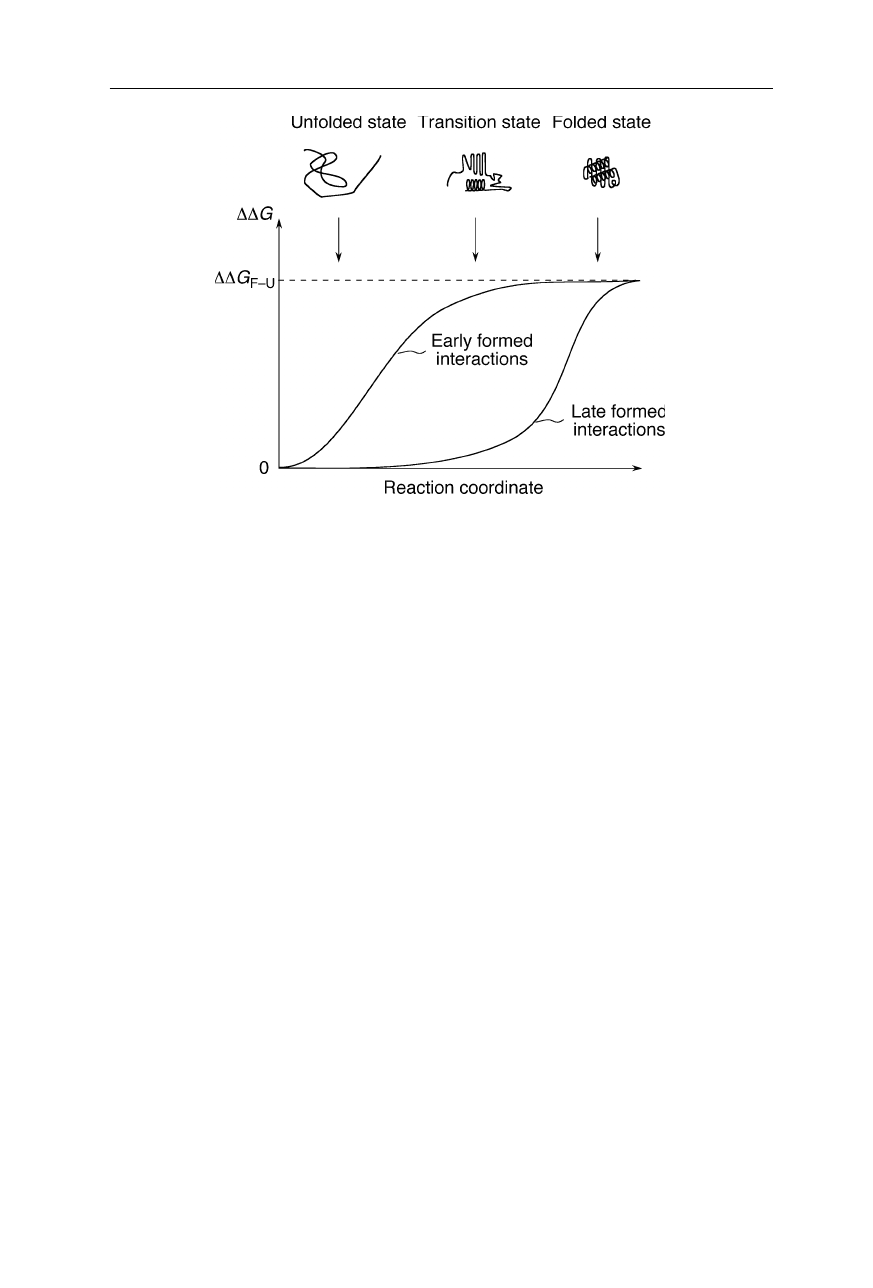
Fig. 11.2
Built-up of an energy difference,
∆∆
G, between wild-type protein and a mutant
in the course of the folding reaction.
∆∆
G
F–U
is the energy difference between wild-type
and mutant protein in the folded state
an energy difference,
∆∆
G, builds up between wild-type and mutant protein. This
build-up of
∆∆
G corresponds to the build-up of structure in the molecule. In
particular, the time point in the reaction at which
∆∆
G becomes significant
depends on the time at which the interactions probed by the mutation build up in
the molecule: If the interactions altered by the mutagenesis form early in the
folding reaction (left curve in Fig. 11.2), one usually observes an early increase of
|
∆∆
G|. In contrast, if the interactions probed by mutagenesis are formed late in the
folding reaction, there is usually no significant
∆∆
G till late in the reaction (right
curve in Fig. 11.2). So, by measuring
∆∆
G at the different stages of the folding
reaction one can find out when certain interactions in the molecule are becoming
formed. For the methods of measurement of
∆∆
G see Nölting (2005).
The formation of stable interactions in the molecule is usually expressed by the
Φ
-value which is a measure of the structure consolidation at the position of the
mutation on a scale from 0 to 1.
Φ
is defined as
Φ
=
∆∆
G /
∆∆
G
F–U
, where
∆∆
G
F–U
is the
∆∆
G in the folded state (Nölting, 2005). A
Φ
of 0 at a certain stage of the
folding reaction suggest the absence of stable structure at the position of the
mutation at this time. If structure is completely formed at the position of the
mutation at this stage of the reaction, one would expect a
Φ
-value of 1. Possible
sources of error in this analysis, e.g., the effect of non-native interactions, can be
decreased by using several mutants for the same part of the molecule.
So, in order to obtain information on the structure of a transition state one
simply needs to measure
Φ
of the transition state for many mutants and correlate
the data with the inter-residue contacts in the molecule (Nölting, 1998, 1999a, b).
11.2 High resolution of six protein folding transition states 199
11.2 High resolution of six protein folding transition states
This section presents the structural characteristics of the main transition states of
six proteins obtained by correlation of inter-residue contacts with
Φ
-values (see
also Sect. 11.1; Chap. 1; Nölting and Andert, 2000). The first four proteins,
barstar, barnase, chymotrypsin inhibitor 2 (CI2), and src SH3 domain are mono-
meric in the native state. Arc repressor is dimeric, and p53 is a tetramer with the
structure of a dimer of dimers. In the first five main transition states (Figs. 11.3–
11.7) one can see a very non-uniform consolidation of structure. It has been
shown that for all five proteins the most consolidated clusters (highlighted as
ribbons in Figs. 11.3–11.7; unconsolidated structure is displayed as wires) contain
a relatively higher content of residues which belong to secondary structure
elements than the non-consolidated parts of the molecules. On the other hand, all
six transition states with the exception of the src SH3 domain (Figs. 11.3–11.5,
11.7, 11.8) contain on average a similar content of secondary and tertiary structure
interactions (Nölting and Andert, 2000). This high resolution of folding transition
states led to an understanding of the mechanism of protein folding and of its
astonishing efficiency: folding of many proteins proceeds similar as the growth of
a crystal – largely driven by the propensity of secondary structure formation, but
also by the hydrophobic effect and other forces, a folding nucleus forms early in
the folding reaction. This nucleus then restricts the number of possible confor-
mations and enables further structure growth around it (Nölting and Andert, 2000;
Nölting et al., 2003; Nölting, 2005).
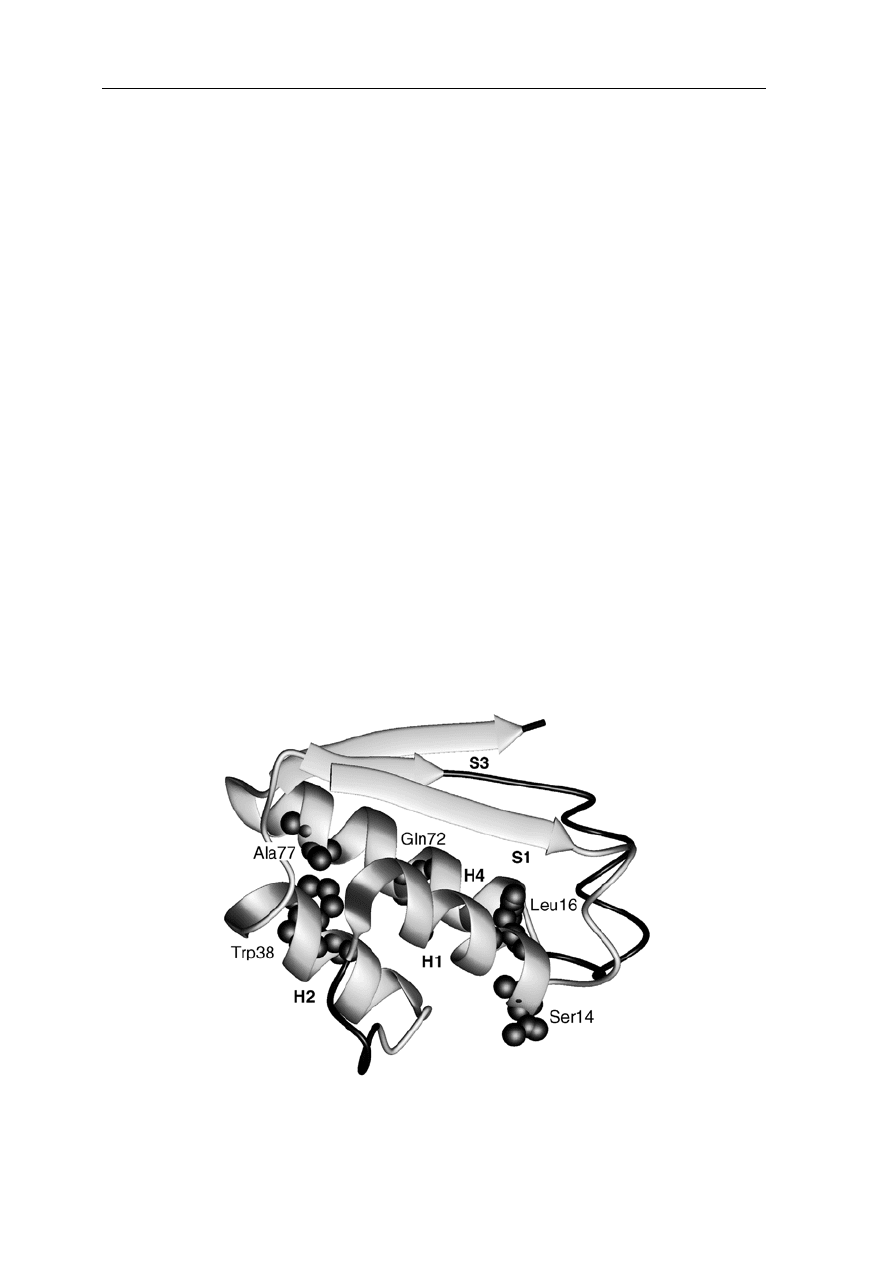
Fig. 11.3
Main transition state structure of barstar (Nölting and Andert, 2000). Consoli-
dated structure is highlighted as ribbons; unconsolidated parts of the molecule are shown
as wires. Amino acid residues with high
Φ
-values are highlighted as spheres. This figure
and the following figures in this section were prepared using MOLMOL, Koradi et al., 1996
200 11
Φ
-Value analysis
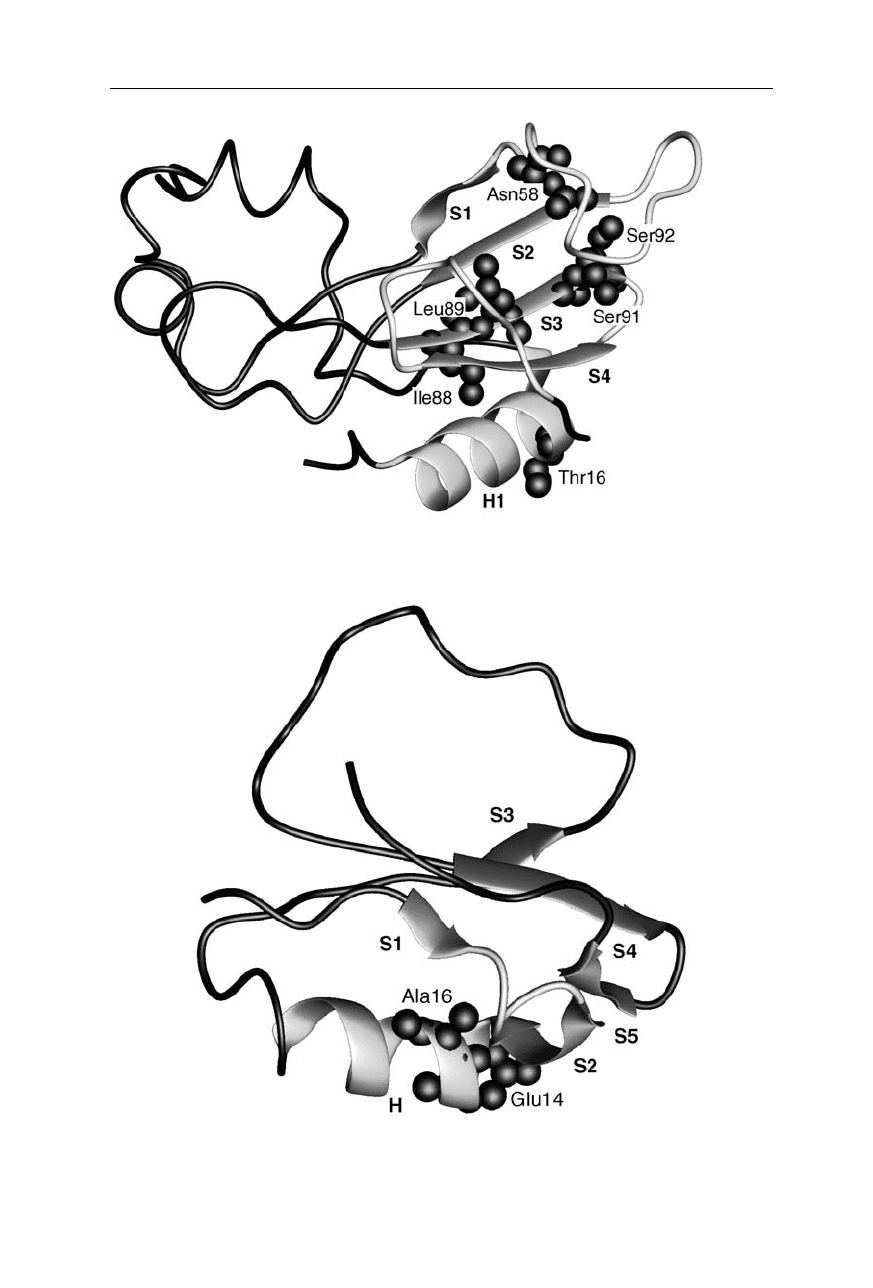
Fig. 11.4
Main transition state structure of barnase (Nölting and Andert, 2000). Consoli-
dated structure is highlighted as ribbons. For further explanation see Fig. 11.3
Fig. 11.5
Transition state structure of CI2 (Nölting and Andert, 2000). Consolidated
structure is highlighted as ribbons. For further explanation see Fig. 11.3
11.2 High resolution of six protein folding transition states 201
Fig. 11.6
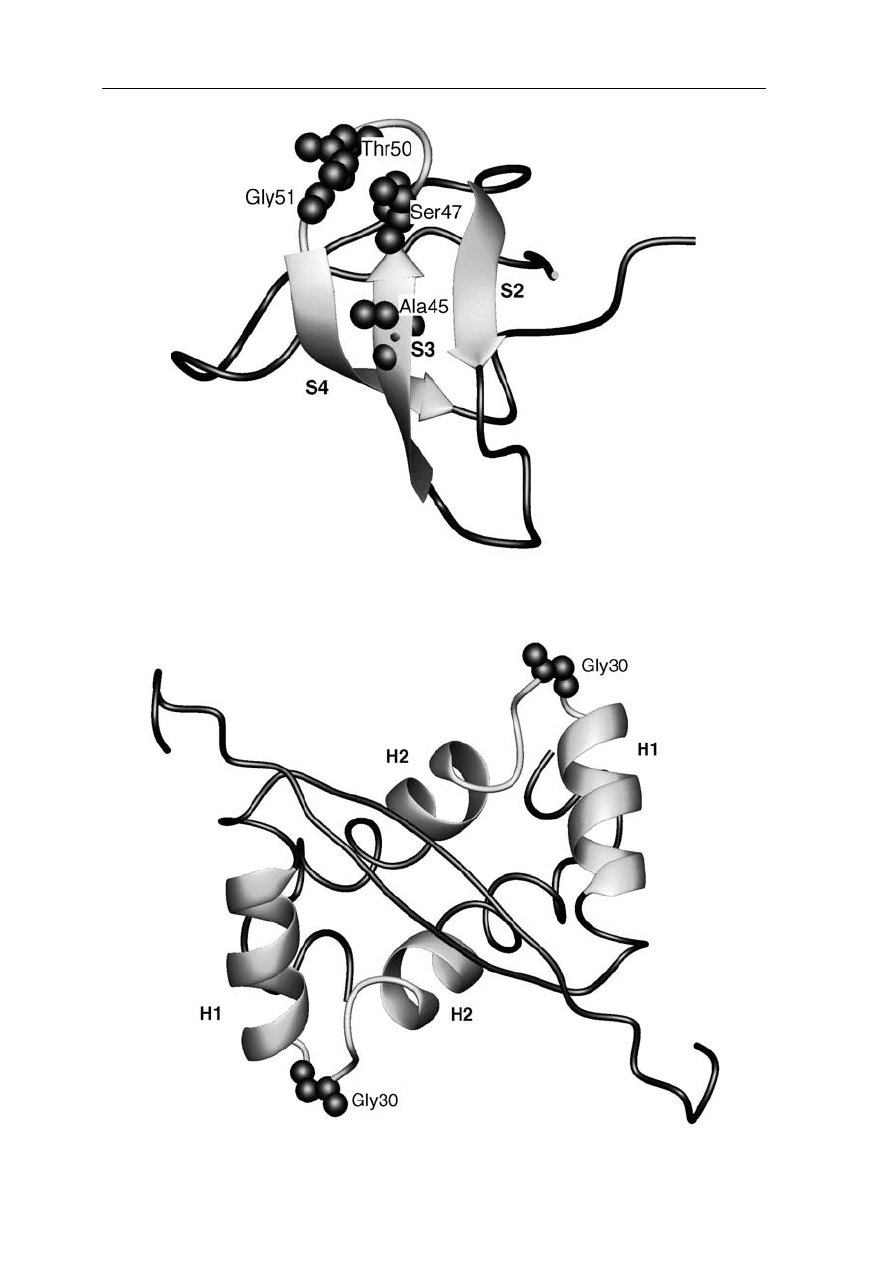
Transition state structure of the src SH3 domain (Nölting and Andert, 2000).
Consolidated structure is highlighted as ribbons. For further explanation see Fig. 11.3
Fig. 11.7
Transition state structure of Arc repressor (Nölting and Andert, 2000). Consoli-
dated structure is highlighted as ribbons. For further explanation see Fig. 11.3
202 11
Φ
-Value analysis
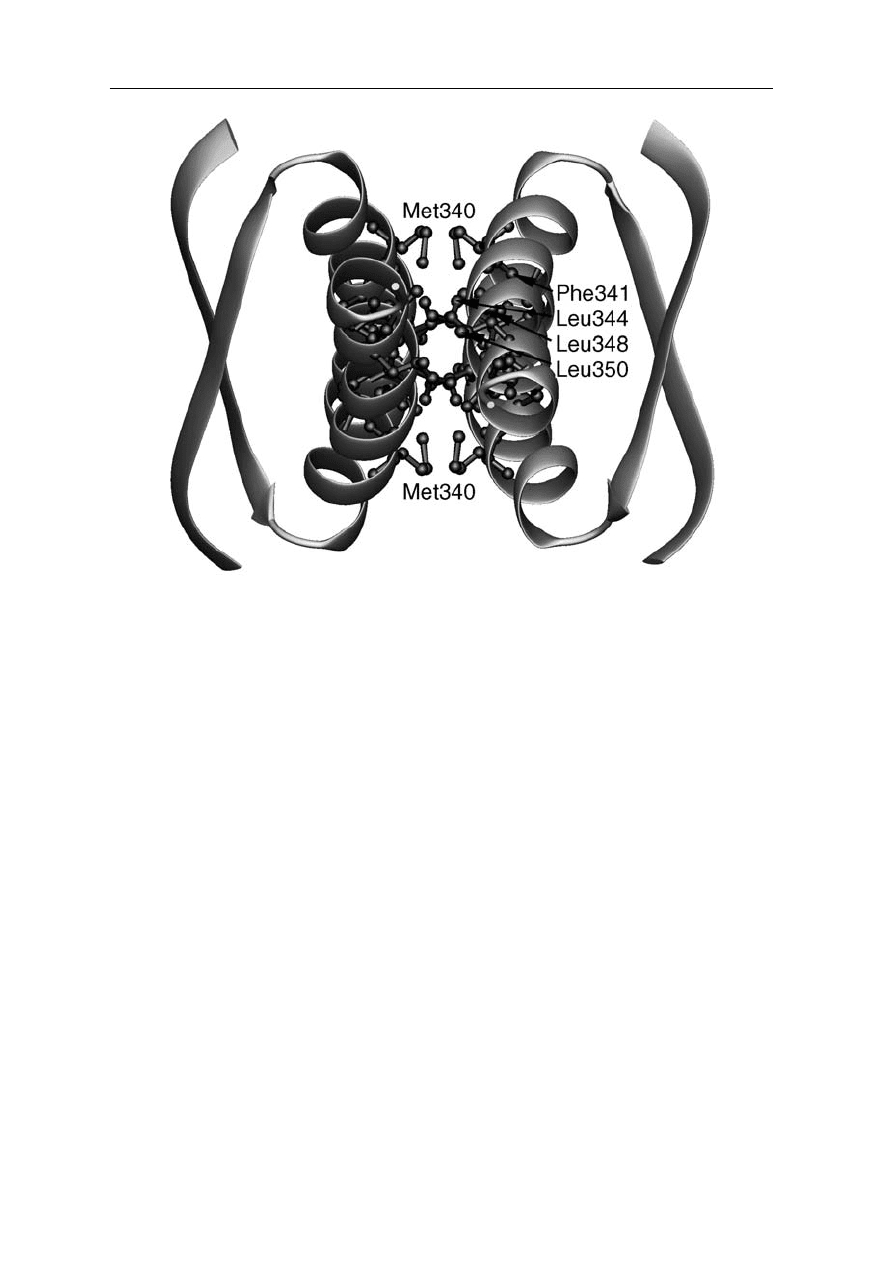
Fig. 11.8
Main transition state structure of p53 (Nölting and Andert, 2000). In this
transition state essentially all parts of the molecule are highly consolidated. Here the
residues with low
Φ
-values are highlighted as small spheres

12 Evolutionary computer programming
12.1 Reasons for the necessity of self-evolving computer
programs
Nature offers a tremendous amount of extremely complicated problems which
cannot easily be rationalized and resolved. Probably one has to accept that there
are scientific and technological problems too complex to be directly rationalized
by humans. A well-known example is the non-periodical movement of many
gravitationally interacting bodies in space. Since we are not able to imagine their
motions with a sufficient degree of perfection, we call it "chaos". Unfortunately,
humans obviously have significant intellectual difficulties to find theoretical
descriptions or models for phenomena which they do not comprehend. Similarly,
as an ape cannot write a mathematical equation beyond its intellectual abilities,
humans are not directly able to establish mathematical structures beyond their
intellectual limits. However, further progression of science and technology
urgently requires to overcome such limits.
One well-known example for a complicated problem is the so-called folding
paradox (see, e.g., Nölting, 2005): how can a protein find its unique native
conformation among the ~10
30
–10
200
possible conformations of an average small
protein in the unfolded state? One of the major difficulties of complex phenomena
like protein folding is often the lack of an efficient and solvable mathematical
description. Often one is able to write down some equations which describe the
physics of the system while not being able to solve them.
This chapter describes a method which can potentially provide solutions
beyond current human intelligence: in order to overcome the limits of rational
design, one lets a computer program evolve itself. The method is exemplarily
applied on protein folding and structure predictions (Nölting et al., 2004) and the
optimization of optical effects of nanoparticle arrays. In Sect. 12.5 the much
wider scope of this method is discussed.
12.2 General features of the method
Figs. 12.1 and 12.3 show the principle of operation of the method of self-evolving
programs. A so-called wild-type computer program is evolved towards higher
efficiency by mutagenesis and selection in a similar way as species evolve in
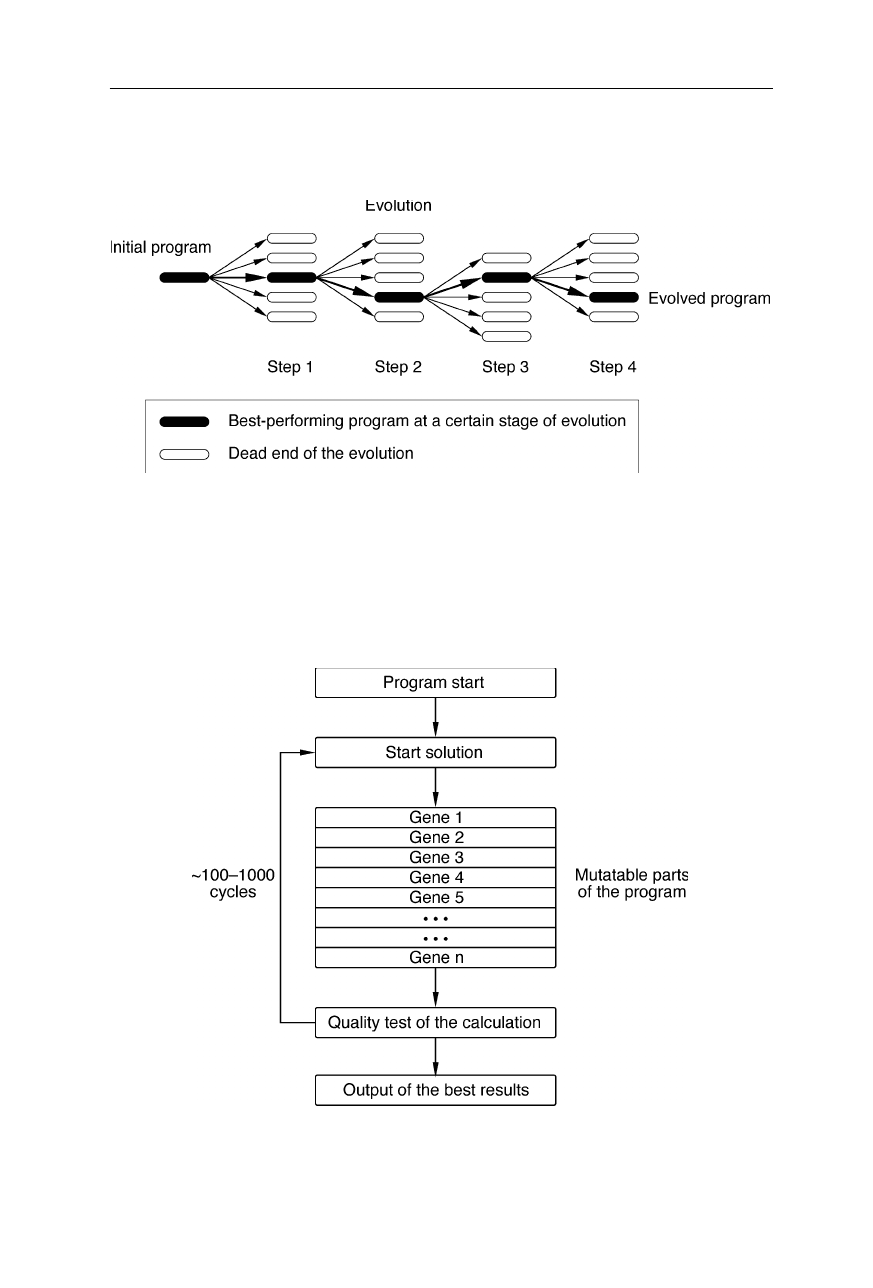
204 12 Evolutionary computer programming
nature: First a number of mutants of the wild-type program is created. The
performance of the mutants is then tested and the best performing program serves
xxxxxx
Fig. 12.1
Scheme of the method of evolutionary computer programming. The best perfor-
ming program at a certain stage of the evolution is shown as a filled rectangle. Open
rectangles indicate less-performing mutants which are usually sorted out. Essentially, the
method is based on the mutation and selection of computer programs similarly as for
species in the biological evolution. In this way, an initial program which contains mu-
tatable parts becomes highly optimized in the course of successive rounds of evolution
Fig. 12.2
A suitable structure of a program for self-evolution. The genes are the mutatable
parts of the program. The genes improve the start solution of the given task
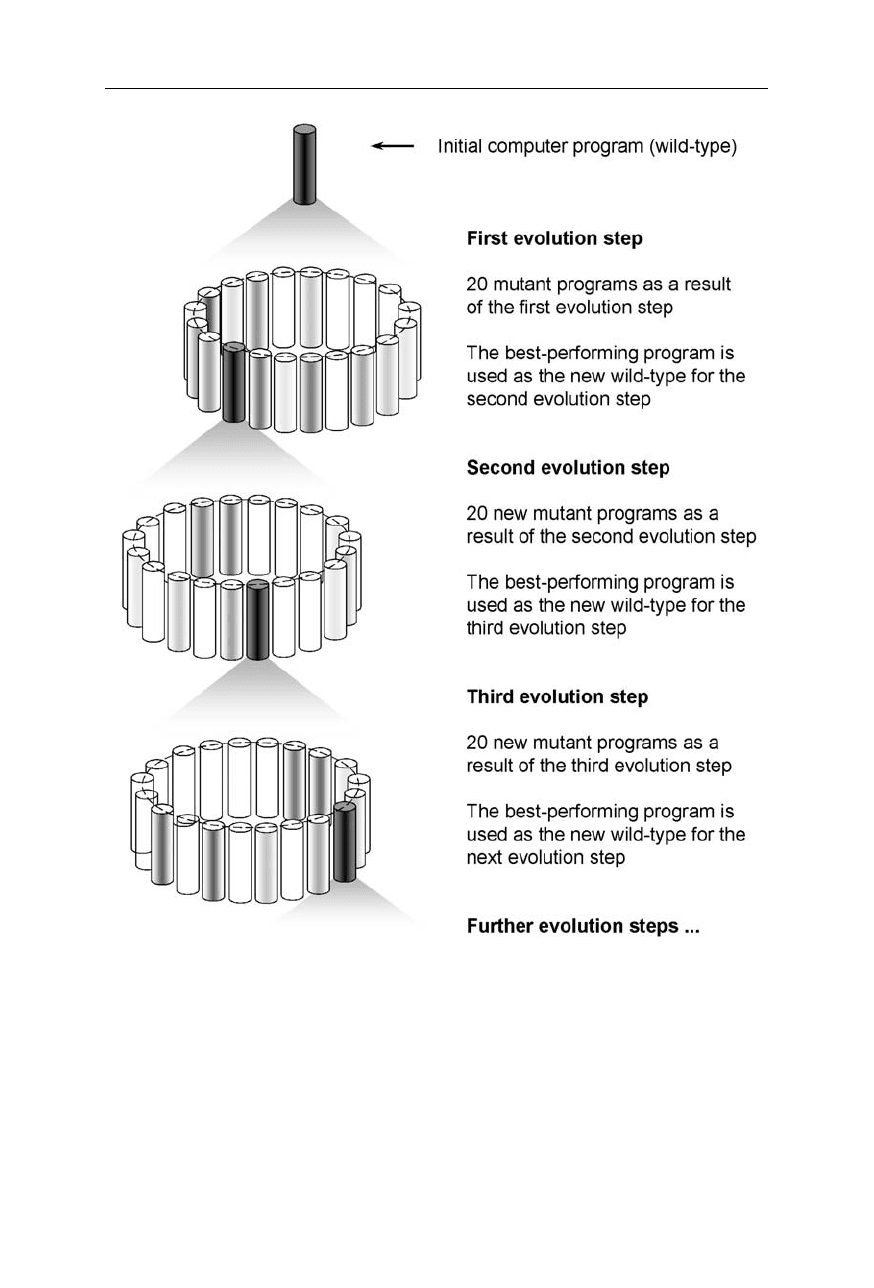
12.2 General features of the method 205
Fig. 12.3
Example of evolutionary computer programming
as a template for further mutagenesis (the next evolution step). The pathway of
evolution is highlighted by filled rectangles in Fig. 12.1. Open rectangles
correspond to dead ends of the evolution, e.g., mutants which did not perform best
