North, Gerald R., Erukhimova Tatiana L. Atmospheric Thermodynamics: Elementary Physics and Chemistry
Подождите немного. Документ загружается.

Problems 187
−40 −30 −20 −10 0 10 20 30 40
600
700
800
900
1000
0.4 1 2 4 7 10 16 24 32 40g/kg132 m
813 m
1537 m
3155 m
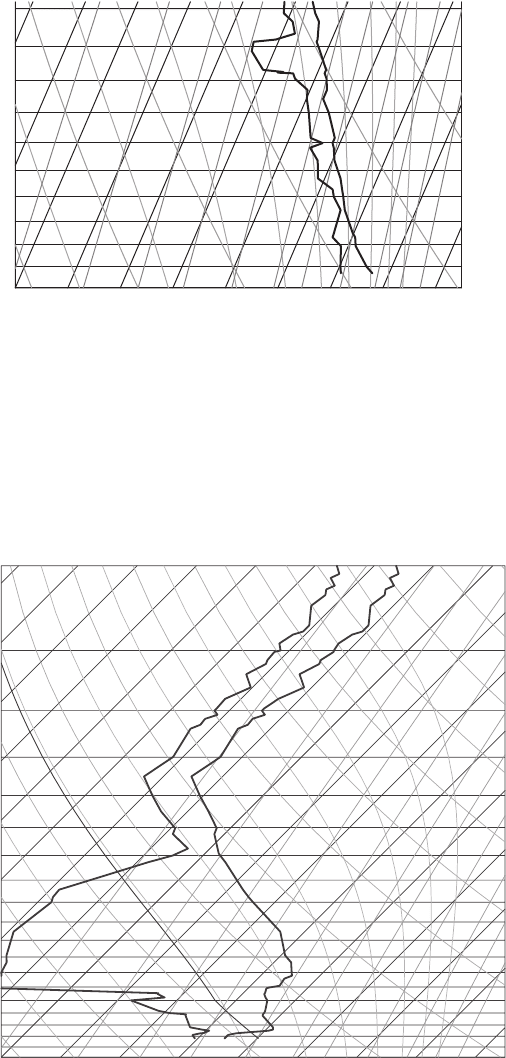
Figure 7.22 A sounding from Lake Charles, LA, at 00Z, June 1, 2007. Taken from
the University of Wyoming website.
(g) Is there a large CAPE?
(h) What is the mixing ratio at 800 hPa?
(i) What is the saturation mixing ratio at 800 hPa?
7.2 Refer to the sounding in Figure 7.23.
–40 –30 –20 –10 0 10 20 30 40
0.4 1 2 4 7 10 16 24 32 40g/kg186 m
798 m
1473 m
2997 m
5560 m
7140 m
9070 m
10250 m
11670 m
13530 m
16140 m
00Z 01 Jan 2007
72764 BIS Bismarck
100
200
300
400
500
600
700
800
900
1000
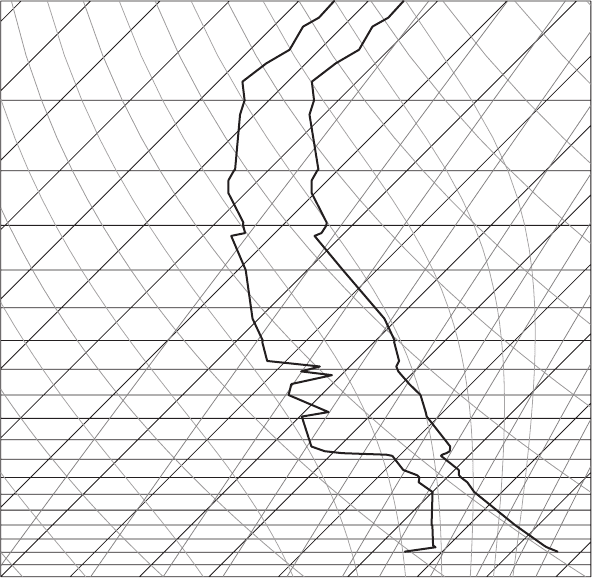
Figure 7.23 A sounding from Bismarck, ND, at 00Z, January 1, 2007. Taken from
the University of Wyoming website.
188 Thermodynamic charts
(a) Where is the tropopause?
(b) Describe the air mass over Bismarck on this day.
(c) Describe the humidity as a function of altitude.
(d) Are there any temperature inversions as a function of altitude?
(e) Is there any CAPE or CIN? Stable?
7.3 Refer to the sounding in Figure 7.24.
(a) Where is the tropopause?
(b) Describe the air mass over Bismarck on this day.
(c) Describe the humidity as a function of altitude.
(d) Are there any temperature inversions as a function of altitude?
(e) Is there any CAPE or CIN? Stable?
−40 −30 −20 −10 0 10 20 30 40
100
200
300
400
500
600
700
800
900
1000
0.4 1 2 4 7 10 16 24 32 40g/kg22 m
725 m
1482 m
3154 m
5880 m
7580 m
9670 m
10910 m
12380 m
14180 m
16650 m
00Z 01 Aug 2007
72764 BIS Bismarck
Figure 7.24 A sounding from Bismarck, ND, at 00Z, August 1, 2007. Taken from
the University of Wyoming website.
Problems 189
−40 −30 −20 −100 10203040
100
200
300
400
500
600
700
800
900
1000
0.4 1 2 4 7 10 16 24 32 40g/kg13 m
678 m
1396 m
3016 m
5650 m
7300 m
9280 m
10470 m
11870 m
13700 m
16200 m
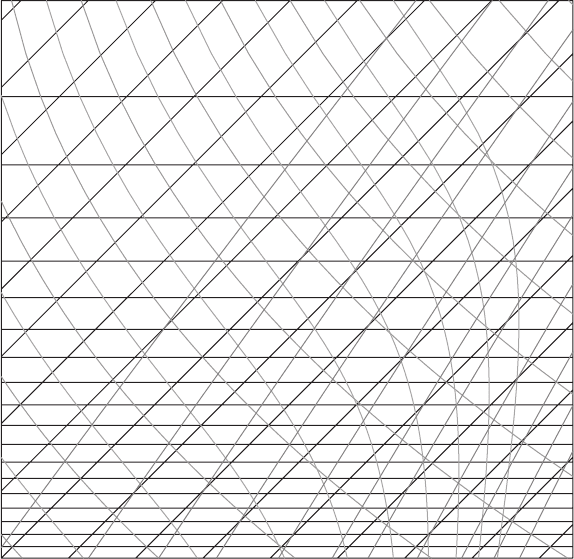
Figure 7.25 A blank skew T chart for Problem 7.4. Taken from the University of
Wyoming website.
7.4 The air at 1000 hPa and 11
◦
C has dew point −0.5
◦
C (see Figure 7.25).
(a) Find the mixing ratio, relative humidity, and the potential temperature using both
the skew T chart and formulas.
(b) Find the lifting condensation level using the chart.
(c) Find the equivalent potential temperature using the chart.
(d) What are the mixing ratio and the potential temperature if the parcel rises to
900 hPa?
(e) What is the equivalent potential temperature if the parcel rises to 600 hPa?
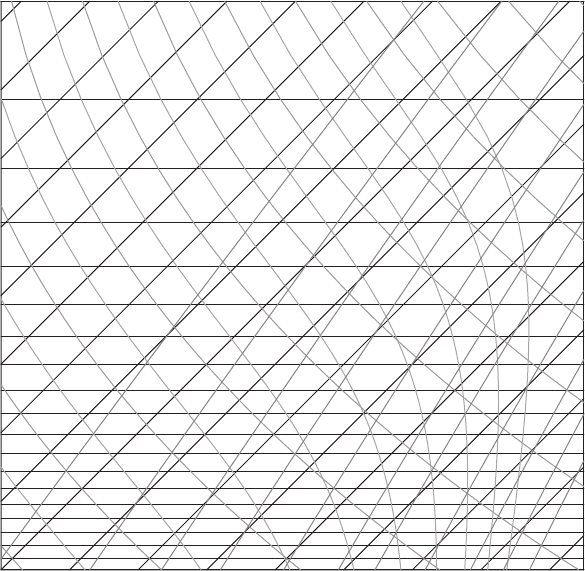
7.5 Consider a parcel of moist air that rises from the surface where p =1000 hPa to 400 hPa.
Assume all of the condensed water is precipitated out during the ascent. The parcel then
descends (unsaturated) back to the surface. If the initial temperature is 20
◦
C and its
initial dew point is 0
◦
C, find the following (use Figure 7.26).
(a) How much water is condensed during the ascent?
(b) The temperature of the parcel and its dew point temperature when it returns to the
surface (1000 hPa).
190 Thermodynamic charts
−40 −30 −20 −10 0 10 20 30 40
100
200
300
400
500
600
700
800
900
1000
0.4 1 2 4 7 10 16 24 32 40g/kg13 m
678 m
1396 m
3016 m
5650 m
7300 m
9280 m
10470 m
11870 m
13700 m
16200 m
Figure 7.26 A blank skew T chart for Problem 7.5. Taken from the University of
Wyoming website.
8
Thermochemistry
Applications of thermodynamics form the heart of physical chemistry. With the
First Law of Thermodynamics we can find some elementary applications of
thermodynamics to processes relevant to the atmosphere. As an example, consider
the elementary reaction
CO
2
(g) + H
2
(g) → CO(g) + H
2
O(g) (8.1)
where the g in parentheses indicates that the chemical species is in the gaseous state
(the solid phase is indicated by s, liquid by l and aqueous solution by aq).
At the molecular level a molecule of CO
2
strikes an H
2
molecule and the
rearrangement collision occurs with a certain probability depending on velocities,
spatial orientation of the colliders, etc. The rate at which a reaction proceeds in
a system is the product of the likelihood of a collision between the important
parties and the probability of rearrangement, given the collision. Sometimes a CO
molecule bumps into an H
2
O molecule and the reverse reaction occurs. That the
reaction might go both ways is indicated by the equation
CO
2
(g) + H
2
(g) CO(g) + H
2
O(g). (8.2)
Once equilibrium is established (rate of reactions proceeding to the right equals
the rate of those going to the left) in a suitable enclosure, we can consider the matter
involved to be a thermodynamic system which can be treated by the methods of
equilibrium thermodynamics. The number of moles of the species ν
CO
2
, ν
H
2
, ν
CO
and ν
H
2
O
become thermodynamic coordinates or functions along with those we are
already acquainted with,
M, p, V , U , H, S, G, and T . In fact, we want to know how
these coordinates (equilibrium concentrations) vary as a function of the temperature
if the pressure is held constant. Fixed pressure is the usual condition for gas phase
reactions in the atmosphere since they can be taken as occurring in a small parcel or
volume element, whose pressure inside quickly adjusts to that outside (which in our
191

192 Thermochemistry
case depends on altitude). In the real atmosphere there are many chemical species
in various states of equilibrium. The task of the atmospheric chemist is often to sort
out which reactions are important, what the sources of the various species are, what
is the feasibility and energetics of chemical reactions and how fast the reactions
proceed.
8.1 Standard enthalpy of formation
Consider a general chemical reaction
a A + b B → c C + d D, (8.3)
where A and B are called the reactants, C and D are the products, and a, b, c and d
are integers (sometimes rational numbers) inserted to balance the equation.
Suppose the ingredients on the left-hand side of the equation (the reactants) are
placed in a closed container that is impermeable to matter crossing its bounding
surface. Furthermore, let the reaction (8.3) proceed from left to right at constant
pressure. If no heat is allowed to enter or leave the system during the (irreversible)
process, the final temperature will be different from that before the reaction began.
If the temperature of the system goes up, we say the reaction is exothermic. If the
temperature goes down, it is endothermic. Chemists have found a convenient way
of characterizing the energetics of such reactions. Suppose the reaction goes from
left to right to completion (no reactants remaining), then the heat required to restore
the system to its original temperature at constant pressure is its change in enthalpy
during the irreversible process, H .
In order to find the heat of reaction for a particular chemical process it is
necessary to start with the so-called standard enthalpy of formation of the individual
compounds. These are based upon the enthalpy needed to form the compound from
the state of the individual atomic species most commonly found in nature. For
example, the convention for the element oxygen is to start with the gaseous form
O
2
, not O. Similarly the base state according to the convention for nitrogen is N
2
and for hydrogen it is H
2
. For argon it is the atomic form Ar and for carbon it is C.
The standard enthalpy of chemical reaction, when reactants in their standard state
are converted to products in their standard states, is equal to the difference between
standard enthalpy of formation of products and reactants:
H
◦
=[cH
◦
(C) + dH
◦
(D)]−[aH
◦
(A) + bH
◦
(B)]
= [products] − [reactants]. (8.4)
The overbar indicates that 1 mol of the substance is considered, the superscript ◦
refers to the standard state, which is at 1 atm and 25
◦
C by convention (see Table 8.1).
If
H
◦
is negative, heat is released and the reaction is exothermic. Exothermic

8.1 Standard enthalpy of formation 193
Table 8.1 Standard enthalpies of formation for selected
compounds (
H
◦
in units of kJ mol
−1
)
The symbol in parentheses after the compound indicates whether
its physical state is liquid, solid or gas. All values relate to 298 K.
CO
2
(g) −393.51 CO(g) −110.53
CH
4
(g) −74.81 H(g) +217.97
H
2
O(g) −241.82 H
2
O(l) −285.83
O
2
(g) 0 O(g) +249.17
O
3
(g) +142.7 OH(g) +38.96
HNO
3
(g) −135.09 NO
2
(g) +33.19
NO(g) +90.25
reactions can proceed spontaneously in the atmosphere. If the opposite is true,
H
◦
is positive, the reaction is endothermic, and an external source of energy is
needed for the reaction to proceed.
The exothermic reactions can be significant for the thermal budget of the
atmosphere. The classical example is the reaction leading to the formation of ozone.
The heat released in this process dominates the form of the temperature profile in
the stratosphere.
Example 8.1 The main mechanism of ozone formation in the stratosphere is the
recombination of atomic oxygen:
O + O
2
+ M → O
3
+ M, (8.5)
where M is a molecule in the background gas which is needed to carry off the
excess momentum in a two-bodies-to-one molecular collision. Find how much
heat is released by this reaction.
Answer: To find how much heat is liberated, we need to calculate the enthalpy of
the reaction:
H
◦
=[H
◦
(O
3
) + H
◦
(M)]−[H
◦
(O) + H
◦
(O
2
) + H
◦
(M)]. (8.6)
Since
H
◦
(O
2
) = 0,
H
◦
= H
◦
(O
3
) − H
◦
(O). (8.7)
From Table 8.1,
H
◦
= 142.7 kJ mol
−1
−249.17 kJ mol
−1
= −106.4 kJ mol
−1
.
The minus sign indicates that this is an exothermic reaction. Therefore, with the
reaction of ozone formation (8.5) 106.4 kJ per mole is liberated. This liberated heat
warms the stratospheric air and raises its temperature which reaches a maximum
at about 50 km altitude. Note that the concentration of O in (8.5) is determined by
the photodissociation of O
2
,O
3
and other species.

194 Thermochemistry
Example 8.2 Suppose we wanted to know the change in enthalpy for the reaction:
CO
2
+ H
2
→ CO + H
2
O. (8.8)
We form
H
◦
= H
◦
(CO) + H
◦
(H
2
O) − H
◦
(CO
2
) − H
◦
(H
2
)
=+(−110.53) + (−241.82) − (−393.51) − (0.0)(kJ mol
−1
)
= 41.16 kJ mol
−1
.
H
◦
is positive, which means that this reaction is endothermic, and heat is absorbed
during the process.
8.2 Photochemistry
Further examples of endothermic reactions include the photochemical reactions.
In this case the additional source of energy necessary for the endothermic
reaction to proceed is solar radiation which can break the chemical bonds of
atmospheric species. In this book we will consider only one photochemical process:
photodissociation.
1
Physics refresher Solar radiation consists of electromagnetic waves.
Electromagnetic radiation has a dual wave-particle nature. This means that
electromagnetic radiation exhibits both wave-like and particle-like properties. In its
wave form electromagnetic radiation can be thought of as a group of superimposed
waves sometimes referred to as an ensemble propagating in vacuum with the speed of
light c = 2.998 × 10
8
ms
−1
independent of wavelength. Each wave in this ensemble
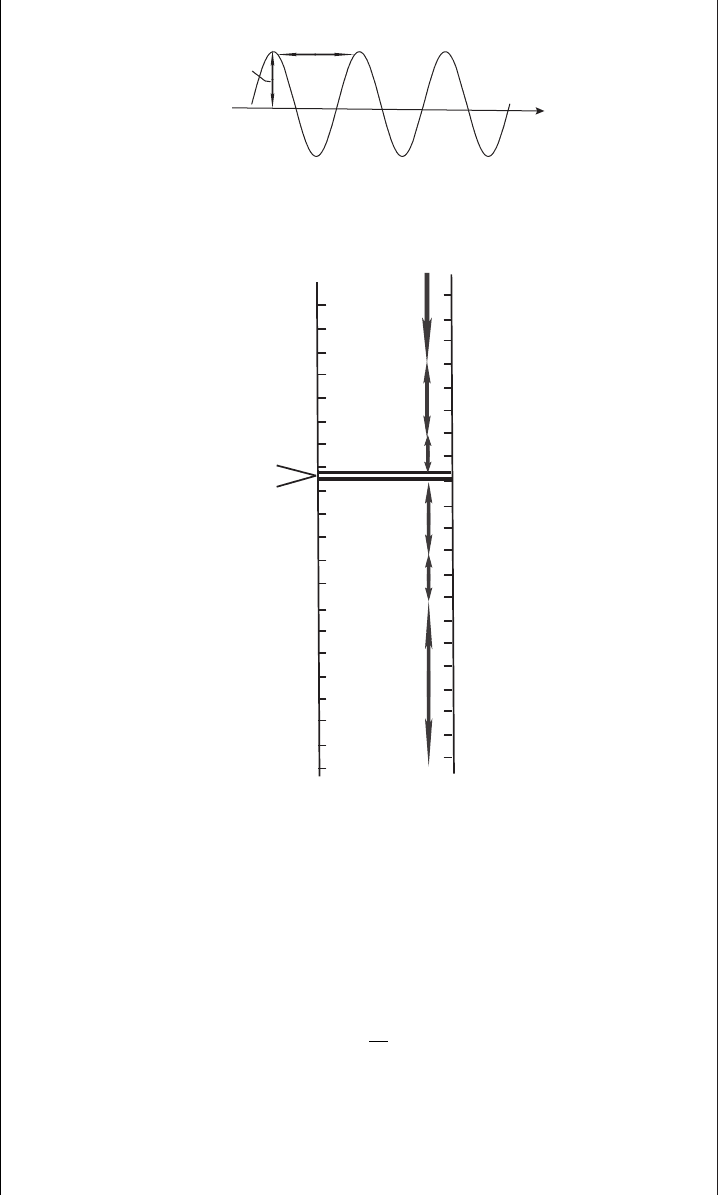
can be treated as a simple sinusoidal function (see Figure 8.1) with a certain
wavelength, frequency, and amplitude. The wavelength, λ, is the distance between
two successive peaks of the wave. The units of λ are meters. The frequency of a wave,
f , is the number of cycles that pass an observer in a second. The unit of frequency is
the hertz (1 Hz is one oscillation per second). The product of wavelength and
frequency for an individual wave is equal to the speed of light (speed is distance
divided by time): c = λ × f . From this equation one can see that waves with higher
frequencies have shorter wavelengths, and waves with lower frequencies have longer
wavelengths.
When radiation interacts with atoms or molecules, it can be absorbed or emitted
only by certain discrete amounts of energy. In other words, electromagnetic radiation
is quantized. The waves may be thought of as a beam of particles called photons
carrying discrete amounts or packages of energy. The energy of a photon of
1
Interested readers are referred to Basic Physical Chemistry for the Atmospheric Sciences by Peter V. Hobbs
(2000) for more information on photochemical reactions.
8.2 Photochemistry 195
x
λ
Amplitude
Figure 8.1 A sinusoidal wave of a given wavelength.
10 = 10 km
4
10 = 100 km
5
10 = 1000 km
6
10 =1km
3
10
2
10
1
10 = 10 cm
–1
10 =1 cm
–2
10 =1mm
–3
10 = 100 µm
–4
10 = 10 µm
–5
10 = 1 µm
–6
10 = 100 nm
–7
10 = 10 nm
–8
10 = 1 nm
–9
10 =
–10
10 =
–11
10 =
–12
10 =
–13
10 =
–14
10 nm
–1
10 nm
–2
10 nm
–3
10 nm
–4
10 nm
–5
Wavelength (m)
Frequency (Hz)
Visible (400–700 nm)
Gamma
rays
X-rays
Ultraviolet
Infrared
Microwaves
Radio waves
10
4
10
2
10
6
10
8
10
10
10
12
10
14
10
16
10
18
10
20
10
22
Figure 8.2 Electromagnetic spectrum.
frequency f is
E = hf (8.9)
where h is Planck’s constant, h = 6.62 × 10
−34
J s. If we express f in terms of λ we
obtain
E =
hc
λ
. (8.10)
Hence, the shorter wavelength (higher frequency) photons are more energetic. The
electromagnetic spectrum from radio waves to gamma rays is illustrated in
Figure 8.2. The most energetic photons are gamma rays. As one moves vertically
down the spectrum from gamma rays towards radio waves, the energy decreases and
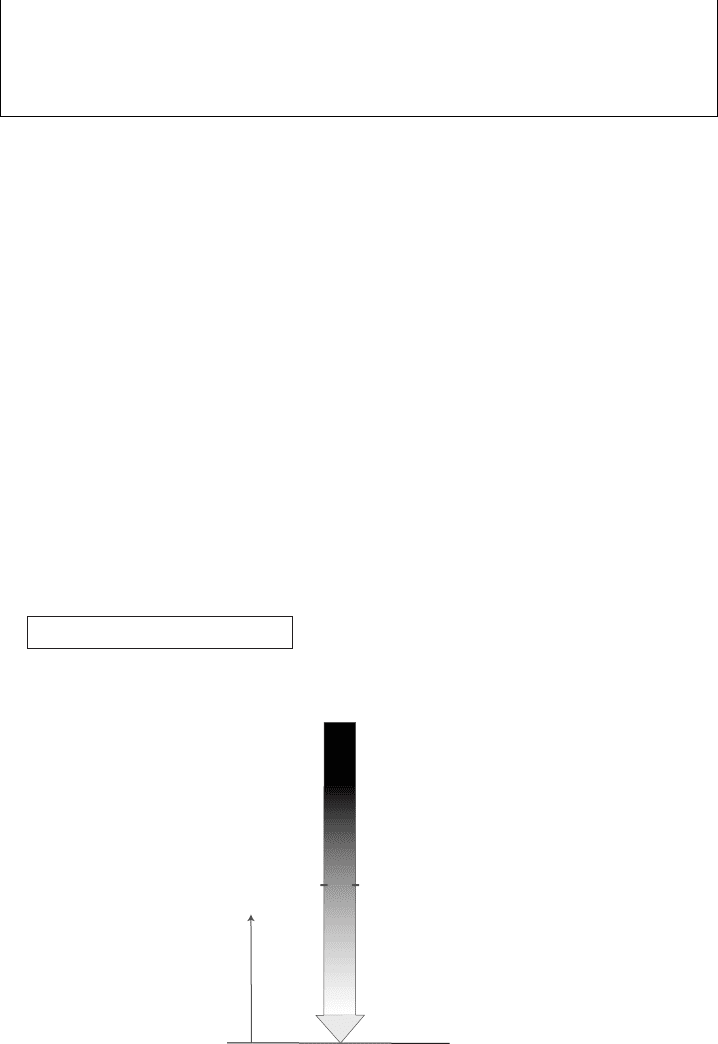
196 Thermochemistry
so does the frequency, while the wavelength increases. A narrow band of the spectrum
corresponds to the visible light. Photons in the visible range (approximately
400–700 nm) can be detected by a human eye. Ultraviolet radiation has shorter
wavelength (higher frequency) than the visible part of the spectrum.
To describe the radiation penetrating the atmosphere it is useful to introduce the
idea of an energy flux. The energy flux (energy passing per unit area perpendicular
to the beam, per unit time) is given by
F = energy flux = n
0
hcf (8.11)
where n
0
is the number of photons per unit volume (number density as in a gas).
The energy flux of solar photons at the top of the atmosphere is 1370 W m
−2
.
This parameter is called the solar constant. Solar photons propagating through the
atmosphere can be absorbed and/or scattered by atmospheric constituents. Consider
the attenuation of a photon flux at wavelength λ due to photon absorption assuming
normal incidence for simplicity (the sun is at zenith, directly overhead (Figure 8.3)).
We denote a photon energy flux at wavelength λ as F
λ
; its dimension is energy per
unit area, per unit time, and per unit wavelength. If at the top of the atmosphere the
flux per unit wavelength is F
λ
(top), the flux at height z, F
λ
(z), is described by
F
λ
(z) = F
λ
(top) exp(−τ(z)) [attenuation of a vertical solar beam]. (8.12)
F
λ
(top)
F
λ
(z)
z
z =0
z
Figure 8.3 Schematic diagram of a solar beam coming from directly overhead
with attenuation of the beam’s intensity indicated by shading.
