Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

82 Fluorine in Medicinal Chemistry and Chemical Biology
widely distributed and was primarily excreted in the urine as the parent compound or as
a single reductive metabolite, (1 R ,2 S ,4 R ,5 S ,6 S ) - 2 - amino - 6 - fl uoro - 4 - hydroxybicyclo[3.1.0]
hexane - 2,6 - dicarboxylic acid. Compound (+) - 7b showed a low brain - to - plasma ratio at
effi cacious doses in rats and was eliminated more slowly in rat brain than in plasma.
Plasma concentration was proportional to the drug dosage (1 mg/kg and 10 mg/kg) in rats,
with good bioavailability (70.7% and 75.3%, respectively). This pharmacokinetic profi le
supported the oral antipsychotic - like effects of (+) - 7b in rats. In dogs, oral bioavailabilities
of (+) - 7b were 63.4% and 69.0% at 0.1 and 0.3 mg/kg dosages, respectively; and no reduc-
tive metabolite was detected. In monkeys, however, the bioavailability was only 20.3%
and a reductive metabolite was found at a relatively high level in the plasma. In vitro
metabolic studies of (+) - 7b in liver subcellular fractions (microsomes and cytosol) showed
the presence of the reductive metabolite in specimens from rats, monkeys, and humans,
but not in specimens from dogs. The metabolism of (+) - 7b was not detected in liver
microsomes from any of the examined species. Similar to the in vivo result, (+) - 7b was
metabolized to the reductive metabolite in the cytosol in a stereospecifi c manner. The order
of in vitro metabolite formation (monkey > > rat ∼ human > > dog) was consistent with the
in vivo results in rats, dogs, and monkeys.
3.5 m G lu R 2/3 Antagonist 14a ( MGS 0039) and Its Prodrug
15a ( MGS 0210)
The original synthesis, pharmacology, and pharmacokinetics of compound 14 and its
prodrug 15 are discussed in this section [25, 34] .
3.5.1 Synthesis of 14a ( MGS 0039), Its Analogue, and Prodrugs of 14a ( MGS 0039)
The syntheses of mGluR2/3 antagonists, 13a , 13b , and 14 , as well as prodrug 15 (a prodrug
of 14a , MGS0039) from ( − ) - 25 (see Scheme 3.3 ) via key intermediates, 50 and 52 , are
illustrated in Schemes 3.8 3.9 [25, 39] .
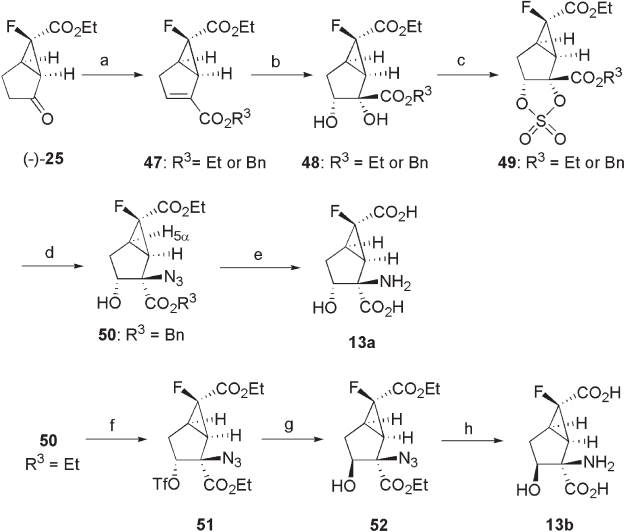
First, the synthesis of mGluR2/3 antagonists, 13a , 13b , is discussed (see Scheme 3.8 )
[25] . Ketone ( − ) - 25 was converted to the corresponding enol trifl ate by reacting with N - p
henylbis(trifl uoromethanesulfonimide) and LHMDS, which was then subjected to the Pd -
catalyzed carboalkoxylation with an alcohol (R
1
OH) at room temperature and atmospheric
pressure of carbon monoxide to give α , β - unsaturated ester 47 (71% (Et) and 33% (Bn)
yields). Compound 47 was converted to key intermediate 50 using the protocol
reported by Shao [54] . Thus, the stereoselective dihydroxylation of 47 with OsO
4
and N -
methylmorphine - N - oxide (NMO) afforded 48 (91% (Et and Bn) yields) as single product,
wherein OsO
4
reacted exclusively with the olefi n moiety of 47 from the opposite face ( exo
face) of the fused cyclopropane ring. Then, 48 was converted to 2,3 - cyclic sulfi tes with
SOCl
2
, followed by oxidization to cyclic sulfate 49 (93% (Et and Bn) yields). The regio -
and stereoselective nucleophilic ring - opening of 49 with NaN
3
, followed by hydrolysis
gave key intermediate 50 (91% (Et) and 89% (Bn) yields).
Fluorinated Conformationally Restricted Glutamate Analogues 83
Scheme 3.8 Synthesis of 13a and 13b . Reagents and conditions: (a) (i) LHMDS, TfNPh,
THF, (ii) CO, Pd(OAc)
2
, ( i - Pr)
2
NEt, PPh
3
, EtOH or PhCH
2
OH, DMF; (b) OsO
4
, NMO, MeCN;
(c) (i) SOCl
2
, Et
3
N, CH
2
Cl
2
, (ii) NaIO
4
, RuCl
3
, H
2
O, CCl
4
, MeCN; (d) (i) NaN
3
, DMF – H
2
O,
(ii) 20 % H
2
SO
4
; (e) (i) 10 % Pd/C, H
2
, AcOH, H
2
O, (ii) 10% HCl. (f) Tf
2
O, pyridine,
CH
2
Cl
2
; (g) KNO
2
, 18 - crown - 6, DMF, rt; (h) (i) Me
3
P, THF, H
2
O, rt or 10% Pd/C, H
2
, AcOH,
H
2
O (ii) LiOH, THF, H
2
O.
Compound 13a was obtained from key intermediate 50 through reduction (hydroge-
nation) of the azido group to an amino group as well as deprotection (hydrogenolysis) of
the benzyl ester over 10% Pd on carbon as the catalyst, followed by hydrolysis of the ethyl
ester moiety with 10% HCl (79% yield) [25] .
Compound 13b was synthesized in four steps via key intermediate 52 , in which the
confi guration of the hydroxyl group at the C - 3 position of 50 was inverted. Compound 50
was reacted with trifl uoromethanesulfonyl anhydride and pyridine to give trifl ate 51 (96%
yield), which was then reacted with KNO
2
in the presence of 18 - Crown - 6, followed by
post - treatment of the resulting nitrous ester with water to afford key intermediate 52 (80%
yield) [55, 56] . Reduction of the azide moiety of 52 by trimethylphosphine or hydrogena-
tion on Pd on carbon, followed by hydrolysis of the ester moieties gave 13b (48%
yield).
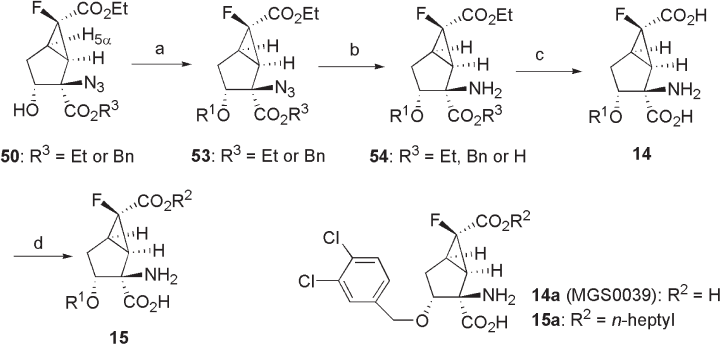
Etherifi cation of the hydroxyl group at the C - 3 position of 50 through benzylation
using benzyl trichloroacetimidate under acidic conditions [57, 58] or an alkyl trifl ate under
basic conditions gave 53 (42% (Et) and 17 – 90% (Bn) yields). Azide 53 was reduced to
84 Fluorine in Medicinal Chemistry and Chemical Biology
amine 54 by the Staudinger reaction [59, 60] or catalytic hydrogenation over Pd on carbon
(89% (Et) and 59 – 81% (Bn) yields). Amino - diester 54 was hydrolyzed under basic (LiOH)
or acidic (HCl) conditions to afford 14 (18 – 82% yield). Finally, alkyl ester prodrugs 15
were obtained by the esterifi cation of 14a (MGS0039) with an alcohol R
2
OH (see Table
3.4 for R
2
) and thionyl chloride (40 – 81% yield). The structure of 15a is shown as an
example.
3.5.2 Pharmacology and Pharmacokinetics of 14a ( MGS 0039) and Its Analogue
mGluR2/3 antagonists bearing a bicyclo[3.1.0]hexane skeleton have been reported [24,
25, 61] . Among them, 3 - alkoxy - 2 - amino - 6 - fl uorobicyclo[3.1.0]hexane - 2,6 - dicarboxylic
acids 14 , especially 14a (MGS0039), is one of the best known mGluR2/3 antagonists,
based on their pharmacological [23 – 31] and pharmacokinetic profi les [25] .
3.5.2.1 In Vitro Pharmacology of Compound 14a ( MGS 0039) and Its Derivatives
The in vitro pharmacological profi les of 14a (MGS0039) and its analogue are summarized
in Table 3.3 [24, 25, 61] . Optically active 13a , bearing a hydroxyl group at the C - 3 α posi-
tion of ( − ) - 7a (MGS0022), exhibits binding affi nities for mGluR2 ( K
i
= 32.9 nM) and
mGluR3 ( K
i
= 67.1 nM), which are similar to those of ( − ) - 7a (mGluR2, K
i
= 22.5 nM;
mGluR3, K
i
= 41.7 nM) as shown in Tables 3.1 and 3.3 . Interestingly, however, 13a shows
a moderate antagonist activity (IC
50
= 476 nM), but no signifi cant agonist activity
(EC
50
> 100 000 nM) for mGluR 2. This makes a sharp contrast with ( − ) - 7a (MGS0022),
which is a strong mGluR2 agonist (EC
50
= 16.6 nM) as shown in Table 3.1 . The (3 S ) -
isomer ( β - OH) 13b , exhibits a 3 - fold lower affi nity for mGluR2 than its (3 R ) - isomer
Scheme 3.9 Synthesis of 14a (MGS0039) and related compounds. Reagents and conditions:
(a) R
1
OC( = NH)CCl
3
, TfOH, CHCl
3
, cyclohexane or R
1
OTf, 2,6 - tert - butylpyridine; (b) Me
3
P,
THF, H
2
O or 10% Pd/C, H
2
, AcOH, H
2
O; (c) LiOH, THF, H
2
O or 10 % HCl; (d) R
2
OH,
SOCl
2
.
Fluorinated Conformationally Restricted Glutamate Analogues 85
Table 3.4 In vitro pharmacological data for optically active m G lu R 2/3 antagonists 11 , 12 , 13 and 14
CO
2
H
X
H
H
CO
2
H
O
NH
2
R
1
Compound X R
1
Binding affi nity
a
Antagonist activity
b
Agonist activity
c
K
i
(nM)
IC
50
± SEM (nM) EC
50
± SEM (nM)
mGluR2 mGluR3 mGluR7 mGluR2 mGluR3 mGluR2 mGluR3
11 3.13 3.04 110
23.2 ± 8.75 14.2 ± 4.34 > 100 000 > 100 000
12a H H
52
d
89
d
– – – – –
13a F H 32.9 67.1 –
476 ± 134
–
> 100 000
–
13b F
H ( β - OH)
105 – – 803 – – –
14a (MGS0039) F 3,4 - Cl
2
- PhCH
2
2.38 4.46 664
20.0 ± 3.67 24.0 ± 3.54 > 100 000 > 100 000
14b F Me 39.2 88.1 –
229 ± 77.3
– – –
14c F
n - Pr
5.17 – – – – – –
14d F PhCH
2
7.14 15.9 –
131 ± 44.9
– – –
14e F 4 - Cl - PhCH
2
3.17 4.77 –
29.1 ± 8.11
– – –
14f F 3,4 - F
2
- PhCH
2
2.27 3.00 –
40.8 ± 12.6
– – –
14g F Ph
2
CH 2.58 3.93 –
24.4 ± 6.53
– – –
14h F 2 - NapCH
2
2.53 5.43 –
22.7 ± 7.06
– – –
14i H 3,4 - Cl
2
- PhCH
2
2.51 – – 34.2 –
> 100 000
–
– : not determined.
EC
50
: 50% effective concentration.
IC
50
: 50 inhibitory concentration.
K
i
: inhibition constant.
a
Binding affi nities for mGluR2 and mGluR3, and affi nities for mGluR7 were determined by binding study utilizing [
3
H]MGS0008 and [
3
H] - 11 , respectively.
b
Compounds 14a (MGS0039) and 11 exhibited no signifi cant antagonist activities for mGluR1 (IC
50
= 93 300 ± 14 600 and 8990 ± 907 nM), mGluR4 (IC
50
= 1740 ± 1080 and
2650 ± 521 nM), mGluR5 (IC
50
= 117 000 ± 38 600 and 11 400 ± 2700 nM), and mGluR6 (IC
50
= 2060 ± 1270 and 1140 ± 378 nM) expressed in CHO cells, respectively.
c
Compound 14a (MGS0039) exhibited no signifi cant agonist activities for mGluR1, mGluR4, mGluR5, and mGluR6 expressed in CHO cells (ED
50
> 100 000 nM).
d
Displacement of [
3
H] - LY354740 binding in rat brain [36] .
86 Fluorine in Medicinal Chemistry and Chemical Biology
( α - OH) 13a . The result indicates that the binding affi nity of 13 depends on the stereo-
chemistry of the hydroxyl group at the C - 3 position of the bicyclo[3.1.0]hexane ring and
the (3 R ) - confi guration ( α - OH) is critical for a high affi nity for mGluR2.
The introduction of a methoxy group in place of the hydroxyl group at the C - 3 posi-
tion of 13a , which provides 14b , does not change the binding affi nity for mGluR2
( K
i
= 39.2 nM) or mGluR3 ( K
i
= 88.1 nM) or the antagonist activity for mGluR2
(IC
50
= 229 nM). However, the introduction of a larger substituent as R
1
, such as n - propyl
( 14c ) and benzyl ( 14d ) groups, resulted in binding affi nities for mGluR2 several - fold
higher than that of 14b . 3 - Benzyloxy derivative 14d exhibits a moderate antagonist activity
for mGluR2 (IC
50
= 131 nM).
These fi ndings indicate that the agonist/antagonist activities of 2 - amino - 6 - fl uorobicy
clo[3.1.0]hexane - 2,6 - dicarboxylic acids, 13 and 14 , for mGluR2/3 are controlled by the
C - 3 substituent in a size - independent manner. This observation is supported by the results
on nonfl uorinated congeners, 3 - alkoxy - 2 - aminobicyclo[3.1.0]hexane - 2,6 - dicarboxylic
acids 14i [61] , 12a (Ro 653479) [36] , and 12b [37] .
Since 14d , bearing a benzyl group at the C - 3 position, showed promising binding
affi nity and antagonist activity for mGluR2/3, the optimization of 14d by varying the
substituents on the benzene ring and replacing the phenyl group with other aryl groups
has been undertaken to fi nd better mGluR2/3 antagonists. It has been found, to date, that
3,4 - dichlorobenzyl derivative 14a (MGS0039) is the best mGluR2/3 antagonist with
regard to binding affi nity, antagonist activity, selectivity, oral bioavailability, and BBB
penetration.
Compound 14a (MGS0039) exhibited a high affi nity for mGluR3 ( K
i
= 4.46 nM) as
well as mGluR2 ( K
i
= 2.38 nM). Furthermore, 14a exhibited a lower affi nity ( K
i
= 664 nM)
for mGluR7 than the standard antagonist 11 (LY341495) ( K
i
= 110 nM). Compound 14a
exhibited potent antagonist activities for both mGluR2 and mGluR3 (IC
50
= 20.0 nM and
24.0 nM, respectively), and much weaker antagonist activities for mGluR4 (IC
50
= 1740 nM),
mGluR6 (IC
50
= 2060 nM), mGluR1 (IC
50
= 93 300 nM) and mGluR5 (IC
50
= 117 000 nM).
In addition, 14a did not exhibit signifi cant agonist activities for mGluR2, mGluR3,
mGluR4, mGluR6, mGluR1, and mGluR5 (EC
50
> 100 000 nM). In contrast, 11 (LY341495)
possesses an affi nity for mGluR7 and mGluR8 [62] . Compound 14a exhibited a 300 - fold
lower affi nity for mGluR7 than that for mGluR2, while 11 exhibited a 35 - fold lower
affi nity for mGluR7 than for mGluR2, as determined by [
3
H] - 11 binding to recombinant
mGluR7. Thus, 14a may possess a greater specifi city for mGluR2/3, although its effects
on mGluR8 have yet to be determined. It should be noted that 14a did not interact with
other receptors and transporters, including N - methyl - d - aspartic acid (NMDA), α - amino -
3 - hydroxy - 5 - methylisoxazole - 4 - propionate (AMPA), and kainite receptors [24] . More-
over, in a preliminary experiment, 14a did not inhibit glutamate transport through glutamate
transporters, such as excitatory amino acid transporter (EAAT) 1, EAAT2, and EAAT3,
even at 10 µ M concentration [24] . These fi ndings indicate that 14a (MGS0039) is one of
the most potent and selective antagonists for mGluR2/3 developed to date.
3.5.2.2 Behavioral Pharmacology of 14a ( MGS 0039)
Antidepressant - like and anxiolytic - like activities of 14a (MGS0039) have been studied in
experimental animal models [24, 29, 30] . Compound 14a exhibited an antidepressant - like
Fluorinated Conformationally Restricted Glutamate Analogues 87
effect when evaluated using the forced swimming test in rats (lowest active dose 1 mg/kg,
i.p.), the tail suspension test in mice (lowest active dose 1 mg/kg, i.p., both acutely and
subchronically for 5 days) [24] , and the learned helplessness test (escape failure) in rats
(lowest active dose 10 mg/kg, i.p. for 7 days) [29] . In addition to these antidepressant - like
effect, 14a also showed anxiolytic - like activities when evaluated using the conditioned
fear stress test in rats (lowest active dose 2 mg/kg, i.p.) [29] , the marble - burying behavior
test in mice (lowest active dose 3 mg/kg, i.p.) [30] , and the stress - induced hyperthermia
test in single housed mice (lowest active dose 1 mg/kg, i.p.) [31] .
3.5.2.3 Pharmacokinetics of 14a ( MGS 0039) and its Derivatives
The pharmacokinetic profi les of 14a (MGS0039) and 14e – 14h , selected from 3 - alkoxy -
2 - amino - 6 - fl uorobicyclo[3.1.0]hexane - 2,6 - dicarboxylic acids 14 as typical mGluR2/3
antagonists, are summarized in Table 3.5 [25] . As Table 3.5 shows, 14a (MGS0039)
exhibits the best pharmacokinetic parameters among these compounds. The oral adminis-
tration of 3, 10, and 30 mg/kg of 14a to fasting rats resulted in almost dose - dependent
pharmacokinetic parameters ( C
max
= 214 ng/mL at 2.0 h, 932 ng/mL at 2.7 h and 2960 ng/mL
at 3.3 h, t
1/2
= 2.15 h, 2.76 h and 2.77 h, AUC
inf
= 1240 ng h/mL, 6260 ng h/mL and
19 300 ng h/mL, respectively). At doses of 3, 10, and 30 mg/kg, the ratios of C
max
were 1.0,
4.4, and 13.9, respectively, while the ratios of AUC
inf
were 1.0, 5.1, and 15.6, respectively.
The mean maximum plasma level of 14a (MGS0039) was 492.3 ng/mL at 6 h. After
peaking, the plasma concentrations decreased with an estimated half - life of 2.3 h. The
AUC
inf
was 6813.0 ng h/mL [25] .
The brain and plasma levels and pharmacokinetics parameters after oral administra-
tion were compared for compounds 14a (MGC0039) and 14e – 14h (see Table 3.4 ) [25] .
Again, 14a exhibited the best BBB penetration among the compounds evaluated. The mean
maximum cerebral level of 14a was 13.22 ng/g at 6 h. After peaking, the cerebral concen-
trations decreased with an estimated half - life of 10.9 h. The cerebrum/plasma ratios of 14a
at 1, 3, 6, and 24 h were 0.01, 0.02, 0.03, and 1.99, respectively. The rate of elimination
from the cerebrum was slower than that from the plasma.
Based on these pharmacokinetic data, the ability of 14a to penetrate the BBB appears
to be acceptable or even better than that of other known mGluR2/3 antagonists, but the
oral bioavailability of 14a might be insuffi cient for its development as a drug for the treat-
ment of depression and/or anxiety.
3.5.3 Pharmacokinetics of 14a ( MGS 0039) Prodrugs
Various prodrugs of 14a (MGS0039) have been examined and reported. [39, 40] The
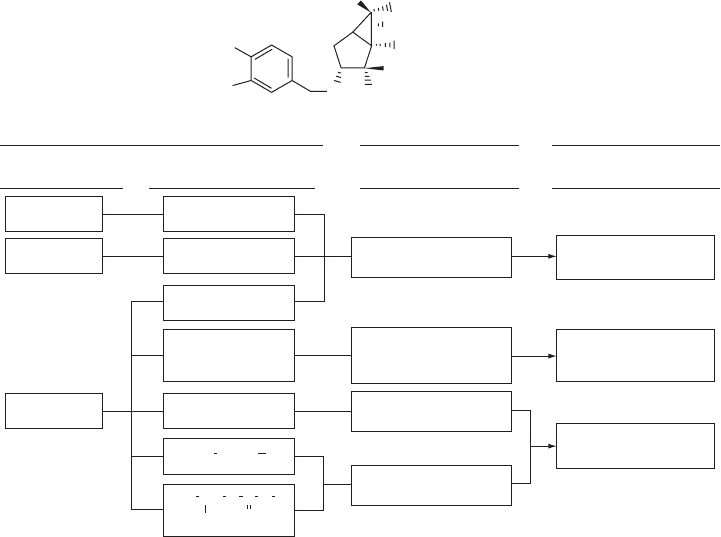
strategy for the development of prodrugs of 14a is summarized in Figure 3.3 . The synthe-
sized prodrugs were initially evaluated in human liver S9 fractions. In this study, the pro-
drugs that were effi ciently transformed to their active form, 14a , were selected for further
evaluation in monkey S9 and rat S9 fractions. The compounds selected in the S9 studies
were then further evaluated using in vivo pharmacokinetic studies in monkeys and rats,
and preclinical candidates were selected on the basis of the transformation of the prodrug
to the active form of 14a as well as its in vivo pharmacokinetics profi le in monkeys.
88 Fluorine in Medicinal Chemistry and Chemical Biology
Table 3.5 Brain and plasma levels and pharmacokinetics parameters of m G lu R 2/3 antagonists 14a , 14e , 14f , 14g , and 14h after peroral
dosing to rats at dose of 10 mg/kg
CO
2
H
F
H
H
CO
2
H
O
NH
2
R
1
Compound R
1
Tissue
Plasma / brain concentrations (ng/mL or g)
a
Parameters
b
in plasma and brain
1 h 6 h 24 h
t
max
(mg/kg)
C
max
(ng/mL)
t
1/2
Lambda z
(h)
AUC
inf (predicted)
(h ng/mL)
14a (MGS039) 3,4 - Cl
2
- PhCH
2
Plasma
364 ± 273 492 ± 344 2.10 ± 0.20
6.0 492 2.3 6810
Brain
3.88 ± 2.46 13.2 ± 6.75 4.22 ± 1.30
6.0 13.2 10.9 269
14e 4 - Cl - PhCH
2
Plasma
176 ± 39.3 98.2 ± 7.7 0.00 ± 0.00
3.0 225 2.5 1330
Brain
2.51 ± 0.20 3.39 ± 1.08 0.00 ± 0.00
3.0 3.66
NA
c
NA
c
14f 3,4 - F
2
- PhCH
2
Plasma
213 ± 11.4 41.1 ± 9.8 0.80 ± 0.70
1.0 213 2.8 1210
Brain
3.06 ± 0.34 2.40 ± 0.40 1.39 ± 1.21
3.0 3.11 19.5 88.7
14g Ph
2
CH Plasma
61.3 ± 24.0 23.1 ± 11.5 0.20 ± 0.10
1.0 61.3 2.8 431
Brain
0.00 ± 0.00 2.04 ± 0.60 0.51 ± 0.89
6.0 2.04 9 32.6
14h 2 - NapCH
2
Plasma
121 ± 9.0 210 ± 11.1 2.20 ± 1.20
3.0 252 2.9 3040
Brain
1.42 ± 0.21 8.72 ± 6.64 0.25 ± 0.02
6.0 8.72 7.1 132
a
Results are expressed as the mean ± SD, n = 3.
b
t
max
: time to reach maximum plasma or brain concentration; C
max
: maximum plasma or brain concentration; t
1/2
Lambda z: the terminal elimination half - life; AUC
inf
: area under the
concentration – time curve from time 0 to infi nite time.
c
Not applicable.
Fluorinated Conformationally Restricted Glutamate Analogues 89
Preparation of prodrugs In vitro PK study In vivo PK study
MGS0039
plasma level
Transformation to
active form in liver S9
Type of prodrug
Target moiety
for prodrug
2-COOH
6-COOH
Alkyl ester
Alkyl ester
Dipeptide
Dipeptide
Benzyl ester
2-NH
2
CO
2
H
CO
2
H
NH
2
O
H
H
2
6
X
CI
CI
0%
(human, monkey, rat)
Low
(rat)
Low
(monkey, rat)
F: 10–50%(monkey)
F: 58–74% (rat)
Correlate
human-monkey
100% (rat)
Species difference
human < monkey
Non-enzymatic
degradation
CO
2
(CH
2
)
n
Y
CO
2
CH O
O
OR"
R'
C
Figure 3.3 Summary of strategy for discovering a prodrug of 14a (MGS0039) (X = F).
To prepare the prodrug for 14a , the functional groups in 14a – two carboxylic acids
at the C - 2 and C - 6 positions and an amino group at the C - 2 position – were exploited. It
was found that the C - 2 ester was not transformed to the active form 14a in human, monkey,
or rat liver S9 fractions. A dipeptide prodrug of 4 (LY354740) with natural amino acids,
particularly with alanine at the 2 - NH
2
position, has been reported as an effective prodrug
[52] . However, in the case of 14a , dipeptides, formed through coupling of the C - 2 car-
boxylic acid with natural amino acids, such as leucine, or coupling of the C - 2 amino group
with natural amino acids, such as alanine, were surprisingly stable in human, monkey and
rat liver S9 fractions. Thus, these dipeptide derivatives were not effective prodrugs of 14a .
Regarding the formation of prodrugs with modifi cations at the C - 6 carboxylic acid, it was
found that substituted alkyl esters, such as morpholinoethyl ester and alkoxycarbonyloxy-
methyl ester, were too unstable under nonenzymatic conditions to be used as prodrugs.
The transformation of benzyl ester to 14a revealed a large difference between humans and
monkeys, in that transformation in a human liver S9 fraction was lower than that in a
monkey liver S9 fraction. Finally, C - 6 alkyl esters showed good to excellent transformation
to 14a in S9 fractions from both human and monkey.
The metabolic stability (transformed percentage to 14a ) of typical C - 6 alkyl ester
prodrugs of 14a in liver S9 fractions from rats, monkeys, and humans as well as their
pharmacokinetics parameters in rats and monkeys are summarized in Table 3.6 . Linear
Table 3.6 Transformation (%) from prodrug 15 to active substance 14a and 14i by liver S9 fractions from rats, monkeys and humans, pharmacokinetics
parameters for active substances, 14a and 14i , after oral dosing (10 mg/kg) of 14a , 14i , and prodrug 15 to rats and monkeys, and antidepressant - like activity
of 14a and its prodrug 15a ( MGS 0210)
CO
2
R
2
X
H
H
CO
2
H
O
NH
2
Cl
Cl
Compound X R
2
Metabolic stability Pharmacokinetic parameter
a
of active form after oral administration of prodrug
In vivo
pharmacology
Transformed % to active
form in liver S9 fractions
Rat Monkey Forced swimming
test in rats /Tail
suspension test in
mice
Rat Monkey Human Dose
( µ mol/kg)
t
max
(h)
C
max
( µ M)
F
(%)
Dose
( µ mol/kg)
t
max
(h)
C
max
( µ M)
F
(%)
Lowest active
dose (mg/kg)
14a (MGS0039) F H NA
b
NA
b
0.2 26.4
2.7 ± 1.2 2.5 ± 0.6
10.9 26.4
1.3 ± 0.6 0.8 ± 0.5
12.6 1.0 (i.p.)/1.0 (i.p.)
14i H H NA
b
NA
b
NA
b
27.8
4.7 ± 3.1 0.4 ± 0.1
3.6 NA
b
NA
b
NA
b
NA
b
NA
b
15a (MGS0210) F
n - Hep
98.0 42.6 76.9 21.0
1.3 ± 0.6 12.9 ± 0.9
73.0 21.0
3.7 ± 3.8 4.0 ± 1.6
38.6 3.0 (p.o.)/3.0 (p.o.)
15b F Me 86.6 26.4 44.2 23.3
1.0 ± 0.0 28.9 ± 7.2
70.6 23.3
3.3 ± 1.2 1.3 ± 0.6
16.9 NA
b
15c F Et 95.9 15.8 28.2 22.6
1.0 ± 0.1 20.7 ± 1.3
66.6 22.6
2.0 ± 0.0 1.4 ± 0.2
10.3 NA
b

15d F n - Pr 100 39.2 39.2 NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
15e F
n - Bu
100 64.2 52.1 21.2
1.3 ± 0.6 17.0 ± 0.5
54.9 21.2
2.7 ± 1.2 4.0 ± 1.0
29.7 NA
b
15f F
n - Pen
100 68.4 43.9 22.3
1.3 ± 0.6 23.9 ± 4.0
59.1 22.3
3.3 ± 1.2 3.3 ± 0.7
32.9 NA
b
15g F
n - Hex
99.0 54.0 65.3 21.6
1.0 ± 0.0 20.8 ± 5.2
46.5 21.6
2.3 ± 1.5 3.3 ± 1.4
30.9 NA
b
15h F
n - Oct
94.9 43.2 68.8 20.4
1.0 ± 0.0 17.7 ± 4.2
43.6 20.4
1.3 ± 0.6 2.3 ± 0.6
25.2 NA
b
15i F
n - Dec
NA
b
54.1 55.0 NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
15j F
i - Pr
100 5.9 5.4 NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
15k F
i - Bu
100 57.8 31.1
21.2
0.8 ± 0.3 24.1 ± 6.6
74.1 21.2
4.7 ± 3.1 3.8 ± 1.0
50.7 NA
b
15l F 3 - Me - Bu 93.1 72.8 56.4 22.3
1.3 ± 0.6 17.8 ± 5.4
44.7 22.3
2.0 ± 0.0 5.0 ± 1.8
44.5 NA
b
15m F 4 - Me - Pen 99.1 66.4 44.2 NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
15n F 5 - Me - Hex 94.6 43.4 71.1 21.0
0.5 ± 0.0 17.3 ± 1.3
39.4 21.0
4.3 ± 3.5 2.7 ± 0.3
44.0 NA
b
15o F 6 - Me - Hep 90.6 33.3 74.9 21.6
0.7 ± 0.3 15.0 ± 1.2
36.3 21.6
2.0 ± 0.0 2.8 ± 0.6
21.8 NA
b
15p F cyclo - Hex 100 19.4 12.3 NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
15q F cyclo - Hex - Me 99.1 69.0 10.0 NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
NA
b
15r H Et 17.8 NA
b
NA
b
23.5
2.0 ± 0.0 3.7 ± 0.2
20.2 NA
b
NA
b
NA
b
NA
b
NA
b
a
t
max
: time to reach maximum plasma concentration; C
max
: maximum plasma concentration; F : oral bioavailability.
b
Not applicable.
