Peube J-L. Fundamentals of fluid mechanics and transport phenomena
Подождите немного. Документ загружается.


Thermodynamics of Continuous Media 73
3) Mixed condition: where a linear relationship exists between the wall flux-
density and the temperature difference between the wall temperature T
p
(M) and
a given temperature T
0
:
0
)( TTh
n
T
Mq
p
p
Tp
¸
¸
¹
·
¨
¨
©
§
w
w
O
The coefficient h of the above relation is called the heat-transfer coefficient
of the wall. It can represent diverse phenomena, such as an external wall, heat
transfer across the boundary layer of a flowing fluid, etc.
Furthermore, as we have already said, it is necessary to fix the initial
temperature distribution in the domain being studied.
In addition to a detailed knowledge of temperature fields, other important
unknown quantities are:
– either the wall heat-flux-density and the thermal power issuing from the
domain studied when the wall temperature is given;
– or the wall temperature in the case where the heat-flux wall is given.
Finally, in the case where chemical reactions occur at the boundary
(evaporation, fusion, catalytic reactions, oxidation, electrolytic reactions, etc.),
we have more complex conditions. So, it is often at the boundary that heat is
released (positively or negatively) with a power proportional to the speed of a
reaction. As the reaction is often limited with respect to the transfer towards the wall
of certain reactants present in the fluid, we have a coupling between a thermal
problem and a problem related to the diffusion of the fluid constituents in which the
presence of flows plays an essential role. For example, in evaporation of a wet wall
in an air flow (temperature measured by a wet-bulb thermometer), the quantity of
liquid evaporated and heat absorbed in the wall surface are increasing functions of
the flow velocity, but the wall temperature is quite independent of the flow velocity
([BIR 01]).
2.4. Diffusion
2.4.1. Introduction
2.4.1.1. Definition
When a fluid F
1
is carefully introduced to a recipient which contains a fluid F
2
which is miscible with F
1
, after a certain duration we notice that movements related
74 Fundamentals of Fluid Mechanics and Transport Phenomena
to mixing have ceased, and that hydrostatic equilibrium is attained; however, an
inhomogeneity can persist in the concentration (this can be observed by differences
in color for example). This indicates that the phenomenon is an extremely slow
function of time. This migration of the components with respect to one another
constitutes a phenomenon known as material diffusion which is characterized by the
fact all of the components of a mixture do not have exactly the same speed, and
these differences lead to variations in the composition of the mixture.
The molecular velocity of a body of given chemical species which makes up a
mixture is thus engendered by two causes: the diffusion and the bulk movement of
the mixture (convection). The separation of these two causes must be very carefully
considered, as the bulk movement results from the behavior of all of the
constituents. Interactions between the two kinds of phenomena (convection and
diffusion) are encountered in all mixing processes with or without chemical
reactions (for example, sugar, which dissolves in coffee).
2.4.1.2. Microscopic interpretation of diffusion
This difference in the behavior of the two components is obviously related to
molecular agitation, which leads to a macroscopic displacement and is different for
the two components. The molecular agitation can be directly visible under certain
conditions in the presence of very small particles (in the order of a few micrometers)
in suspension in a fluid. Examined under a microscope these particles present erratic
behavior: this is known as Brownian movement, which is a macroscopic
manifestation of molecular agitation. These movements obey diffusion equations.
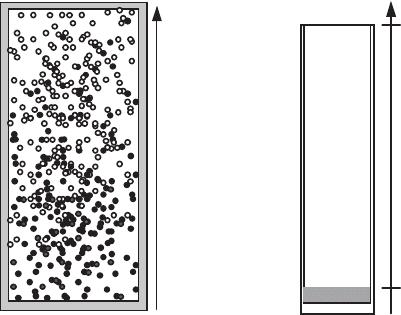
Let us consider two molecular species (black and white molecules in Figure
2.9a). By supposing that the two categories of molecules have the same energy (in
other words the same temperature), each molecular species moves as a result of
collisions. These collisions have a random character; for a resting gas made up of
one or many species of molecule, uniformly distributed, we see that the molecules
are statistically stationary, because each species only permutes between themselves.
During collisions and molecular interactions in the volume shown in Figure 2.9a,
there are more black (or respectively white) molecules at the bottom (or at the top),
but they will end up uniformly distributed in the container considered. The statistical
aspects of the second principle of thermodynamics leads to a complete mixing of the
two species, whose concentrations become uniform.
However, in this container, where the pressure is supposed constant, the total
molar concentration remains constant at all points, with the species whose
concentration decreases being replaced by the other species whose concentration
increases. This pressure constraint leads to a relation between migrations of the two
species.
Thermodynamics of Continuous Media 75
x
O
A
(a) (b)
n
s
n
2a
x
Figure 2.9.
(a) Gaseous diffusion in a volume; (b) evaporation of a liquid
Evaporation towards the atmosphere of a liquid contained in a tube (Figure 2.9b)
is another example of gaseous diffusion where we have a vapor flux from the liquid
towards the exterior across air, which is at rest in the tube. Note that the vapor
crosses the free surface of the liquid (at the lower wall where evaporation takes
place) and diffuses towards the exterior air, whereas the air, which does not dissolve
in the liquid, does not cross the free surface.
For the sake of simplicity, we will consider the case of mixing between two
perfect gases: gravity here often plays a negligible role at the laboratory scale.
Kinetic gas theory allows the modeling of phenomena at the molecular scale. We
will limit the discussion to cases where the temperature of the systems studied is
uniform; if this was not the case, it would be necessary to introduce a further
phenomenon: thermal diffusion. The interested reader should refer to classic texts on
the subject (see section 2.4.5).
It should nevertheless be noted that, depending on the problem studied, the
influence of external forces or accelerations on diffusive phenomena should be taken
into account (centrifugation for example).
2.4.1.3. Extensive variables of a mixture
The composition of a continuous medium where k chemical species are present
can be characterized by the local reduced extensive concentration variables which
can be either:
76 Fundamentals of Fluid Mechanics and Transport Phenomena
the number of moles n
i
of each species per unit volume (molar
concentration);
the partial density
U
i
of the species i, which is the mass per unit volume
(
iii
Mn
U
) of the molecules of species i, of molar mass M
i
(mass
concentration);
the partial pressure p
i associated with the molecules of species i supposed
only to occupy an elementary local volume of the mixture.
As the preceding quantities are additive, we can define the total number of moles
n (or molecules) per unit volume, and the density
U
of the mixture:
¦¦¦
k
i
ii
k
i
i
k
i
i
Mnnn
111
;
UU
[2.20]
If the system comprises a perfect gas, which we assume in what follows, the
total pressure is equal to the sum of the partial pressures of the different
constituents.
We also consider the concentrations of the species i by means of relative values:
– molar fraction
i
J
;
– mass fraction
i
Z
:
nn
ii
/
J
;
U
U
Z
/
ii
[2.21]
According to [2.20], these variables respect the relations:
1;1
11
¦¦
k
i
i
k
i
i
ZJ
While the density
U
or the number of moles n per unit volume of a mixture
appear in the global mixing equations, the composition of the mixture is in fact
characterized by the given concentrations of only k 1 components.
In a liquid medium, the molar concentration C
i
(also called molarity) is
expressed as the number of moles of a species contained in a volume equal to 1 liter.
It often happens that the mixture contains a constituent 1, which is dominant, and
a weak proportion of a species i:
Thermodynamics of Continuous Media 77
111
or: or: ....
iii
nn pp
All relative concentrations of species 1 then have the same first order value. For
example, it is thus possible to confuse the molar fraction
J
i
= n
i
/ n with the quantity
J
i
= n
i
/ n
1
, the errors thus committed being in the order of (n
i
/ n
1
)
2
. The same goes
for the mass fraction
Z
i
=
U
i
/
U
and the quantity
U
i
/
U
1
, the partial pressure p
i
/ p
and p
i
/ p
1
. All these variables of relative concentration are proportional. We will
note as c
i
the species concentration i present in small quantities in a mixture.
2.4.2. Molar and mass fluxes
2.4.2.1. Flux of a component
We have seen (see section 2.1.3.1) that for any scalar extensive quantity G, we
can identify a corresponding vector flux density
G
q
G
, whose flux across the surface
(S) (equation [2.3]) represents the quantity of G which crosses S per unit time. The
extensive quantities of a pure body (volume, energy, etc.) are associated with the
matter which we suppose fixed in the reference frame. In the presence of diffusion,
not only does this reference not exist, but the flux densities of the components, as a
result of their movement, depend on the particular reference which is chosen. We
will return to this choice at a later stage...
It can easily be seen that the matter which crosses a surface ds at a velocity
V
G
over a period of length
G
t, comprises a cylinder of length
tV
G
G
whose volume is
equal to
G
G
V.n į t.
ds
S
V
G
t
G
.V
G
n
G
Figure 2.10.
Balance in an elementary displacement across ds
78 Fundamentals of Fluid Mechanics and Transport Phenomena
Letting
g
be the volume density of the quantity G (see section 2.1.1), we can
derive expressions for the vector flux density
g
q
G
of G
2
and the flux
GS
M
of the
quantity
G across the surface S:
³³
SS
GGSg
dsnVgdsnqVgq
G
G
G
G
G
G
..
M
By considering
g
to be the number of moles per unit volume, we obtain
respectively for the
molar flux density
ni
q
G
and the molar flux
ni
M
across (S) of the
constituent
i with velocity
i
V
G
:
³³
S
ii
S
nniii
ni
dsnVndsnqVnq
i
G
G
G
G
G
..;
M
[2.22]
If we now choose mass to be the extensive quantity, we obtain, respectively for
the mass flux density
mi
q
G
and the mass flux
mi
M
of the component i across (S):
³³
S
ii
S
mimiiimi
dsnVdsnqVȡq ...
G
G
G
G
G
G
UM
[2.23]
The molar flux
ni
M
and the mass flux
mi
M
represent, respectively, the number
of moles and the component mass i crossing S per unit time.
2.4.2.2. Balance equations
The balance equation of each constituent i (i =1, 2,…, k) can be obtained by
applying equation [2.8] to the volumic number of moles n
i
:
¸
¸
¹
·
¨
¨
©
§
w
w
w
w
w
w
nini
i
j
nij
ni
i
qdiv
t
n
x
q
t
n
G
VV
The volume source
V
ni
is the (volumetric) volumic number of moles in
component i created by homogenous chemical reactions. By replacing the molar flux
density with expression [2.22]:
.,..2,1 kiun
xt
n
niiji
j
i
w
w
w
w
V
[2.24]
2 We shall study convective fluxes in more detail later (section 3.3.3.2).

Thermodynamics of Continuous Media 79
Balance equations can be written for the density
U
i
, which are equivalent to
[2.24], by multiplying each of these by the molar mass M
i
of the corresponding
component:
.,..2,1 kiMu
xt
niiiji
j
i
w
w
w
w
VU
U
[2.25]
2.4.2.3. Global fluxes and mean velocities
For the set of components of a mixture, we define:
– the total density
n
q
G
of the molar flux and the total molar flux
n
M
:
i
k
i
i
k
i
inn
Vnqq
G
G
G
¦¦
11
³
S
nn
dsnq
G
G
.
M
[2.26]
– the
total density
m
q
G
of the mass flux
and the total mass flux
m
M
:
i
k
i
i
k
i
imm
Vqq
G
G
G
¦¦
11
U
³
S
mm
dsnq
G
G
.
M
[2.27]
The global molar and mass balances for the mixture allow average mixing
velocities to be defined according to the methodology described in section 1.4.2.5
for the intensive quantities
3
. We thus define:
– the
molar average velocity
*
G
V
(which is independent of the molar mass) using
the molar balance [2.26]:
¦
k
i
ii
Vn
n
V
1
*
1
G
G
[2.28]
– the
mass average velocity
V
G
(velocity of the inertia center of a fluid particle
4
),
using mass balance [2.27]:
¦
k
i
m
ii
q
VV
1
1
U
U
U
G
GG
[2.29]
where
iii
Mn
U
is the partial density of the species i of molar mass M
i
.
3 The velocity is an intensive quantity (see section 1.2.1).
4 See section 3.2.1 and following sections.

80 Fundamentals of Fluid Mechanics and Transport Phenomena
The velocities
V
G
and
*
V
G
are the same only if the continuous medium comprises
a homogenous mixture of constant composition or of identical molecules (a pure
substance).
The molar fluxes
n
M
and
m
M
represent, respectively, the number of moles and
the total mass of a mixture which are crossing a surface
S per unit time:
³³
SS
nn
dsVndsq ..
*
G
G
M
³³
SS
m
m
dsVdsq ..
G
UM
By summing equations [2.24], term by term, and taking account of definition
[2.28], we obtain the balance equation for the total volumic number of moles
n:
¦
w
w
k
i
ni
Vndiv
t
n
1
*
V
G
[2.30]
The quantity
¦
k
i
ni
1
V
is the number of moles created by chemical reactions.
We do the same for equations [2.25] and [2.29]; however, the sum
¦
k
i
nii
M
1
V
is
zero as there is no mass source. We obtain the conservation equation for the total
mass:
0
w
w
Vdiv
t
G
U
U
[2.31]
We will encounter the preceding equation again in the next chapter, during our
study of fluid flows, where it plays a fundamental role.
The reader will note that the
k equation [2.24] (or [2.25]) is equivalent to the
system of
k-1 equation [2.24] (or [2.25]) and equation [2.30] (or [2.31]). In other
words, it suffices to describe
k-1 component balance equations in addition to the
global balance equation for the mixture.
2.4.3. Choice of reference frame
2.4.3.1. Introduction
In this chapter, we have already studied the transfer of diverse quantities G
(energy, entropy, etc.) with respect to matter which was supposed stationary; in
Thermodynamics of Continuous Media 81
Chapter 3 we will examine the transport of a quantity G associated with convection
of matter, in other words associated with a bulk movement of the continuous
medium.
In an inhomogenous mixture, each component
i has its own velocity
i
V
G
. There is
therefore no preferred reference frame in which all of the matter is stationary. It is
for this reason that we did not specify the reference frame used in the preceding
definitions of flux.
As diffusion is a
differential phenomenon between the components, it is
necessary to identify a global condition which defines the bulk movement of the
matter, in other words it is necessary to fix the local mean velocities field for the
matter, as diffusion is a phenomenon which must be considered relative to a mean
velocity. The reference frame chosen for a study of diffusion is thus determined by
the choice of reference frame for the mean velocity. It depends on the kind of
problem studied, as the following examples demonstrate.
The quantities which characterize diffusion (velocities, flux densities), which
depend on the reference frame
R chosen for their study, will be represented between
brackets
>@
*
with a superscript (*,G or 1) indicating the reference frame.
2.4.3.2.
Diffusion in a fluid at mechanical rest
Let us now consider an isothermal fluid mixture containing k components.
Diffusion phenomena are here characterized by very slow velocities associated with
negligible accelerations. The local composition is characterized by the number of
moles
n
i
per unit volume for each species. Suppose that the total number of moles n
per unit volume is constant at all points in the mixture (for example, in an ideal
isothermal gas). In the absence of chemical reactions, equation [2.30] can thus be
written:
0
*
Vdiv
G
[2.32]
We will limit our discussion to a simple case involving a configuration in stable
equilibrium (section 2.2.1.6), in which the concentration is constant in horizontal
planes, the diffusion velocities being vertical and parallel to the axis Ox. The field
txV
,
*
G
, which thus respects the condition
0
*
dxdV
, derived from [2.32], is
uniform at each instant and the molar average velocity
tV
*
G
is only a function of
time.
The preceding situation is observed for isothermal gaseous mixtures whose
pressure is uniform and constant. In a gravitational field, diffusion only occurs
82 Fundamentals of Fluid Mechanics and Transport Phenomena
without natural convection for fluids in situations of 1D equilibrium. In other cases,
the inevitable density gradients created by variations in the component
concentrations must be taken into account: these induce complex movements due to
natural convection in the fluid.
2.4.3.3.
Diffusion in a closed container
Let us now consider a perfect, isothermal, gaseous mixture, contained in a fixed
container (Figure 2.9a), whose total volumic number of moles
n is constant. We will
suppose that the walls are impermeable; this results in a
molar average velocity
which is zero in the reference frame of the container
, which can thus be considered a
preferred reference frame:
**
**
1
0, with:
k
ii i i
i
nV n V V V V
¯ ¯
¡° ¡°
¢± ¢±
GG GGG
This results in the molar flux densities playing an important role in this reference
frame:
**
*
, with: 0
ii
ni ni
i
qnV q
¯ ¯
¯
¡°
¡° ¡°
¢±
¢± ¢±
GG
G
2.4.3.4
. Diffusion in steady evaporation
Let us now examine the 1D problem of steady evaporation of a liquid contained
in a vertical tube as shown in Figure 2.9b. As before, the pressure is constant in the
tube. We suppose that the temperature is constant and the composition is
independent of time, but not uniform in space. The total volumic number of moles
n
and the molar velocity
*
V
G
(section 2.4.3.2) are steady and uniform in space. The
molar flux densities of air (species 1) and vapor (species 2) are steady (section
2.4.3.2).
As the free surface of the liquid at the bottom of the tube is impermeable to air,
the flux density and velocity of the air are both zero at all points within the tube:
the
air is at rest in the reference frame of the tube
(
>@
0
1
1
V
G
) and so diffusion velocity
of vapor in air can be written:
>@
12
1
2
VVV
GGG
Since we have a steady evaporation regime, we can conclude that the
flux of
liquid evaporated
>@
1
22
Vn across a cross-section of the tube is independent of the
