Water and Wastewater Engineering
Подождите немного. Документ загружается.

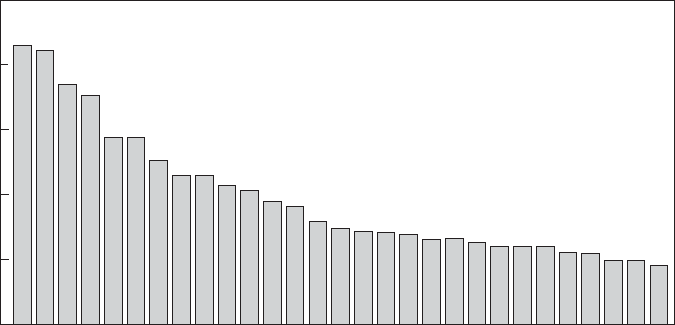
14-20 WATER AND WASTEWATER ENGINEERING
14-9 PHARMACEUTICALS AND ENDOCRINE-DISRUPTING
CHEMICALS (EDCs)
Pharmaceutical compounds, such as sulfamethoxazole, carbamazepine, and ibuprofen, and
endocrine-disrupting compounds, such as 17b-estradiol, testosterone, and bisphenol-A, have
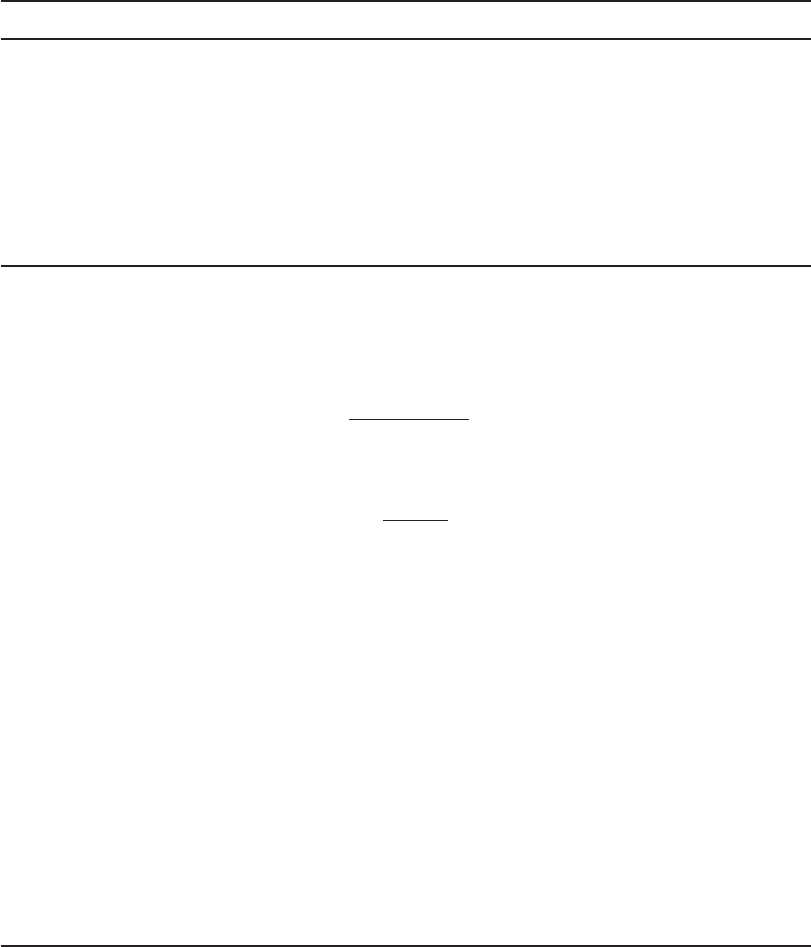
been identified in the surface waters of the United States. Figure 14-6 shows the most frequently
detected pharmaceuticals and EDCs. Typical concentrations are low. For example, in surface
water supplies they are generally less than 1 g/L (Hullman, 2009).
Although research on removal of pharmaceuticals and EDCs is in its infancy, some early
results are useful in pointing to potential technologies. For a selected set of pharmaceuticals (bezaf-
ibrate, clofibric acid, carbamazepine, and diclofenac), Ternes et al. (2002) reported the following:
• Conventional sand filtration and coagulation with ferric chloride provide no significant
removal,
• Ozonation was effective for some compounds, and
• Ozonation followed by granular ac tivated carbon filtration was very effective for all
compounds investigated but clofibric acid.
For selected sets of pharmaceuticals (sulfamethoxazole, carbamazepine, diclofenac, and
ibuprofen) and EDCs (estradiol, es
trone, testosterone, and progesterone), several authors
Coprostanol(5)
Cholesterol(5)
N-N-diethyltoluamide(4)
Tri(2-chloroethyl) phosphate(4)
4-nonylphenol(4)
4-nonylphenol monoethoxylate(4)
4-nonylphenol diethoxylate(4)
5-methyl-1H-benzotriazole(4)
1,4-dichlorobenzene(4)
Fluoranthene(4)
Pyrene(4)
1,7-dimethylxanthine(3)
Ethanol, 2-butoxy-phosphate(4)
4-octylphenol monoethoxylate(4)
4-octylphenol diethoxylate(4)
Bis
phenol-A(4)
Cotinine(3)
Diazinon(4)
4-methyl phenol(4)
Acetaminophen(3)
Tetrachloroethylene(4)
Trimethoprim(3)
Erythromycin-H2O(1)
Estriol(5)
Lincomycin(1)
Sulfamethoxazole(3)
Phthalic anyhydride(4)
Triclosan(4)
Caffeine(4)
Frequency of detection (%)
0
20
40
60
80
FIGURE 14-6
Most frequently detected pharmaceuticals and endocrine-disrupting compounds. ( Source: U.S.G.S., 2002.)
REMOVAL OF SPECIFIC CONSTITUENTS 14-21
(Drewes et al., 2005; Nghiem et al., 2004 and 2005) indicate NF and RO are highly effective in
removing these compounds. Ozonation also appears to be highly effective for the destruction of
these compounds (Westerhoff et al., 2005). UV irradiation of pharmaceuticals appears to be only
effective in the presence of hydrogen peroxide (Rosenfeldt and Lin
den, 2004).
14-10 RADIONUCLIDES
The most common radionuclides of concern in natural waters are radium-226 (
226
Ra) radium-
228 (
228
Ra), radon-222 (
222
Rn), and uranium-234 (
234
U). In groundwater,
226
Ra and
228
Ra exist
primarily as divalent cations . Radon-222 is a volatile gas. Uranium-234 exists as uranyl ion. It
readily complexes with carbonate and hydroxide. The revised Radionuclide Rule outlining the
concentration limits for these compounds was publi
shed on December 8, 2003.
The U.S. EPA has designated the following as best available technologies (BAT) for removal
of the radionuclide shown (U.S. EPA, 2005):
• Radon-222: air stripping.
• Radium-226 and radium-228 together: coagulation/flocculation/sedimentation/filtration.
• Radium-226 and radium-228 separately: ion exchange, RO or lime-soda softening.
• Uraniu
m-234: ion exchange; coagulation/flocculation/sedimentation/filtration.
Table 14-7 summarizes the performance of specific tec hnologies for specific radionuclides.
Sludge and resin disposal alternatives are discussed in Chapter 15.
TABLE 14-7
Performance of specific technologies for specific radionuclides
Removal efficiency, %
Technology Radon Radium Uranium
Activated alumina up to 99
Aeration, diffused bubble to 99
Aeration, spray 70–95
Air stripping, packed
tower
to 99
Coagulation-filtration 85–95
GAC adsorption 62–99
Greensand
19 to 82
a
Anion exchange up to 95
Cation ion exchange 81–97
Lime-soda softening 75–90
16 to 97
b
Reverse osmosis 90–95 90
a
19 to 63% for radium-226 and 23 to 82% for radium-228.
b
pH of at least 10.6.
A dapted from Lowery and Lowery, 1988; U.S EPA, 2005.

14-22 WATER AND WASTEWATER ENGINEERING
14-11 SYNTHETIC ORGANIC CHEMICALS (SOCs) AND VOLATILE
ORGANIC CHEMICALS (VOCs)
Synthetic organic chemicals include pesticides, polycyclic aromatic hydrocarbons, and polychlo-
rinated biphenyls. SOC is considered a regulatory term rather than a chemical description. Some
of the SOCs are volatile organic chemicals. The term volatile organic chemical (VOC) refers to
a compound with a high vapor pressure that causes it to evaporate readily. In water treatm ent
practice, they are classified into three broad groups:
• Those found in petroleum products (e.g., benzene, toluene, and xylene).
• Halogenated compounds used as solvents, degreasers, and intermediates
(e.g., tetrachloro-
ethylene and methylene chloride).
• Disinfection byproducts like the trihalomethanes.
With the exception of the THMs, these compounds enter the water from industrial waste dis-
charge, leaking fuel storage tanks, and uncontrolled waste disposal sites.
Treatment Strategies
The U.S. EPA designated air stripping and granular activated c arbon (GAC) as best available
technology (BAT) for treatment of VOCs except for vinyl chloride and methylene chloride. For
these two chemicals, only air stripping is recognized as BAT. As a rule of thumb, chemicals hav-
ing a dimensionless Henry’
s law coefficient 7.5 10
4
at 20 C can be removed by packed
tower stripping (54 FR 22062). Air stripping is more economical than GAC for removal of VOCs
if the off-gas can be directly discharged without treatment (Ball and Edwards, 1992; Gross and
TerMaath, 1985; Hand et al., 1986). The economic advantage of air stripping over GAC dimin-
ishes when off-gas treatment is requ
ired and other strategies should be investigated (Ball and
Edwards, 1992).
For SOCs that cannot be stripped, GAC is the BAT technology.
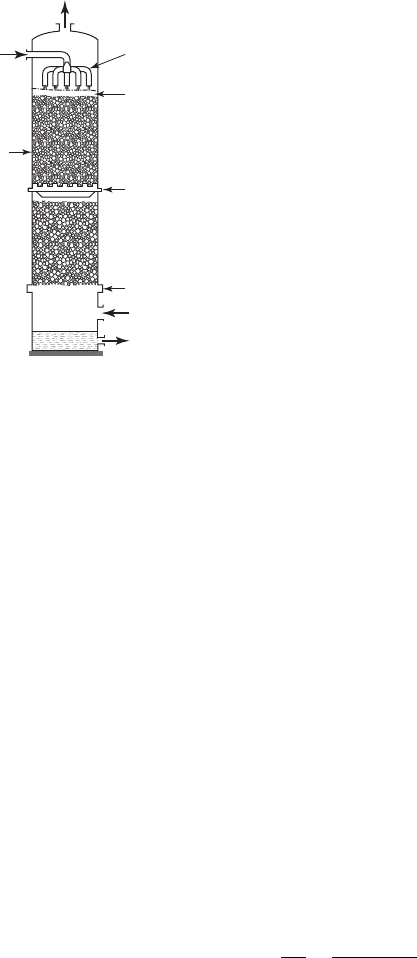
Stripping. Although there are a number of air stripping systems available, packed towers
( Figure 14-7 ) serve as a primary method for removal of VOCs. The relevant equations are
( LaGrega et al., 2001):
Z ()()HTU NTU (14-13)
HTU
Q
AKa
L
()( )
(14-14)
NTU
S
S
CC S
S1
11
⎛
⎝
⎞
⎠
⎡
⎣
⎢
⎤
⎦
⎥
ln
/
in eff
()()
(14-15)
S
HQ
Q
a
()( )
(14-16)
where Z packing height, m
HTU height of transfer unit, m
Q flow rate of water, m
3
/ s
REMOVAL OF SPECIFIC CONSTITUENTS 14-23
A cross-sectional area of column, m
2
K
L
a
overall transfer rate constant, s
1
NTU number of transfer units
S stripping factor
C
in
influent concentration, mg/L
C
eff
effluent concentration, mg/L
H Henry’s constant, dimensionless
Q
a
flow rate of air, m
3
/ s
The overall transfer rate constant ( K
L
a
) is a complex function of the diffusion coefficients,
liquid mass loading, liquid viscosity, and packing size. The Sherwood and Holloway equation and
the Onda correlations (see LaGrega et al., 2001) are two techniques for estimating K
L
a
. However,
for design purposes, K
L
a
should be determined experimentally. A factor of safety is required for
both the pilot scale data and the estimating techniques because they overestimate K
L
a
. They are
between 33 percent and 47 percent high (Djebbar and Narbaitz, 1995; MWH, 2005).
Dimensionless Henry’s constants for selected compounds are given in Appendix A.
Air-to-water ratios ( Q
a
/ Q ) range from 5:1 to 300:1 (Kavanaugh and Trussel, 1980; LaGrega et al.,
2001). For chemicals with dimensionless Henry’s law coefficients from 0.003 to 0.3, the air-to-water
ratio that provides the minimum tower volume and power requirement is approximately 3.5 times the
minimum air-to-water ratio needed to meet a treat
ment objective concentration of C
eff
(Hand et al.,
1986; MWH, 2005). The minimum air-to-water ratio is calculated with the following equation:
Q
Q
CC
HC
a
in eff
in
()( )
(14-17)
where Q
a
/ Q air-to-water ratio
C
in
influent liquid concentration, mg/L
C
eff
effluent liquid concentration, mg/L
H Henry’s law coefficient, dimensionless
Liquid in
Gas in
Liquid out
Shell
Packing suppor
t
Liquid
redistributor
Packing
restrainer
Liquid
distributor
Random
packing
Gas out
FIGURE 14-7
Packed tower stripping column.

14-24 WATER AND WASTEWATER ENGINEERING
For VOC stripping, the ratio should be selected to achieve the desired concentration of VOC in
the effluent while maintaining a height-to-diameter ratio greater than 1:1. In general, it is more
economical to provide higher air-to-water ratio because it lowers the tower height. The trade-off
is between the operating cost for the blower to provide air and the capital cost for a taller tower.
The pressure drop per unit of tower height (
P / Z ) in the packed tower is a function of
the superficial gas velocity and the friction factor for dry packing ( F
p
). The proportionality
e xpression is:
P
Z
Fv
Pg
()()
2
(14-18)
where
P / Z pressure drop per unit length of packed column, Pa/m
F
P
friction factor or, more commonly, packing factor, dimensionless
v
g
superficial gas velocity, m/s
The packing factor i s specific to the shape and size of the packing. A few examples are shown in
Table 14-8 .
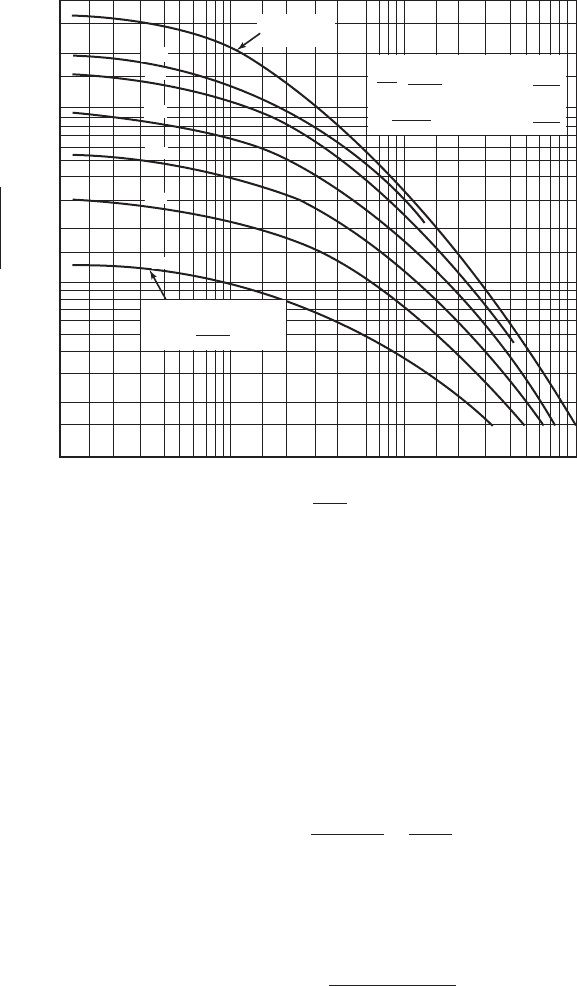
The cross-sectional area of the column (A) is estimated using the Eckert pressure drop curves
shown in Figure 14-8 (Eckert, 1961).
The estimating procedure is
1 . Specify the water temperature, packing factor ( F
p
), air-to-water ratio ( Q
a
/ Q ), and gas
pressure drop (
P / Z ).
2. Compute the value of the ratio G
m
/ L
m
using the following expression:
G
L
Q
Q
m
m
a
g
w
⎛
⎝
⎜
⎞
⎠
⎟
⎛
⎝
⎜
⎞
⎠
⎟
r
r
(14-19)
TABLE 14-8
Selected packing factors and specific surface area
Type Nominal diameter, mm
Packing factor, F
p
Plastic saddles
a
50 20.0
75 16.0
Plastic tripacks
b
5015.0
90 14.0
Flexring
c
50 24.0
90 20.0
IMPAC
d TM
85 15.0
140 6.0
LANPAC
d TM
60 21.0
90 14.0
a
Norton Co., Akron, OH.
b
Jaeger Co., Houston, TX.
c
Ceilcote Co., Cleveland, OH.
d
LANTEC Co., Los Angeles, CA.
REMOVAL OF SPECIFIC CONSTITUENTS 14-25
where G
m
air mass loading rate, kg/m
2
· s
L
m
water mass loading rate, kg/m
2
· s
g
air density, kg/m
3
w
water density, kg/m
3
3. Compute the value of x on the x -axis of the Eckert curve using
x
G L
mm
g
1
0 5
/
(
)
⎡
⎣
⎢
⎤
⎦
⎥
⎡
⎣
⎢
⎤
⎦
⎥
.
r
r
w
–
r
g
(14-20)
4. Determine y on Figure 14-8 using the computed value of x and the specified pressure drop.
5. With the value of y, solve the following expression for G
m
:
G
y
m
g
0.5
()( )(r
F
p
01.
()()m
⎡
⎣
⎢
⎢
⎤
⎦
⎥
⎥
r
w
–
r
g
)
(14-21)
where y numerical value on y-axis found in step 4
F
p
packing factor, dimensionless
dynamic viscosity of water, Pa · s
0.4
0.2
0.10
0.08
0.06
0.04
0.02
0.01
0.008
0.006
0.004
0.002
0.001
0.01 0.02 0.04 0.1 0.2 0.4 1.0 2 4 10
p
Z
lb
r
/ft
2
ft
N/m
2
m
N/m
2
m
6.37 10
–3
1.224 10
–3
inH
2
O
ft
Approximate
flooding
Gas pressure drop
N/m
2
m
1200
800
400
200
100
50
L
m
G
m
1/2
r
g
)(
G
2
F
p
r
g
(r
w
–r
g
)
0.1
r
w
r
g
m
FIGURE 14-8
Flooding and pressure drop in randomly packed towers.
( Source: Treybal, R. E. 1980. Mass Transfer Operations. New York: Chem. Engrg. Series, McGraw-Hill, 3rd ed.)
Reproduced with permission of The McGraw-Hill Companies.
14-26 WATER AND WASTEWATER ENGINEERING
6. Determine the water mass loading rate.
L
G
QQ
m
m
agw
()()//rr
(14-22)
7. Determine the cross-sectional area of the packed tower.
A
Q
L
w
m
()( )r
(14-23)
T ypical design ranges are given in Table 14-9 .
Prefabricated tower dimensions are limited by highway transportation clearances . Multiple
towers in series are used to meet height requirements when tower heights exceed 9 m. Multi-
ple towers in parallel are used when tower diameters exceed 3.6 m. The tower height is
greater
than the height of the packing. Approximately 1 to 2 m may be added to the packing height for
support structures and internal distribution piping. Prefabricated units are generally built in 0.3 m
increments.
Potential operating problems arise when the water contains appreciable quantities of iron,
manganese, and/or hardness. The aeration will oxidize the iron/manganese, which will then pre-
c
ipitate on the packing and foul it. Preoxidation and filtration may be used to avoid this problem.
Because CO
2
i s being stripped, the pH of the water will rise. This may lead to calcium carbonate
precipitation in the packing. Using a larger packing diameter ( 50 mm), careful monitoring, and
periodic cleaning of the packing may be required. A design with capability to periodically acid
wash the packing has also been used
when high iron/manganese/calcium was encountered (Ball
and Edwards, 1992).
Example 14-2. Springfield has a well field that is contaminated with trichloroethylene (TCE).
The design flow rate is 8,200 m
3
/ d. A packed tower has been selected to remove the TCE. A
treatment objective of 95 percent removal of TCE has been selec ted. Design a packed tower to
strip TCE from the water. The following design data have been provided:
TABLE 14-9
Typical packed column design ranges
Parameter ValueComment
Tower height 1.5 to 9 m Prefab will be sized to fit on flat
bed trailer
Diameter 0.3 to 3.6 m Restriction to 3.6 m for transport
of prefab units
Height:diameter
1:1
Without liquid redistribution use
4:1 for proper liquid distribution
Pressure drop 50 to 100 Pa/m of packing Economics favor 50 Pa/m
Q
a
/Q
5:1 to 300:1
Ratio of diameter to packing size 8:1 to 15:1
15:1 preferred
Sources: Dyksen, 2005; Hand et al., 1999; LaGrega et al., 2001; MWH, 2005; Treybal, 1980.

REMOVAL OF SPECIFIC CONSTITUENTS 14-27
Raw water TCE 72.0 g/L
T e mperature 10 C
K
L
a
0.0128 s
1
P a cking plastic tripack with diameter of 50 mm
P / Z 50 Pa/m
g
1.2 kg/m
3
w
1,000 kg/m
3
H at 10 C 0.116
Determine the following to complete the design:
Q
a
/ Q
Q
a
Column diameter
Stripping factor
Height of packing
Overall height of packed tower
Solution:
a . Determine the minimum air to water ratio ( Q
a
/ Q ) from Equation 14-17. At 95 percent
removal, the effluent concentration of TCE is 3.60 g/L.
Q
Q
a
72 0 3 60
0 116 72 0
8
..
..
g/L g/L
g/L()( )
..19
To achieve the air-to-water ratio that provid es the minimum tower volume and power
requirement, multiply the minimum air-to-water ratio by 3.5.
Q
Q
a
()()819 35 28 66 30.. .or
b. Determine the diameter of the column
(1) Compute the value of the ratio G
m
/ L
m
.
G
L
m
m
()30
12
1 000
00360
3
3
.
,
.
kg/m
kg/m
⎛
⎝
⎜
⎞
⎠
⎟
(2) Compute the value of x on the Eckert curve using Equation 14-20.
x
1
00360
12
1 000 1 2
0 5
3
3
.
.
.
.
⎡
⎣
⎢
⎤
⎦
⎥
,
kg/m
kg/m
kkg/m
3
096
⎡
⎣
⎢
⎤
⎦
⎥
.
(3) Find y on Figure 14-8 using x 0.96 and the
P / Z 50 Pa/m curve:
y 00039.
(4) With the value of y, solve Equation 14-21 for G
m
. The packing factor of 15.0 for
50 mm tripacks is found in Table 14-8 .
14-28 WATER AND WASTEWATER ENGINEERING
G
m
0 5
33
00039 1 2 1 000 1 2
.
.. , .()( )(kg/m kg/m kgg/m
kg/
3
3 01
15 01307 10
0 778
)
()( )..
.
.
⎡
⎣
⎢
⎤
⎦
⎥
mms
2
(5) Determine the water mass loading rate.
L
m
0.778 kg/m ·s
(30)(1.2 kg / m /1,000 kg/m
2
3
33
2
)
21.61kg/ ms
(6) Determine the cross-sectional area of the packed tower.
A
()( )
(
8 200 1 000
21 61
33
2
,,
.
m /d kg/m
kg/ms
))( )86 400
4 39
2
,
.
s/d
m
(7) Determine the diameter of the column.
D
0 5
2
4 394
2 3624
.
.
..
()()m
or m
⎛
⎝
⎜
⎞
⎠
⎟
c. The height of a transfer unit (HTU) is a function of the area of the column. Using the
area of 4.39 m
2
HTU
m /d
ms
8 200
4 39 0 0128 86 400
3
21
,
.. ,()( )(
ss/d
m
)
169.
d. With the Henry’s law constant of 0.116, the stripping factor is
S ()()0 116 30 3 48..
e. The number of transfer units (NTU) is
NTU ln
g/L/ g/L
3 48
3 48 1
72 0 3 60.
.
..
⎛
⎝
⎞
⎠
()(
3348 1 1
3 48
3 76
.
.
.
) +
⎡
⎣
⎢
⎤
⎦
⎥
f. The height of the packing ( Z ) is then
Z ()()169 3 76 6 35...mm
The height to diameter ratio is greater than 1:1 so this design is acceptable.
g. The estimated overall height of the tower will be 6.35 m 1.0 m for distribution piping,
etc. 7.35 m. Because prefabricated units are
usually constructed in 0.3 m increments,
the actual final dimensions will be about 2.4 m in diameter and 7.5 m in height.
Comments:
1 . The overall tower height meets the 9 m criterion for prefab units. If it d id not, alter-
native designs would be considered including increasing the air-to-water ratio and
/or
splitting the tower into two sections and using them in series.
2. The K
L
a
for this problem was selected arbitrarily. It is not based on experimental data for
the water or packing specified and should NOT be used for actual design.

REMOVAL OF SPECIFIC CONSTITUENTS 14-29
3. A spreadsheet was used to perform the computations and explore several variables. Other
solutions provide acceptable answers.
Granular Activated Carbon (GAC). The removal of a chemical from solution by activated
carbon is a mass-transfer proc ess in which the chemical is bonded to the s
olid. This process is
called adsorption. The chemical (the adsorbate ) penetrates into the pores of the solid (the adsor-
bent ), but not into the lattice itself. The bond may be physical or chemical. Electrostatic forces
hold the chemical when physical binding is predominant. Chemical bonding is b
y reaction with
the surface. Activated carbon (the adsorbate) is made from various materials such as wood, coco-
nut shells, coal, and lignite. The manufacturing process is essentially a carbonization of the solid
followed by activation using hot air or steam. Like ion exchange resins, the num
ber of active sites
is finite and the carbon becomes saturated with use over time. It is regenerated by heating with
hot air or steam.
Adsorption isotherms are used to select one of the manufactured activated carbons for remov-
ing the SOCs of concern in the water supply. The isotherms are prepared from experim
ental data.
The selected activated carbon is placed in a solution containing the chemical or chemicals of
interest. The solution is agitated to provide adequate contact between the granules of carbon and
the chemical. The slurry is mixed until equilibrium is a
chieved. In general, this will take about
one to four hours. The initial concentration will decrease to an equilibrium value. By employing
a series of slurry tests, a plot can be made that describes the relationship between the equilibrium
concentration and the mass of SOC ( x ) adsorbed per unit mass of activated carbon (
m ). Because
the adsorption phenomenon is very temperature and pH dependent, the experimental temperature
is controlled, that is, the experiment is isothermal, and the pH must be the same as that used in the
full-scale treatment process. As a consequence, the data are only relevant for the temperature and
pH at which the experiment is conducted.
Freundlich (1906) developed an empirical equation that is used to describe the results of the
adsorption isotherm experiment. A form of the equation is
q
x
m
KC
ee
n
()
1/
(14-24)
where q
e
mass of solute adsorbed per mass of activated carbon, mg/g
K Freundlich adsorption capacity parameter, (mg/g)(L/mg)
1/ n
C
e
equilibrium concentration of solute, mg/L
1/ n Freundlich adsorption intensity parameter, dimensionless
The linear form of the Freundlich equation is
log log log()qK
n
C
ee
1
⎛
⎝
⎞
⎠
(14-25)
A log-log plot will yield a straight line with a slope of 1/ n. With C
e
equal to 1.0, K q
e
.
EPA has published isotherm data for individual chemicals that may be used for preliminary
feasibility studies (Dobbs and Cohen, 1980). For actual selection of a manufactured carbon, iso-
therm experiments with the actu al raw water are necessary be
cause there are usually multiple
chemicals that compete for adsorption sites and many other constituents that are not SOCs will
adsorb.
