Andrews Dawid G. An Introduction to Atmospheric Physics
Подождите немного. Документ загружается.


149 Problems
wavelength is greater than a certain value, L say. Calculate L at 60
◦
Nfor(a)U = 50 m s
−1
and (b) U = 150 m s
−1
. Compare this with the length of a latitude circle and comment.
Problem 5.8 Take (u, v) = (u
p
+ u
τ
, v
p
+ v
τ
),whereu
p
(x, y), v
p
(x, y) is a geostrophic
flow and u
τ
(z) and v
τ
(z) satisfy the steady laminar Ekman-layer equations
−f v
τ
= ν
∂
2
u
τ
∂z
2
, fu
τ
= ν
∂
2
v
τ
∂z
2
,
(f is a constant, taken to be positive, for the Northern Hemisphere). The boundary conditions
are (u, v) = 0onz = 0and(u, v) → (u
p
, v
p
) as ζ →∞,whereζ = z/h and h = (2ν/f )
1/2
.
Put λ
τ
= u
τ
+ iv
τ
and show that
λ
τ
=−(u
p
+ iv
p
)e
−(1+i)ζ
. (5.68)
Hence verify that
u = u
p
(1 − e
−ζ
cos ζ)− v
p
e
−ζ
sin ζ ,
v = v
p
(1 − e
−ζ
cos ζ)+ u
p
e
−ζ
sin ζ .
Choose axes such that v
p
= 0 and draw the Ekman spiral, showing how the vector (u, v)
varies as ζ increases from 0 to ∞. Note that friction causes a significant cross-isobar flow
in the Ekman layer: in which direction is this flow, in general? Sketch the relative directions
of the pressure-gradient force, the Coriolis force and the viscous force at some point above
the ground but well within the boundary layer, assuming that u
p
> 0.
Using equation (5.68), calculate
∞
0
u
τ
dz and
∞
0
v
τ
dz. What do these quantities
represent? Show that the vertical velocity at the top of the Ekman layer is given by
w
τ
=
ν
2f
1/2
∂v
p
∂x
−
∂u
p
∂y
.
If the boundary layer depth is of order 1 km and f = 10
−4
s
−1
, estimate the order of
magnitude of ν. If the geostrophic wind changes by 20 m s
−1
over a horizontal distance of
1000 km, estimate the order of magnitude of w
τ
. Give the direction and order of magnitude
of the horizontal stress at the ground at a point where the geostrophic wind is 10 m s
−1
.
(Assume that ρ = 1kgm
−3
.)
Problem 5.9 Suppose that friction is represented by linear Rayleigh friction
F
(x)
=−ru, F
(y)
=−rv,
rather than by an eddy-viscosity assumption. Use this representation in the linear Boussinesq
momentum equations on an f plane to model the following situation.
During the daytime, convection is active, leading to a strong frictional coupling with the
ground (modelled by taking r to be large, r = 10
−5
s
−1
) and the atmosphere can be assumed
to be in a steady state at this time. At sunset, convection is quenched and r becomes zero.
Assuming that the pressure gradient is purely in the y direction, is independent of height
and does not change with time, show that the subsequent motion consists of geostrophic

150 Further atmospheric fluid dynamics
motion, superimposed on which is an inertial oscillation of amplitude ru
g
/(r
2
+ f
2
)
1/2
,
where u
g
is the geostrophic velocity.
Calculate the amplitude of the oscillation at 30
◦
N when the flow is 20 m s
−1
from the
west. Show that after 12 h the meridional flow will be the reverse of its daytime value.
(Assume that night-time conditions last this long.)
Problem 5.10 Consider a region of the atmosphere, in the Northern Hemisphere, that is
in geostrophic and hydrostatic balance. Make the Boussinesq approximation and assume
that the wind is in the zonal direction and increases linearly with height, while the density
ρ decreases linearly with height.
Show that the surfaces of constant density slope upwards towards the pole at an angle α
to the horizontal given by
tan α = f Λ/N
2
B
,
where Λ is the vertical wind shear and N
B
is the buoyancy (Brunt–Väisälä) frequency.
Now suppose that two air parcels of equal volume V , densities ρ
1
and ρ
2
(where ρ
2
>ρ
1
)
and heights z
1
and z
2
are interchanged, while the rest of the atmosphere remains undisturbed.
Under what condition is potential energy released by this process?
Suppose that δρ = ρ
2
−ρ
1
and the distance δs between the parcels are small. Show that
the amount of potential energy released is given by
P = ρ
0
V(N
B
δs)
2
(sin φ cos φ tan α − sin
2
φ),
where φ is the angle between the line joining the parcels and the horizontal. [Hint: note
that δρ = (∂ρ/∂y) δy + (∂ρ/∂z) δz,whereδy = δs cos φ, δz = δs sin φ.]
Show that P reaches a maximum P
max
when φ = α/2. Given that α is small, so that
α ≈ tan α, show that
P
max
≈ ρ
0
V
f Λδs
2N
B
2
.
Estimate the angle to the horizontal at which air parcels would move to maximise the
release of potential energy near 60
◦
N, given a vertical windshear of 2 m s
−1
km
−1
and a
buoyancy period of 8 min.
Problem 5.11 Apply the Eady model of baroclinic instability to a region of the atmosphere
near 50
◦
N in which the mean zonal wind varies by 30 m s
−1
over a depth of 10 km and
N
B
10
−2
s
−1
. Estimate the time (in days) taken by the fastest-growing Eady mode to
grow in amplitude by a factor of e and estimate the zonal wavelength of this mode. What is
the shortest zonal wavelength for which instability can occur under the given conditions?

6
Stratospheric chemistry
In keeping with the emphasis on atmospheric physics in this book, the purpose of the
present chapter is to illustrate the use of basic physical principles in the study of some
aspects of atmospheric chemistry, rather than to provide a comprehensive treatment of
atmospheric chemistry as a whole. We therefore focus on stratospheric chemistry, which
provides some simple yet important applications of the basic principles and also some
examples of interactions between chemistry and dynamics.
In Section 6.1 we outline some of the basic thermodynamics of chemical reactions, while
in Section 6.2 we introduce some elementary aspects of chemical kinetics, including the
concepts of reaction rates and chemical lifetimes. In Section 6.3 we focus on bimolecular
reactions and show how physical reasoning can give an expression for the reaction rate.
The process of photo-dissociation is introduced in Section 6.4. Once these basic ideas have
been established, we apply them to stratospheric ozone in Section 6.5, first describing
the Chapman theory (which involves oxygen compounds only) and then introducing the
effects of catalytic cycles. The principles of chemical transport by atmospheric flows are
discussed in Section 6.6, with a qualitative description of the main global-scale meridional
transport structures in the middle atmosphere. Finally, in Section 6.7, we bring several of
these ideas together in a general description of the processes implicated in the formation of
the Antarctic ozone hole.
6.1 Thermodynamics of chemical reactions
Consider a chemical reaction in which reactants A and B lead to products C and D:
A + B → C + D. (6.1)
In the laboratory this reaction takes place within a fixed reaction vessel; in the atmosphere
we have to imagine it taking place within a given ‘parcel’ or ‘blob’ of air, with no transfer
of mass into or out of the parcel. If the reaction takes place at constant pressure p,which
is usually true for reactions in the atmosphere, then it follows from the First Law of
Thermodynamics in the form (2.18) and from equation (2.19) that the heat Q supplied to
the parcel is given, assuming reversibility, by
Q = H.

152 Stratospheric chemistry
Here H is the enthalpy of reaction, that is, the sum of the enthalpies of the products
minus the sum of the enthalpies of the reactants. If H > 0, so that heat must be supplied
for the reaction to proceed, the reaction is called endothermic, w hereas if H < 0, so that
heat is liberated, the reaction is called exothermic.
The standard molar enthalpy of formation of a compound is the enthalpy of reaction
associated with the formation of one mole of the compound from its constituent elements at
a standard temperature (e.g. 25
◦
C = 298.15 K) and pressure (1 atm). This is often denoted
by H
f
, the superscript referring to the standard temperature and pressure. Tables of
H
f
for a variety of atmospheric gases are available in atmospheric chemistry texts. (The
corrections required to convert the enthalpy of reaction to other atmospheric temperatures
and pressures are fairly small.)
Applying these ideas to reaction (6.1) we obtain, at standard temperature and pressure
(STP), the molar enthalpy of reaction
H
= H
f
(C) + H
f
(D) − H
f
(A) − H
f
(B). (6.2)
Given the enthalpies of formation on the right-hand side of this equation, we can deter-
mine whether the reaction is endothermic or exothermic. As an example, consider the
recombination of oxygen (O) atoms to molecular oxygen (O
2
) near the mesopause,
O + O + M → O
2
+ M. (6.3)
Here M is an arbitrary air molecule, which is required to satisfy conservation of energy
and momentum in the reaction. Typically M is either of the most abundant atmospheric
molecules, N
2
or O
2
. Reaction (6.3) is found to have a negative enthalpy of reaction
H ≈−500 kJ mol
−1
, and is thus exothermic; it therefore contributes to the diabatic
heating of the atmosphere; see Section 4.10.
Conversely, the photolysis (or photo-dissociation) of O
2
to form two O atoms has H ≈
+500 kJ mol
−1
(the same enthalpies of formation apply as those in reaction (6.3)), so this
reaction is endothermic: some source of energy is required to drive it. One possibility, which
actually occurs in the atmosphere, is that this energy is supplied by solar photons. A simple
calculation (see Problem 6.1) shows that the relevant photons must have wavelengths of
less than about 240 nm. The standard notation for such a reaction is
O
2
+ hν → 2O, (6.4)
hν here referring to the photon energy required for photolysis.
Thermodynamics also allows us to predict the direction in which a reaction will proceed;
for example, whether A + B → C + DorC+ D → A + B. Consider a natural (or spon-
taneous) change for a ‘system’ (here taken to be our parcel of reacting gases) immersed in a
‘heat bath’ (the surrounding atmosphere) at fixed temperature and pressure. The Second Law
of Thermodynamics implies that the Gibbs free energy G = U +pV −TS (see Section 2.10)
153 Chemical kinetics
of the system (excluding the surroundings) must decrease during this change;
1
i.e.,
G < 0. (6.5)
An equation analogous to equation (6.2) relates the overall molar Gibbs free energy of
reaction at standard temperature and pressure, G
, to the molar Gibbs free energies of
formation of the gases taking part in a reaction. Tabulations of the latter then allow us to
determine whether G
is negative (so that the reaction can occur spontaneously at STP),
positive (so that it cannot) or zero (so that there is equilibrium between the gases involved).
Since the enthalpy H = U +pV and T = T
0
is constant, we have the following relation
between the molar Gibbs free energy of reaction G , the molar enthalpy of reaction H
and the change in molar entropy of the system S:
G = H − T
0
S.
6.2 Chemical kinetics
We now consider how rapidly chemical reactions occur, starting with the simplest first-
order or unimolecular reaction, in which one molecule of a single reactant A breaks down
to one molecule of product B and one molecule of product C:
A → B + C.
Representing the number density of A as [A] molecules per unit volume, we define the
reaction rate R
A
as the rate of decrease of [A] or equivalently the rate of increase of [B]
or [C]:
R
A
=−
∂[A]
∂t
=
∂[B]
∂t
=
∂[C]
∂t
≡ k
A
[A]. (6.6)
The term on the far right-hand side of this equation defines the rate coefficient k
A
,which
has units of s
−1
; its inverse, the chemical lifetime τ
A
of A, satisfies
τ
A
=
1
k
A
=
[A]
∂[A]
∂t
.
In practice the rate coefficient is independent of [A]and t, so we can integrate equation (6.6)
to get
[A]=[A]
0
e
−k
A
t
=[A]
0
e
−t/τ
A
,
where the subscript 0 indicates the value at time t = 0. This shows that the number density
of A decays exponentially in time, decreasing by a factor e in one chemical lifetime.
1
The proof starts with the statement that the entropy of the system plus the surroundings cannot decrease during
the change. Consideration of the heat and work interactions between the system and its surroundings and use
of the First Law and the facts that T = T
0
and p = p
0
are constant during the change then lead to (6.5). Details
are given in standard books on thermodynamics, e.g. Blundell and Blundell (2009).

154 Stratospheric chemistry
Next we consider second-order or bimolecular reactions, of the type considered in
equation (6.1):
A + B → C + D.
In this case the reaction rate R
A
and the rate coefficient k
AB
satisfy
R
A
=−
∂[A]
∂t
=−
∂[B]
∂t
=
∂[C]
∂t
=
∂[D]
∂t
≡ k
AB
[A][B]. (6.7)
We can define an instantaneous lifetime for A for this reaction by
τ
A
=
[A]
∂[A]
∂t
=
1
k
AB
[B]
.
In this case we only get a solution of the form
[A]=[A]
0
e
−t/τ
A
(6.8)
if [B][A] so that [B] can be considered to be approximately constant in time; see
Problem 6.3. Third-order (termolecular) and higher-order reactions can also be considered;
some examples are discussed later.
We now consider several simultaneous reactions. First suppose that A has three loss
processes, but no production, described by
A → products (k
1
), (6.9a)
A + B → products (k
2
), (6.9b)
A + C + D → products (k
3
), (6.9c)
where the k
i
are the rate coefficients. The reaction rate R
A
includes contributions from each
of these processes. It is given by
R
A
=−
∂[A]
∂t
= k
1
[A]+k
2
[A][B]+k
3
[A][C][D].
The corresponding lifetime for A is
τ
A
=
[A]
∂[A]
∂t
=
1
k
1
+ k
2
[B]+k
3
[C][D]
and [A] decays exponentially with time if [B], [C] and [D] are approximately constant.
(The lifetime may still give an instantaneous measure of the fractional rate of change of
[A] even if [B], [C] and [D] are varying.)
Now suppose that, in addition to the loss processes described by reactions (6.9),thereis
also production of A, given by
E + F → A + G (k
4
);
in this case the reaction rate is
R
A
=−
∂[A]
∂t
= k
1
[A]+k
2
[A][B]+k
3
[A][C][D]−k
4
[E][F].
155 Bimolecular reactions
This shows that a steady state is possible, in which ∂[A]/∂t = 0, with a balance between
production and loss of A and an equilibrium number density of A given by
[A]=
k
4
[E][F]
k
1
+ k
2
[B]+k
3
[C][D]
.
It should be emphasised that the equations considered in this section apply to a fixed
volume of reacting chemicals. They need modification when applied to a moving parcel of
air, whose volume may be changing with time; see Section 6.6.
6.3 Bimolecular reactions
Some physical insight into the nature of chemical reactions can be gained by considering a
special case, that of bimolecular reactions, which we now represent by
A + BC → (ABC) → AB + C. (6.10)
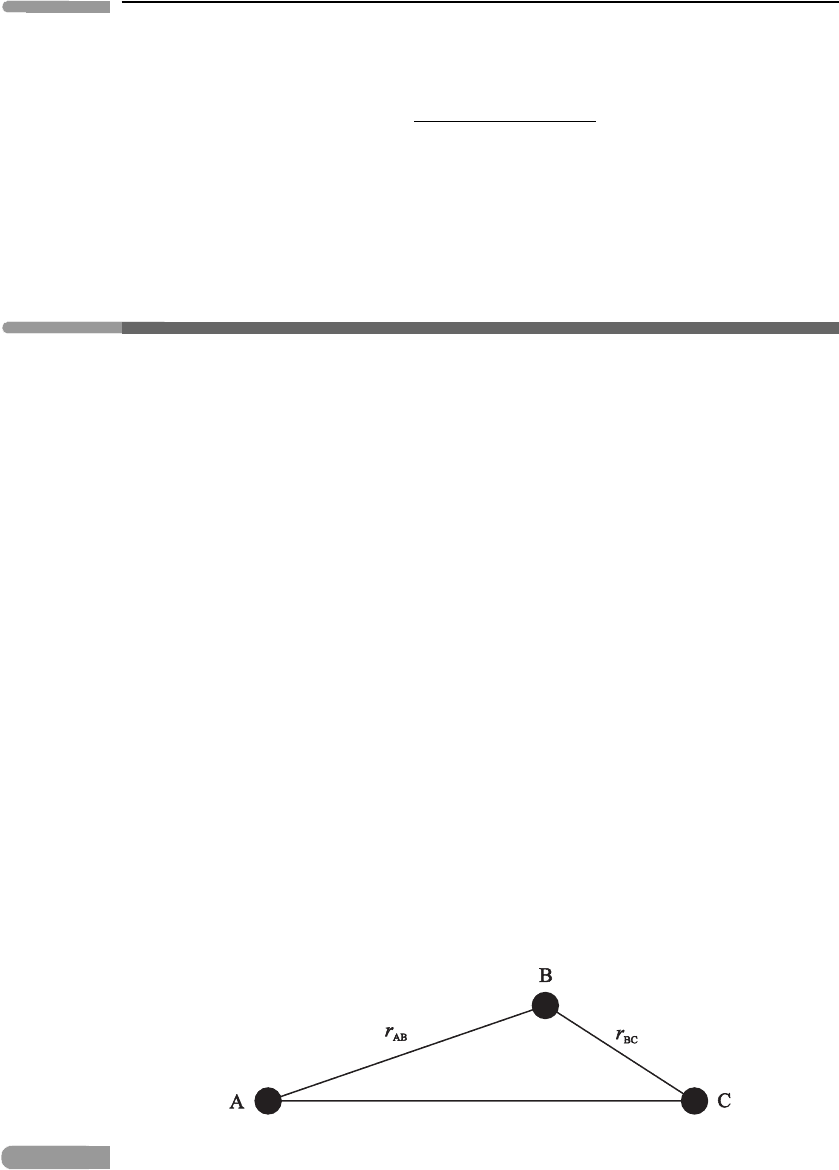
This starts with initial molecules A and BC; BC breaks into fragments B and C and then A,
B and C pass through an intermediate ‘transient reaction complex’ ABC, before forming
the final molecules AB and C. A schematic diagram of the intermediate complex is given
in Figure 6.1: initially the distance r
BC
between B and C is small and the distance r
AB
between A and B is large. During the reaction, the situation is reversed, r
AB
becoming
small and r
BC
becoming large. In the special case in which the angle ∠ABC is fixed, the
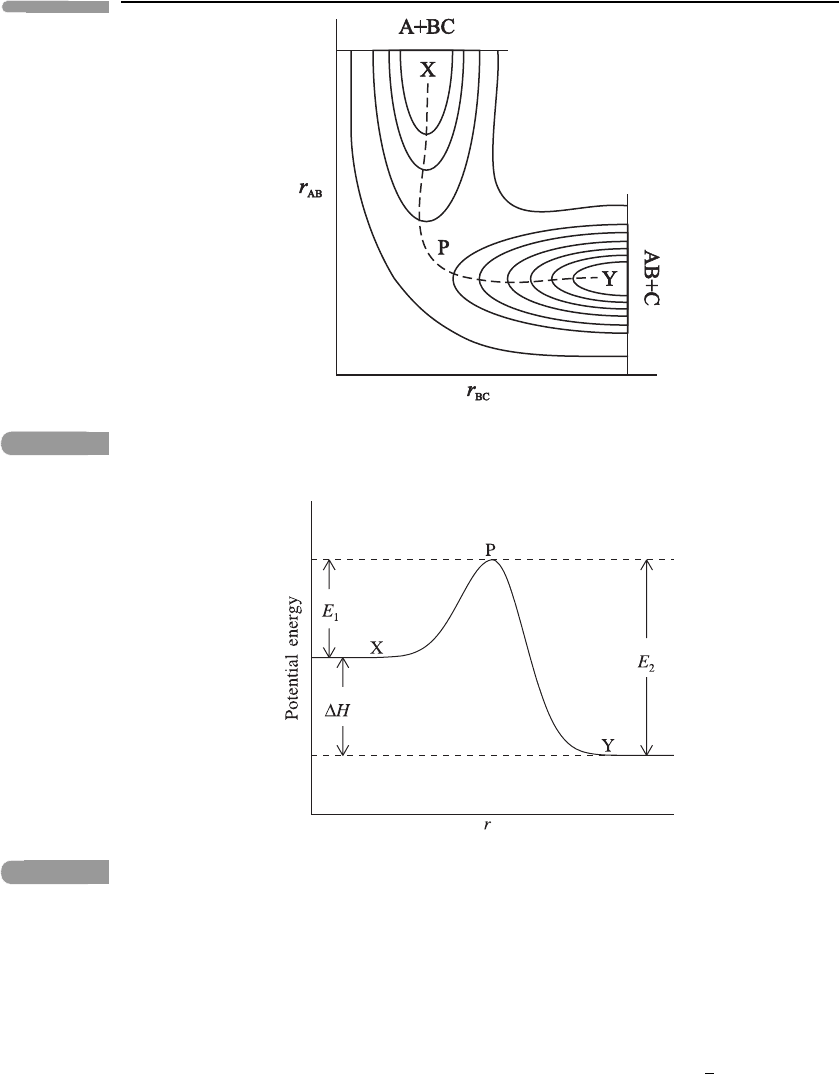
process can be represented in a two-dimensional contour plot of the potential energy as a
function of r
AB
and r
BC
;seeFigure 6.2. This shows that, if sufficient energy is available,
ABC can proceed up the ‘potential energy valley’ X, associated with A + BC, over the
‘saddle point’ or ‘col’ at P and down into the ‘valley’ Y associated with AB + C, following
the dashed line in Figure 6.2.
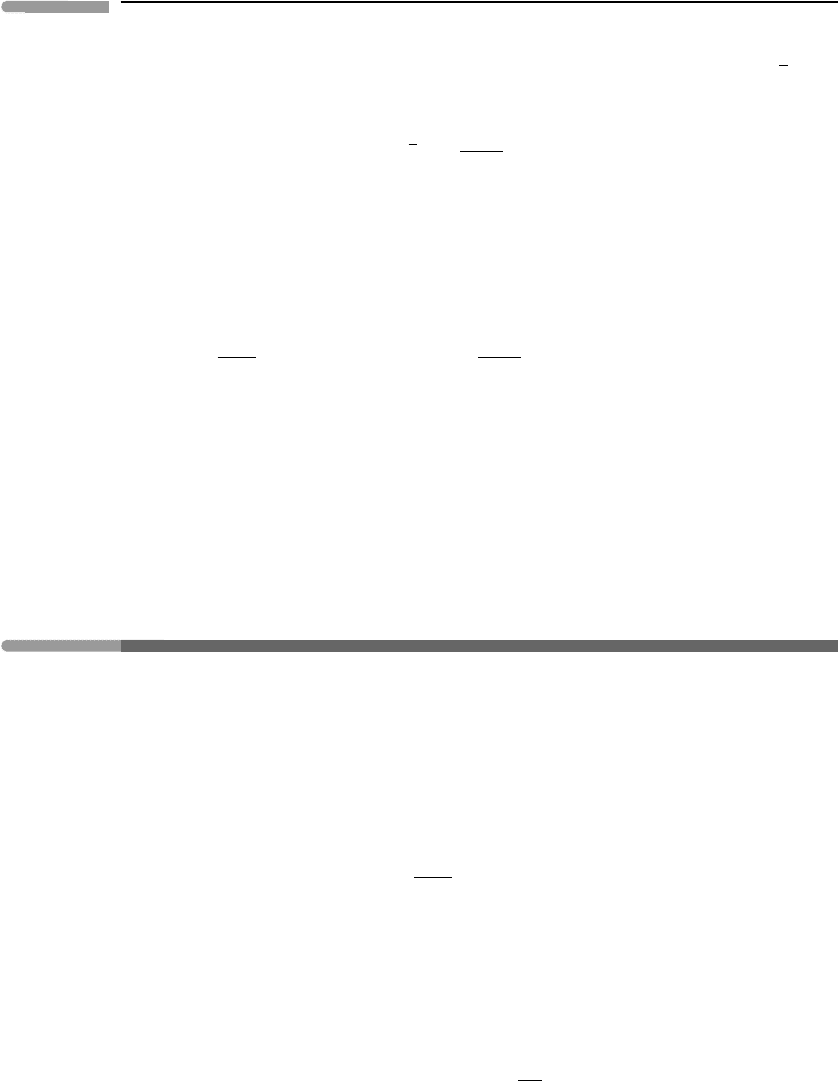
This idea is further illustrated in Figure 6.3, a plot of the potential energy against a
‘reaction coordinate’ r, which indicates how far the reaction has proceeded. This diagram
is a vertical slice, along the dashed line, through the potential energy surface whose
contours are given in Figure 6.2. Reaction (6.10) proceeds from left to right in Figure 6.3:
an activation energy E
1
= H
ABC
− H
A+BC
is required to get from the valley X over the
saddle point at P. In the case shown, a larger amount of energy E
2
= H
ABC
− H
AB+C
is
regained on the further side, in valley Y, so the reaction is exothermic, with an enthalpy of
Fig. 6.1 A schematic sketch of the transient reaction complex (ABC) involved in reaction (6.10).
156 Stratospheric chemistry
Fig. 6.2 A contour plot of potential energy for reaction (6.10), showing the path of the reaction as a
dashed line that proceeds from X, over the saddle point P, to Y.
Fig. 6.3
A plot of the potential energy for reaction (6.10), as a function of the reaction coordinate r.
reaction H = E
1
−E
2
< 0. On the other hand, the reverse reaction AB +C → A +BC,
proceeding from right to left in Figure 6.3, is endothermic, with an enthalpy of reaction
H = E
2
− E
1
> 0.
A crude collision theory provides a simple model for the temperature dependence of the
bimolecular rate coefficient. The kinetic theory of gases shows that the number of collisions,
per unit volume per unit time, between molecules A and BC is [A][BC]σ
c,whereσ is the
collision cross-section
σ = π
(
r
A
+ r
BC
)
2
,
157 Photo-dissociation
r
A
and r
BC
being the notional radii of the molecules (taken to be hard spheres in this
model). The molecules are taken to have masses m
A
and m
BC
, respectively, and c is the
mean relative speed of the molecules at temperature T:
c =
8k
B
T
πm
r
1/2
,
where k
B
is Boltzmann’s constant and m
r
is the reduced mass m
A
m
BC
/(m
A
+ m
BC
) of
the molecules. However, not all collisions lead to reaction: the colliding molecules must
possess sufficient kinetic energy to overcome the energy barrier E
1
; the probability of this
occurring is given by the Boltzmann factor exp[−E
1
/(k
B
T)]. The mean rate of loss of
molecules A per unit volume is therefore
−
∂[A]
∂t
=[A][BC]π(r
A
+ r
BC
)
2
8k
B
T
πm
r
1/2
e
−E
1
/(k
B
T)
≡ k
2
[A][BC],
say. Apart from the weak T
1/2
dependence, the expression for the rate coefficient k
2
is
similar to the empirical Arrhenius expression
k
2
= αe
−E
a
/(k
B
T)
,
where α is a constant and E
a
is an activation energy, provided that E
a
is associated with the
barrier energy E
1
.
6.4 Photo-dissociation
In equation (6.4) we gave an example of photo-dissociation, in which the energy for a
chemical reaction is provided by an incident solar photon that is absorbed by the reactant
molecule (see Section 3.1). In general we can write such a reaction as
A + hν → products,
where the number density [A] satisfies
∂[A]
∂t
=−j
A
[A],
j
A
being the photo-dissociation rate. This implies that, in the absence of other processes,
[A] would decrease exponentially like exp(−j
A
t).
The photo-dissociation rate j
A
depends upon the incident flux of solar photons, the
frequency ν of the photons and the properties of the molecule A, as follows. Between
frequencies ν and ν + dν there is a contribution
dj
Aν
= Φ
Aν
σ
Aν
F
↓
ν
hν
dν,
where Φ
Aν
is the quantum yield (i.e. the number of reactant molecules decomposed
for each absorbed photon) and σ
Aν
is the absorption cross-section for the molecule A
158 Stratospheric chemistry
(see Section 3.5.3). The quantity F
↓
ν
/(hν) is the incident flux of solar photons, i.e. the
number of photons in the given frequency interval crossing unit horizontal area per unit
time; it is equal to the spectral irradiance divided by the energy per photon; see Section 3.6.
We here assume that the Sun is overhead and that there is no scattering, so that all incident
solar photons come from above. For cases of interest to us, the quantum yield is typically
quite close to a step function, being zero for frequencies less than a limit ν
0
that corresponds
to the minimum energy required to dissociate the molecule and approximately unity for
higher frequencies. As shown, for example, in Figure 3.15, the absorption cross-section
can vary rapidly with frequency. The spectral irradiance F
↓
ν
depends on the absorbing gases
present in the path of the solar beam; see Section 3.6. The total photo-dissociation rate is
found by integration over frequency:
j
A
=
∞
ν
0
Φ
Aν
σ
Aν
F
↓
ν
hν
dν.
There is a close relation between the processes of photo-dissociation and thermalisation;
see Section 3.1. For a given frequency interval dν, the quantum yield Φ
Aν
equals the rate
of consumption of photo-dissociation energy per unit volume, dj
Aν
[A]hν, divided by the
heating rate per unit volume, ρQ
Aν
dν, say, due to absorption of photons by molecules A.
This implies that photo-dissociation rates, like heating rates, may form Chapman layers in
the vertical; see Section 3.6.
6.5 Stratospheric ozone
6.5.1 Chapman chemistry
The first attempt to explain the presence of the ‘ozone layer’ – that is, the region of
maximum ozone number density in the lower stratosphere (see Section 1.4.1)–wasmade
by Chapman (1930). He used a set of oxygen-only reactions, starting with the photolysis
of molecular oxygen by ultra-violet photons of wavelength less than about 240 nm:
O
2
+ hν → 2O (j
2
); (6.11a)
cf. reaction (6.4). Next, two fast reactions interconvert O and O
3
: the termolecular reaction
O + O
2
+ M → O
3
+ M (k
2
), (6.11b)
where M is an arbitrary air molecule (cf. reaction (6.3)), and the photolysis of ozone by
photons of wavelength less than 1180 nm,
O
3
+ hν → O + O
2
(j
3
). (6.11c)
These two reactions determine the partitioning between O and O
3
within odd oxygen,
defined as O
x
= O + O
3
. Finally, ozone is destroyed by the slow reaction
O + O
3
→ 2O
2
(k
3
). (6.11d)
