Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.

10-6 What is a copolymer? What is the advantage to
forming copolymers?
10-7 What is the ABS copolymer? State some of the
applications of this material.
Section 10-3 Conditions for Unlimited Solid
Solubility
10-8 Briefly state the Hume-Rothery rules and explain
the rationale.
10-9 Based on Hume-Rothery’s conditions, which of
the following systems would be expected to dis-
play unlimited solid solubility? Explain. (a) Au-
Ag; (b) Al-Cu; (c) Al-Au; (d) U-W; (e) Mo-Ta;
(f) Nb-W; (g) Mg-Zn; and (h) Mg-Cd.
Section 10-4 Solid-Solution Strengthening
10-10 Suppose 1 at% of the following elements is
added to copper (forming a separate alloy with
each element) without exceeding the solubility
limit. Which one would be expected to give the
higher strength alloy? Are any of the alloying
elements expected to have unlimited solid sol-
ubility in copper? (a) Au; (b) Mn; (c) Sr; (d) Si;
and (e) Co.
10-11 Suppose 1 at% of the following elements is
added to aluminum (forming a separate alloy
with each element) without exceeding the sol-
ubility limit. Which one would be expected to
give the least reduction in electrical conduc-
tivity? Are any of the alloy elements expected
to have unlimited solid solubility in aluminum?
(a) Li; (b) Ba; (c) Be; (d) Cd; and (e) Ga.
10-12 Which of the following oxides is expected to
have the largest solid solubility in Al
2
O
3
? (a)
Y
2
O
3
; (b) Cr
2
O
3
; and (c) Fe
2
O
3
.
10-13 What is the role of small concentrations of Mg in
aluminum alloys used to make beverage cans?
10-14 Why do jewelers add small amounts of copper
to gold and silver?
10-15 Why is it not a good idea to use solid solu-
tion strengthening as a mechanism to increase
the strength of copper for electrical applications?
Section 10-5 Isomorphous Phase Diagrams
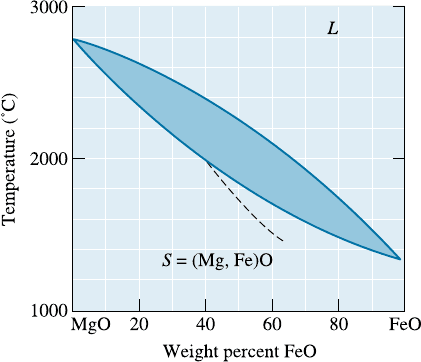
10-16 Determine the liquidus temperature, solidus
temperature, and freezing range for the follow-
ing MgO-FeO ceramic compositions. (See Fig-
ure 10-17.)
(a) MgO-25 wt% FeO; (b) MgO-45 wt% FeO;
(c) MgO-65 wt% FeO; (d) MgO-80 wt% FeO.
10-17 (a) Determine the phases present, the composi-
tions of each phase, and the amount of each
phase in wt% for the following MgO-FeO ce-
ramics at 2000
C. (See Figure 10-17.) (i) MgO-
25 wt% FeO; (ii) MgO-45 wt% FeO; (iii) MgO-
60 wt% FeO; and (iv) MgO-80 wt% FeO. (b)
Consider an alloy of 65 wt% Cu and 35 wt% Al.
Calculate the composition of the alloy in at%.
10-18 Consider a ceramic composed of 30 mol% MgO
and 70 mol% FeO. Calculate the composition
of the ceramic in wt%.
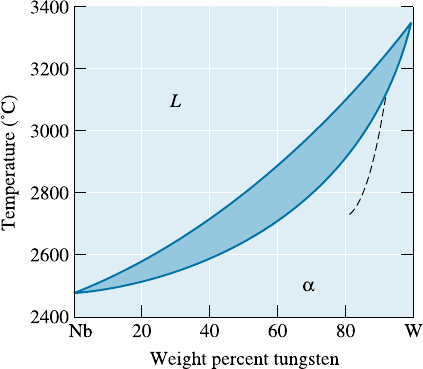
10-19 A Nb-60 wt% W alloy is heated to 2800
C. De-
termine (a) the composition of the solid and
liquid phases in both wt% and at%; (b) the
amount of each phase in both wt% and at%;
and (c) assuming that the density of the solid
is 16.05 g/cm
3
and that of the liquid is 13.91
g/cm
3
, determine the amount of each phase in
vol%. (See Figure 10-18.)
10-20 How many grams of nickel must be added to
500 grams of copper to produce an alloy that
has a liquidus temperature of 1350
C? What is
the ratio of the number of nickel atoms to cop-
per atoms in this alloy?
10-21 How many grams of nickel must be added to
500 grams of copper to produce an alloy that
contains 50 wt% a at 1300
C?
10-22 How many grams of MgO must be added to
1 kg of NiO to produce a ceramic that has a
solidus temperature of 2200
C?
Figure 10-17 The equilibrium phase diagram for the
MgO-FeO system (for Problems 10-16, 10-17, 10-24,
10-25, 10-32 and 10-36). The dashed curve
represents the solidus for non-equilibrium cooling.
C H APT ER 1 0 Solid Solutions and P hase Equilibrium320
10-23 How many grams of MgO must be added to
1 kg of NiO to produce a ceramic that contains
25 mol% solid at 2400
C?
10-24 We would like to produce a solid MgO-FeO
ceramic that contains equal mol percentages of
MgO and FeO at 1200
C. Determine the wt%
FeO in the ceramic. (See Figure 10-17.)
10-25 We would like to produce a MgO-FeO ceramic
that is 30 wt% solid at 2000
C. Determine the
original composition of the ceramic in wt%.
(See Figure 10-17.)
10-26 A Nb-W alloy held at 2800
C is partly liquid
and partly solid. (a) If possible, determine the
composition of each phase in the alloy; and (b)
if possible, determine the amount of each phase
in the alloy. (See Figure 10-18.)
10-27 A Nb-W alloy contains 55% a at 2600
C. De-
termine (a) the composition of each phase; and
(b) the original composition of the alloy. (See
Figure 10-18.)
10-28 Suppose a 544-kg bath of a Nb-40 wt% W alloy
is held at 2800
C. How many kilograms of
tungsten can be added to the bath before any
solid forms? How many pounds of tungsten
must be added to cause the entire bath to be
solid? (See Figure 10-18.)
10-29 A fiber-reinforced composite material is pro-
duced, in which tungsten fibers are embedded
in a Nb matrix. The composite is composed of
70 vol% tungsten. (a) Calculate the wt% of
tungsten fibers in the composite; and (b) sup-
pose the composite is heated to 2600
C and held
for several years. What happens to the fibers?
Explain. (See Figure 10-18.)
Section 10-6 Relationship between Properties
and the Phase Diagram
10-30 What is brass? Explain which element strength-
ens the matrix for this alloy.
10-31 What is the composition of Monel alloy?
Section 10-7 Solidification of a Solid-Solution
Alloy
10-32 Equal moles of MgO and FeO are combined
and melted. Determine (a) the liquidus temper-
ature, the solidus temperature, and the freezing
range of the ceramic; and (b) determine the
phase(s) present, their composition(s), and their
amount(s) at 1800
C. (See Figure 10-17.)
10-33 Suppose 75 cm
3
of Nb and 45 cm
3
of W are
combined and melted. Determine (a) the liquidus
temperature, the solidus temperature, and the
freezing range of the alloy; and (b) determine the
phase(s) present, their composition(s), and their
amount(s) at 2800
C. (See Figure 10-18.)
10-34 A NiO-60 mol% MgO ceramic is allowed to
solidify. Determine (a) the composition of the first
solid to form; and (b) the composition of the last
liquid to solidify under equilibrium conditions.
10-35 A Nb-35% W alloy is allowed to solidify. De-
termine (a) the composition of the first solid to
form; and (b) the composition of the last liquid
to solidify under equilibrium conditions. (See
Figure 10-18.)
10-36 For equilibrium conditions and a MgO-65 wt%
FeO ceramic, determine (a) the liquidus tem-
perature; (b) the solidus temperature; (c) the
freezing range; (d) the composition of the first
solid to form during solidification; (e) the com-
position of the last liquid to solidify; (f) the
phase(s) present, the composition of the phase
(s), and the amount of the phase(s) at 1800
C;
and (g) the phase(s) present, the composition of
the phase(s), and the amount of the phase(s) at
1600
C. (See Figure 10-17.)
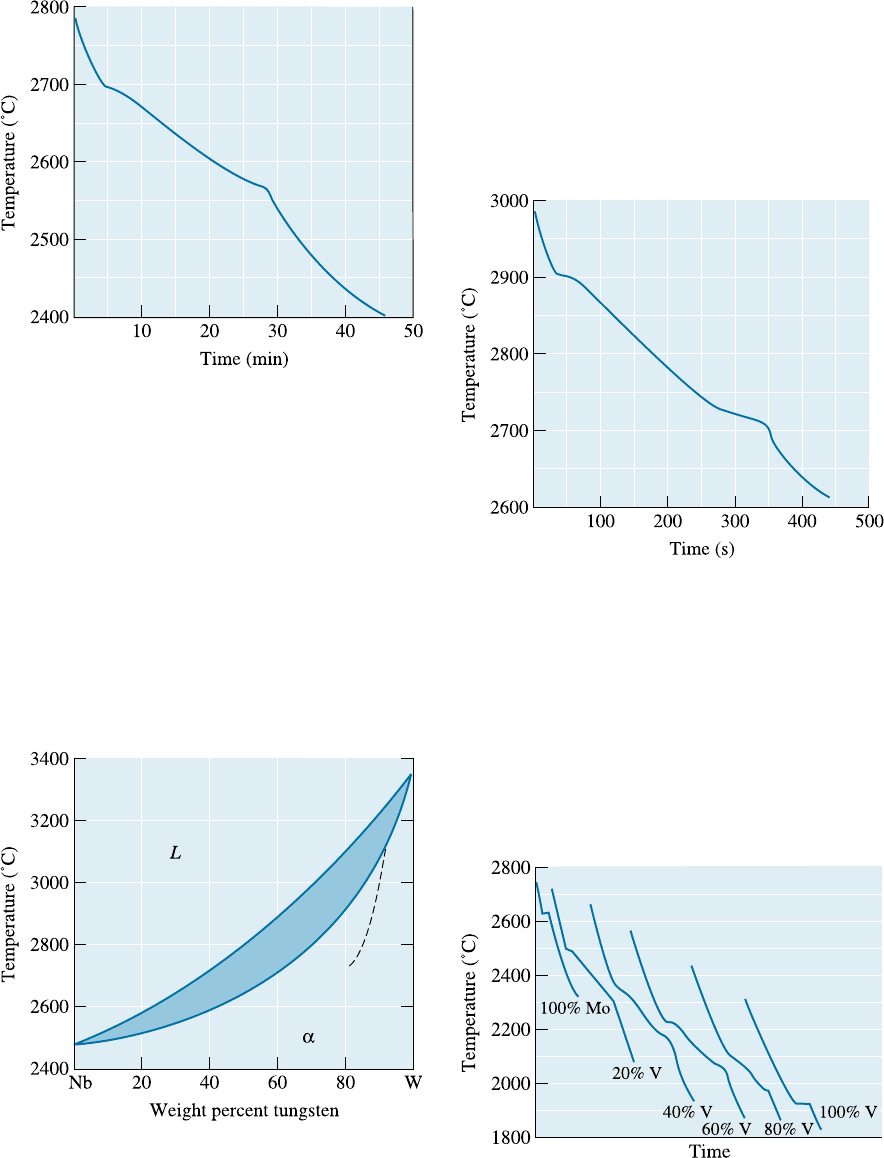
10-37 Figure 10-19 on the next page shows the cooling
curve for a NiO-MgO ceramic. Determine
(a) the liquidus temperature; (b) the solidus tem-
perature; (c) the freezing range; (d) the pouring
temperature; (e) the superheat; (f) the local sol-
idification time; (g) the total solidification time;
and (h) the composition of the ceramic.
Figure 10-18 The equilibrium phase diagram for the
Nb-W system (for Problems 10-19, 10-26, 10-27,
10-28, 10-29, 10-33 and 10-35). The dashed curve
represents the solidus for non-equilibrium cooling.
Problems 321
10-38 For equilibrium conditions and a Nb-80 wt% W
alloy, determine (a) the liquidus temperature;
(b) the solidus temperature; (c) the freezing
range; (d) the composition of the first solid to
form during solidification; (e) the composition
of the last liquid to solidify; (f) the phase(s)
present, the composition of the phase(s), and
the amount of the phase(s) at 3000
C; and (g)
the phases(s) present, the composition of the
phase(s), and the amount of the phase(s) at
2800
C. (See Figure 10-18.)
10-39 Figure 10-20 shows the cooling curve for a
Nb-W alloy. Determine (a) the liquidus temper-
ature; (b) the solidus temperature; (c) the freez-
ing range; (d) the pouring temperature; (e) the
superheat; (f) the local solidification time; (g)
the total solidification time; and (h) the compo-
sition of the alloy.
10-40 Cooling curves are shown in Figure 10-21 for
several Mo-V alloys. Based on these curves,
construct the Mo-V phase diagram.
10-41 What are the origins of chemical segregation in
cast products?
10-42 How can microsegregation be removed?
Figure 10-18 (Repeated for Problem 10-38). The
equilibrium phase diagram for the Nb-W system. The
dashed curve represents the solidus for non-
equilibrium cooling.
Figure 10-19 Cooling curve for a NiO-MgO ceramic
(for Problem 10-37).
Figure 10-20 Cooling curve for a Nb-W alloy (for
Problem 10-39).
Figure 10-21 Cooling curves for a series of Mo-V
alloys (for Problem 10-40).
C H APT ER 1 0 Solid Solutions and P hase Equilibrium322

10-43 What is macrosegregation? Is there a way to re-
move it without breaking up the cast structure?
10-44 What is homogenization? What type of segre-
gation can it remove?
10-45 What is spray atomization?
Design Problems
g
10-46 Homogenization of a slowly cooled Cu-Ni alloy
having a secondary dendrite arm spacing
(SDAS) of 0.025 cm requires 8 hours at 1000
C.
Design a process to produce a homogeneous
structure in a more rapidly cooled Cu-Ni alloy
having a SDAS of 0.005 cm.
10-47 Design a process to produce a NiO-60% MgO
refractory whose structure is 40% glassy phase
at room temperature. Include all relevant tem-
peratures.
10-48 Design a method by which glass beads (having
a density of 2.3 g/cm
3
) can be uniformly mixed
and distributed in a Cu-20% Ni alloy (density of
8.91 g/cm
3
).
Problems 323

11
Dispersion Strengthening and
Eutectic Phase Diagrams
Have You Ever Wondered?
9 Why did some of the earliest glassmakers use plant ash to make glass?
9 What alloys are most commonly used for soldering?
9 What is a fiberglass?
9 Is there an alloy that freezes at a constan t temperature?
9 What is Pyrex 9?
When the solubility of a material is exceeded by
adding too much of an alloying element or com-
pound, a second phase forms and a two-phase
material is produced. The boundary between the
two phases, known as the interphase interface,isa
surface where the atomic arrangement is not per-
fect. In metallic materials, this boundary inter-
feres with the slip or movement of dislocations,
causing their strengthening. The general term for
such strengthening by the introduction of a sec-
ond phase is known as dispersion strengthening.
In this chapter, we first discuss the fundamentals
of dispersion strengthening to determine the mi-
crostructure we should aim to produce. Next, we
324

examine the types of reactions that produce mul-
tiple-phase alloys. Finally, we examine, in some
detail, methods to achieve dispersion strengthen-
ing by controlling the solidification process. We
will concentrate on eutectic phase diagrams that
involve the formation of multiple phases. In
Chapter 12, we will learn about a special way of
producing a dispersion of a second phase via a
solid-state phase transformation sequence known
as precipitation hardening or age hardening. Pre-
cipitation hardening is a sub-set of the overall
class of dispersion-strengthened materials.
11-1 Principles and Examples of Dispersion Strengthening
Most engineered materials are multi-phase, and many of these materials are designed to
provide a certain level of strength. In simple dispersion-strengthened alloys, tiny par-
ticles of one phase, usually very strong and hard, are created within a second phase,
which is weaker but more ductile. The soft phase, usually continuous and present in
larger amounts, is called the matrix. The hard-strengthening phase may be called the
dispersed phase or the precipitate, depending on how the alloy is formed. In some cases,
a phase or a mixture of phases may have a very characteristic appearance – in these
cases this phase or phase mixture may be called a microconstituent. For dispersion
strengthening to occur, the dispersed phase or precipitate must be small enough to
provide e¤ective obstacles to dislocation movement, thus providing the strengthening
mechanism.
In most alloys, dispersion strengthening is produced by phase transformations. In
this chapter, we will concentrate on a solidification transformation by which a liquid
freezes to simultaneously form two solid phases. This is called the eutectic reaction and
is of particular importance in cast irons and many aluminum alloys. In the next chap-
ter, we will discuss the eutectoid reaction, by which one solid phase leads to simulta-
neously form two di¤erent solid phases; this reaction is key in the control of properties
in steels. In Chapter 12, we will also discuss precipitation, or age, hardening which
produces precipitates by a sophisticated heat treatment.
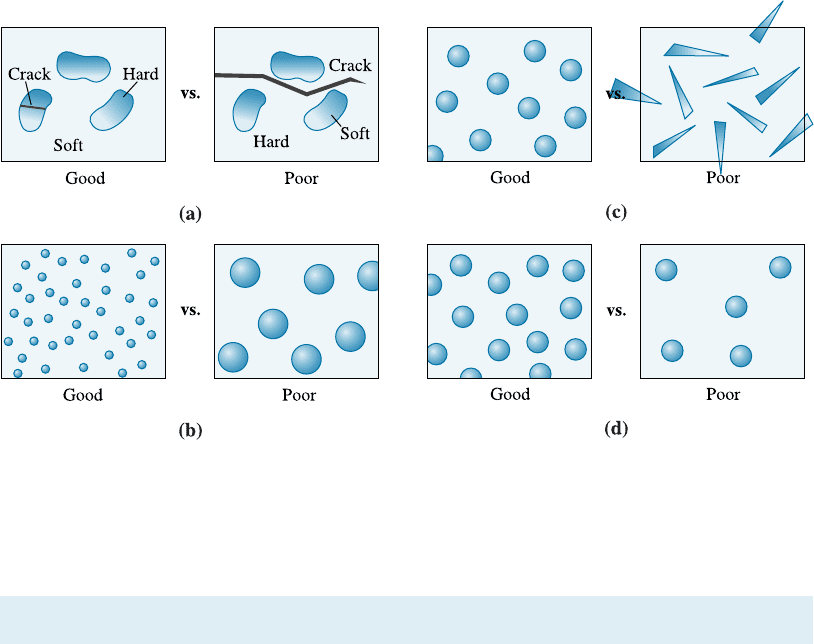
There are some general considerations for determining how the characteristics of
the matrix and the dispersed phase a¤ect the overall properties of an alloy (Figure 11-1).
1. The matrix should be soft and ductile, however, the dispersed phase should be
hard and strong. The dispersed phase particles interfe re with slip, while the matrix
provides at least some ductility to the overall alloy.
2. The hard dispersed phase should be discontinuous, while the soft, ductile matrix
should be continuous. If the hard and brittle dispersed phase were continuous, cracks
could propagate through the entire structure.
3. The dispersed phase particles should be small and numerous, increasing the
likelihood that they interfere with the slip process since the area of the interphase in-
terface is increased significantly.
4. The dispersed phase particles should be round, rather than needlelike or sharp
edged, because the rounded shape is less likely to initiate a crack or to act as a notch.
5. Higher concentrations of the dispersed phase increase the strength of the alloy.
11-1 Principles and Examples of Dispersion Strengthening 325
11-2 Intermetallic Compounds
An intermetallic compound is made up of two or more metallic elements, producing
a new phase with its own composition, crystal structure, and properties. Intermetallic
compounds are almost always very hard and brittle. Intermetallics or intermetallic
compounds are similar to ceramic materials in terms of their mechanical propertie s,
however, in that they are formed by combining two or more metallic elements. Our
interest in intermetallics is two-fold. First, often dispersion-strengthened alloys contain
an intermetallic compound as the dispersed phase. Secondly, many intermetallic com-
pounds, on their own (and not as a second phase) are being investigated and developed
for high-temperature applications. In this section, we will discuss properties of inter-
metallics as stand-alone materials. In the sections that follow, we will discuss how
intermetallic phases help strengthen metallic materials. Table 11-1 summarizes the
properties of some intermetallic compounds.
Stoichiometric intermetallic compounds have a fixed composition. Steels are often
strengthened by a stoichiometric compound, iron carbide (Fe
3
C), which has a fixed
ratio of three iron atoms to one carbon atom. Stoichiometric intermetallic compo unds
are represented in the phase diagram by a vertical line [Figure 11-2(a)]. An example
of a useful intermetallic compound is molybdenum disilicide (MoSi
2
). This material is
used for making heating elements of high temperature furnaces. At high temperatures
(@1000–1600
C), MoSi
2
shows outstanding oxidation resistance. At low temperatures
(@500
C and below), MoSi
2
is brittle and shows catastrophic oxidation known as pesting.
Nonstoichiometric intermetallic compounds have a range of compositions and are
sometimes called intermediate solid solutions. In the molybdenum-rhodium system, the g
phase is a nonstoichiometric intermetallic compound [Figure 11-2(b)]. Because the
Figure 11-1 Considerations for effective dispersion strengthening: (a) The precipitate phase
should be hard and discontinuous, (b) the dispersed phase particles should be small and
numerous, (c) the dispersed phase particles should be round rather than needlelike, and
(d) larger amounts of dispersed phase increase strengthening.
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams326
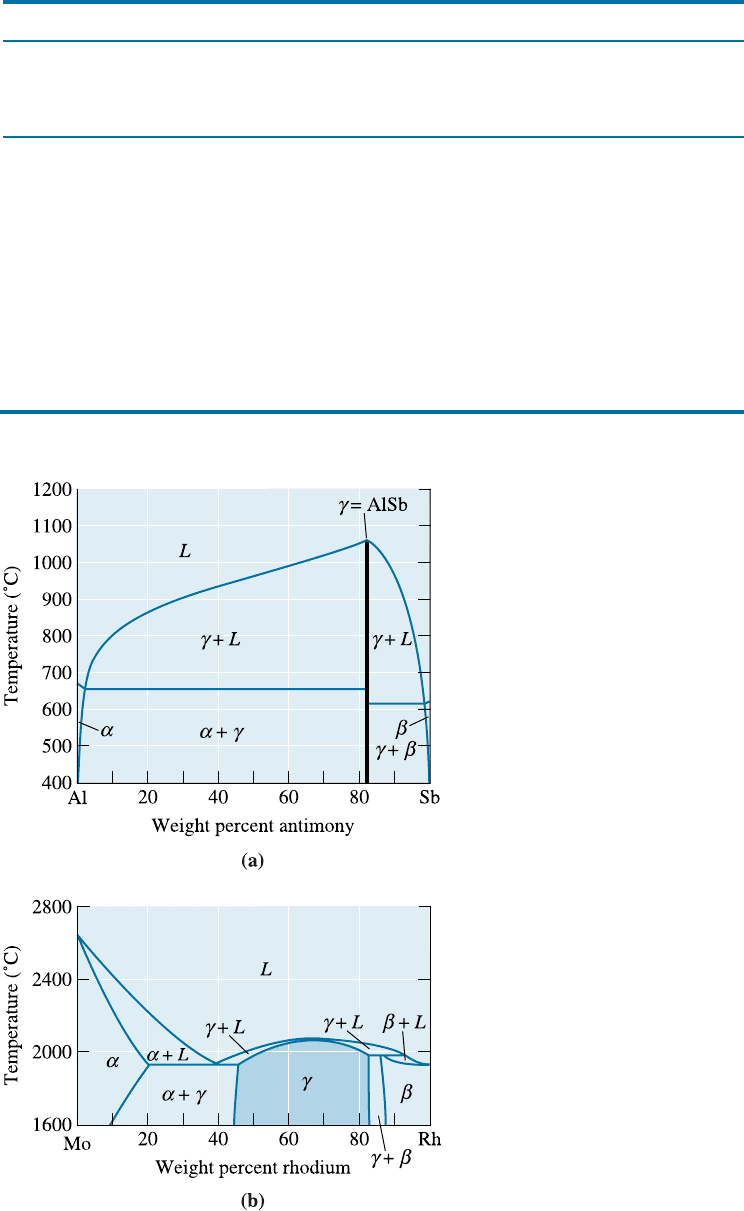
TABLE 11-1 9 Physical and mechanical properties of important intermetallic compounds.
Density
(g/cm
3
)
Crystal
Structure
(ordered)
Young’s
Modulus
(GPa)
Coefficient
of thermal
expansion
(10
C6
/˚C)
Tensile yield
stress (MPa)
Melting
point (˚C)
Al
3
Ti 3.4–4.0 DO
22
(tetr.) 215 12–15 120–425 1350
TiAl 3.8–4.0 Ll
0
(tetr) 160–175 11.7 400–775 1480
Ti
3
Al 4.1–4.7 DO
19
(CPH) 120 12 700–900 1680
MoSi
2
6.1 Tetragonal 380–440 8.1–8.5 200–400 2020
Ni
3
Al 7.4–7.7 Ll
2
(FCC) 180–200 14–16 200–900 1397
NiAl 5.9 B2(FCC) 177–190 14–16 175–300 1638
Ni
5
Si
3
7.2 340 N/A 550 N/A
Fe
3
Al 6.7 DO
3
140–170 19 600–1350 1540
FeAl 5.6–5.8 B2 160–250 21.5 500–700 N/A
(Source: Used with permission from M.A. Meyers & K.K. Chawla, Mechanical Behavior of Materials, 2nd
ed., 2008, Cambridge University Press, UK.)
Figure 11-2
(a) The aluminum-antimony phase
diagram includes a stoichiometric
intermetallic compound g.
(b) The molybdenum-rhodium
phase diagram includes a
nonstoichiometric intermetallic
compound g.
11-2 Intermetallic Compounds 327
molybdenum-rhodium atom ratio is not fixed, the g phase can contain from 45 wt% to
83 wt% Rh at 1600
C. Precipitation of the nonstoichiometric intermetallic copper alu-
minide CuAl
2
causes strengthening in a number of important aluminum alloys.
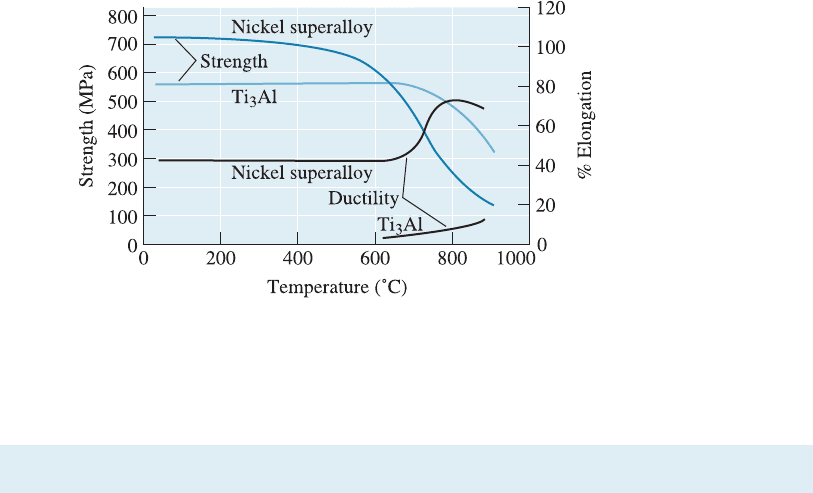
Properties and Applications of Intermetallics Intermetallics such as Ti
3
Al and Ni
3
Al
maintain their strength and even develop usable ductility at elevated temperatures
(Figure 11-3). Lower ductility, though, has impeded further development of these
materials. It has been shown that the addition of small levels of boron (B) (up to 0.2%)
can enhance the ductility of polycrystalline Ni
3
Al. Environmental e¤ects also probably
play a role in limiting the ductility levels in intermetallics. Enhanced ductility levels
could make it possible for intermetallics to be used in many high temperature and load-
bearing applications. Ordered compounds of NiAl and Ni
3
Al are also candidates for
supersonic aircraft, jet engines, and high-speed commercial aircraft. Not all applications
of intermetallics are structural. Intermetallics based on silicon (e.g., platinum silicide)
play a useful role in microelectronics and certain intermetallics such as Nb
3
Sn are useful
as superconductors.
The titanium aluminides, TiAl (also called the gamma (g) alloy) and Ti
3
Al (the
a
2
alloy) are being considered for a variety of applications, including gas turbine engines.
11-3 Phase Diagrams Containing Three-Phase Reactions
Many binary systems produce phase diagrams more complicated than the isomorphous
phase diagrams discussed in Chapter 10. The systems we will discuss here contain reac-
tions that involve three separate phases. Five such reactions are defined in Figure 11-4.
Each of these reactions can be identified in a phase diagram by the following procedure:
1. Locate a horizontal line on the phase diagram. The horizontal line, which in-
dicates the presence of a three-phase reaction, represents the temperature at which the
reaction occurs under equilibrium conditions.
2. Locate three distinct points on the horizontal line: the two endpoints plus a third
point, in between the two endpoints of the horizontal line. This third point represents
the composition at which the three-phase reaction occurs. In Figure 11-4 the point in
Figure 11-3
The strength and ductility
of the intermetallic com-
pound Ti
3
Al compared
with that of a conven-
tional nickel superalloy.
The Ti
3
Al maintains its
strength to higher tem-
peratures than does the
nickel superalloy.
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams328
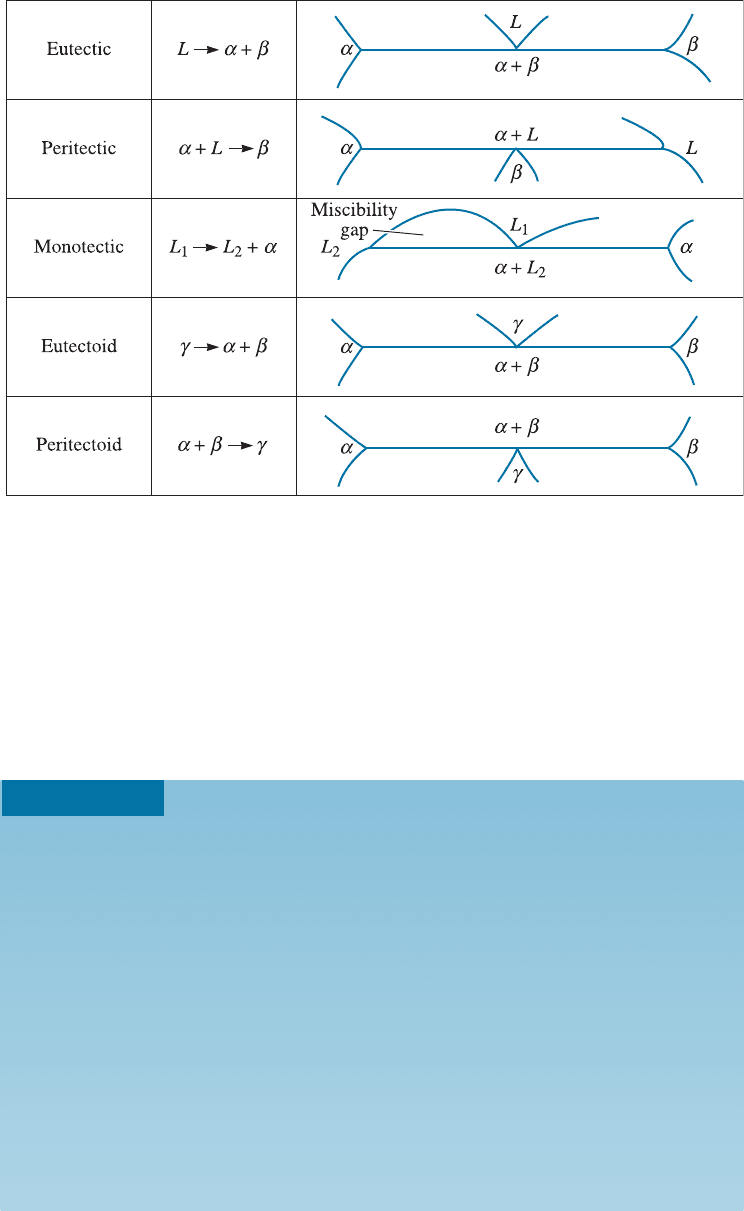
between has been shown at the center. However, on a real phase diagram this point is
not necessarily at the center.
3. Look immediately above the in-between point and identify the phase or phases
present; look immediately below the point in between the end points, and identify the
phase or phases presen t. Then write in the reaction form the phase(s) above the point
which are transforming to the phase(s) below the point. Compare this reaction with
those in Figure 11-4 to identify the reaction.
Figure 11-4 The five most important three-phase reactions in binary phase diagrams.
EXAMPLE 11-1
Identifying Three-Phase Reactions
Consider the binary phase diagram in Figure 11-5. Identify the three-phase
reactions that occur.
SOLUTION
We find horizontal lines at 1150
C, 920
C, 750
C, 450
C, and 300
C:
1150
C: The in-betwen point is at 15% B. d þ L are present above the point, g
is present below. The reaction is:
d þ L ! g; a peritectic
920
C: This reaction occurs at 40% B:
L
1
! g þ L
2
; a monotectic
750
C: This reaction occurs at 70% B:
L ! g þ b; a eutectic
11-3 Phase Diagrams Containing Three-Phase Reactions 329
