Basu P. Biomass Gasification and Pyrolysis: Practical Design and Theory
Подождите немного. Документ загружается.


106
chapter
|
4 Tar Production and Destruction
Gasification in air: Both the yield and the concentration of tar in the
product gas decreases with an increase in the ER. Higher ER (see Section
6.6.2 for a definition) allows greater amounts of oxygen to react with the
volatiles in the flaming pyrolysis zone (see Figure 4.5, page 111). Above an
equivalence ratio of 0.27 phenols are nearly all converted and less tar is
formed (Kinoshita et al. 1994). This decrease is greater at higher tempera-
tures. At a higher ER, the fraction of PAH, benzene, naphthalene, and other
3- and 4-ring aromatics increases. While higher ER reduces the tar, it
reduces the quality of the gas as well. The heating value of the gas is reduced
because of nitrogen dilution from air.
Gasification in steam: When steam reacts with biomass to produce H
2
(Eq. 4.3), the tar-reforming reaction reduces the tar.
C H H O H CO
n x
n n x n+ → +
( )
+
2 2
2 (4.3)
A large reduction in tar yield was seen over an S/B ratio range of 0.5 to 2.5
(Herguido et al., 1992). Further reduction is possible in the presence of
catalyst, which encourages the tar-reforming reaction (García et
al., 1999).
Three main types of catalyst are dolomite, alkali metal, and nickel.
Gasification in a steam–oxygen mixture: The addition of oxygen with
steam further improves tar reduction. Additionally, it provides the heat
TABLE 4.4 Gasification Mediums and Characteristic Parameters
Medium Parameter
Air ER = ratio of air used to stoichiometric air
Steam Steam-to-biomass (S/B) ratio
Carbon dioxide CO
2
-to-biomass ratio
Steam and oxygen Gasifying ratio (GR): (steam + O
2
)-to-biomass ratio
TABLE 4.5 Comparison of Tar Production in Three Gasification Mediums
Medium Operating Condition
Tar Yield
(g/Nm
3
)
LHV (MJ/
Nm
3
dry)
Tar Yield
(g/kg BM
daf
)
Steam S/B = 0.9 30–80 12.7–13.3 70
Steam and oxygen GR = 0.9, H
2
O/O
2
= 3 4–30 12.5–13.0 8–40
Air ER = 0.3; H/C = 2.2 2–20 4.5–6.5 6–30
Source: Data compiled from Gil et al., 1999.

107
4.3 Tar Reduction
needed to make the gasification reaction autothermal. Instead of the S/B
ratio, the ratio of steam–oxygen to biomass, known as the gasification ratio
(GR), is used. The tar yield reduces with an increase in the gasifying ratio.
For example, an 85% reduction in tar is obtained when the GR is increased
from 0.7 to 1.2 (Aznar et
al., 1997). Light tars are produced at a low GR.
Gasification in carbon dioxide: The tar may be reformed on the catalyst
surface in a carbon dioxide medium. Such a reaction is called dry reforming
and is shown here (Sutton et
al., 2001).
C H CO CO x H
n x
+ → +
( )
n n
2 2
2 2 (4.4)
The effect of gasifying agents on tar reduction or tar yield are compared in
Table 4.5 (Gil et al., 1999).
Residence Time
Residence time has a nominal effect on tar yield in a fluidized-bed gasifier.
Kinoshita et al. (1994) noted that with increasing residence time (bed height/
superficial gas velocity), the yield of oxygenated compounds and 1- and 2-ring
compounds (benzene and naphthalene excepted) decreased, but the yield of
3- and 4-ring compounds increased.
Tar Reduction by Additives in Fluidized-Bed Gasifiers
Catalysts accelerate the two main chemical reactions of tar reduction. In a
steam-reforming reaction, we have
C H H O H CO
Catalyst
n x
n n x n+ → +
( )
+
2 2
2 (4.5)
In a dry-reforming reaction, we have
C H CO H CO
Catalyst
n x
n x n+ →
( )
+
2 2
2 2 (4.6)
Catalysts can facilitate tar reduction reactions either in the primary reactor
(gasifier) or downstream in a secondary reactor. In either case, dolomite,
olivine, alkali, nickel, and char have found successful use in catalysts for tar
reduction.
Dolomite
Dolomite (MgCO
3
, CaCO
3
) is relatively inexpensive and is readily available.
It is more active if calcined and used downstream in the secondary reactor at
above 800 °C (Sutton et
al., 2001). The reforming reaction of tar on a dolomite
surface occurs at a higher rate with CO
2
than with steam. Under proper condi-
tions it can entirely convert the tar, but cannot convert methane if that is to be
avoided for syngas production. Carbon deposition deactivates dolomite, which,
being less expensive, may be discarded.

108
chapter
|
4 Tar Production and Destruction
Olivine
Olivine is a magnesium-iron silicate mineral (Mg, Fe
2
)SiO
4
that comes in sizes
(100–400 micron) and density ranges (2500–2900
kg/m
3
) similar to those of
sand. Thus, it is conveniently used with sand in a fluidized-bed gasifier. The
catalytic activity of olivine is comparable to that of calcined dolomite. When
using olivine, Mastellone and Arena (2008) noted a complete destruction of tar
from a fluidized-bed gasifier for plastic wastes, while Rapagnà et
al. (2000)
obtained a 90% reduction in a biomass-fed unit.
Alkali
Alkali metal catalysts are premixed with biomass before they are fed into the
gasifier. Some of them are more effective than others. For example, the order
of effectiveness of some alkali catalysts can be shown as follows:
K CO Na CO Na H CO H O Na B O H O
2 3 2 3 3 3
2
2 2 4 7 2
2 10> >
( )
×
( )
> × (4.7)
Unlike dolomite, alkali catalysts can reduce methane in the product gas, but it
is difficult to recover them after use. Furthermore, alkali cannot be used as a
secondary catalyst. Its use in a fluidized bed makes the unit prone to agglomera-
tion (Mettanant et al., 2009).
Nickel
Many commercial nickel catalysts are available in the market for reduction of
tar as well as methane in the product gas. They contain various amounts of
nickel. For example, catalyst R-67-7H of Haldor Topsøe has 12 to 14% Ni on
an Mg/Al
2
O
3
support (Sutton et al., 2001). Nickel catalysts are highly effective
and work best in the secondary reactor. Use of dolomite or alkali as the primary
catalyst and nickel as the secondary catalyst has been successfully demon-
strated for tar and methane reduction. Catalyst activity is influenced by
temperature, space–time, particle size, and composition of the gas atmosphere.
The optimum operating temperature for a nickel catalyst in a downstream fluid-
ized bed is 780 °C (Sutton et
al., 2001). Steam-reforming nickel catalysts for
heavy hydrocarbons are effective for reduction of tar while nickel catalysts for
light hydrocarbons are effective for methane reduction. Deactivation due to
carbon deposition and particle growth is a problem for nickel-reforming
catalysts.
Char
Char, a carbonaceous product of pyrolysis, also catalyses tar reforming when
used in the secondary reactor. Chembukulam et
al. (1981) obtained a nearly
total reduction in tar with this. As a major gasification element, char is not
easily available in a gasifier’s downstream. Design modification is needed to
incorporate char as a catalyst.

109
4.3 Tar Reduction
Gasifier Design
The design of the gasifier can be a major influence on the amount of tar in the
product gas. For example, a counter current moving-bed gasifier with an inter-
nal recycle and a separate combustion zone can reduce the tar content to as low
as 0.1
g/Nm
3
(Susanto and Beenackers, 1996), while in an updraft gasifier the
tar can well exceed 100
g/Nm
3
. To understand how gasifier design might influ-
ence tar production, we will examine the tar production process.
As we saw in Figure 4.2, primary tar is produced at fairly low temperatures
(200–500 °C). It is a mixture of condensable hydrocarbons that undergoes
molecular rearrangement (reforming) at higher temperatures (700–900 °C),
producing some noncondensable gases and secondary tar. Tar is produced at an
early stage when biomass (or another fuel) undergoes pyrolysis following
drying. Char is produced further downstream in the process and is often the
final solid residue left over from gasification. The gasifier design determines
where pyrolysis takes place, how the tar reacts with oxidants, and the tempera-
ture of the reactions. This in turn determines the net tar production in the
gasifier.
Updraft, downdraft, fluidized bed, and entrained bed are the four major
types of gasifier with their distinct tar formation. Table 4.2 earlier in the chapter
compares their tar production, and a brief discussion of formation of tar in these
reactors follows here.
Updraft Gasifier
Biomass is fed from the top and a gasifying medium (air) is fed from the
bottom. The product gas leaves from the top while solids leave from the bottom.
Figure 4.4 illustrates the motion of biomass, gas, and tar. The temperature is
highest close to the grate, where oxygen meets with char and burns the char.
The hot gas travels up, providing heat to the endothermic gasification reactions,
and meets pyrolyzing biomass at a low temperature (200–500 °C). Primary tar
is produced in this temperature range (Figure 4.4). This tar travels upward
through cooler regions and therefore has no opportunity for conversion into
gases and secondary tar. For this reason, updraft gasifiers generate the highest
amount of tar—typically 10 to 20% by weight of the feed.
Downdraft Gasifier
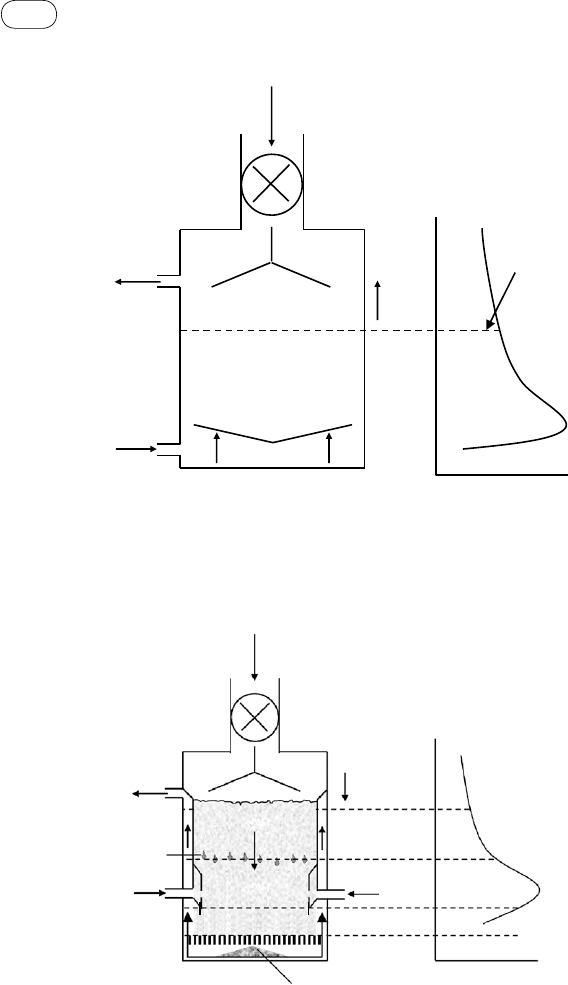
Figure 4.5 shows the tar production in a downdraft gasifier. Here, both gas and
feed travel downward. The temperature is highest in the downstream combus-
tion zone. The tar is produced after drying at lower temperatures (200–500 °C)
close to the feed point. The oxygen in the air, along with the tar, travels down-
ward to the hotter zone. Owing to the availability of oxygen and high tempera-
ture, the tar readily burns in a flame, raising the gas temperature to 1000 to
1400 °C. The flame occurs in the interstices between feed particles, which
remain at 500 to 700 °C (Milne et
al., 1998, p. 14). This phenomenon is called
110
chapter
|
4 Tar Production and Destruction
Air
Air
Ash
Tar
formation
Drying
Biomass
Temperature
200–500 °C (drying)
500–700 °C (pyrolysis)
1000–1400 °C
(combustion)
800–1000 °C
(gasification)
Pyrolysis
Combustion
Gasification
Flaming pyrolysis
Gas, tar
fIGurE 4.5 Tar generation in a downdraft gasifier. The tar produced passes through the highest-
temperature zone and so is easily cracked.
Pyrolysis
Reduction
Combustion
Ash
Biomass
Air
Gas, tar
Temperature
1000 °C
200–500
°C
Tar
Tar formation
fIGurE 4.4 Tar production in an updraft gasifier. Tar passes through only the low-temperature
(200–500 °C) zone, so it has no opportunity to crack.
111
4.3 Tar Reduction
flaming pyrolysis. Since the pyrolysis product, tar, contacts oxygen while
passing through the highest-temperature zone, it has the greatest opportunity to
be converted into noncondensable gases. For this reason, a downdraft gasifier
has the lowest tar production (<1
g/Nm
3
).
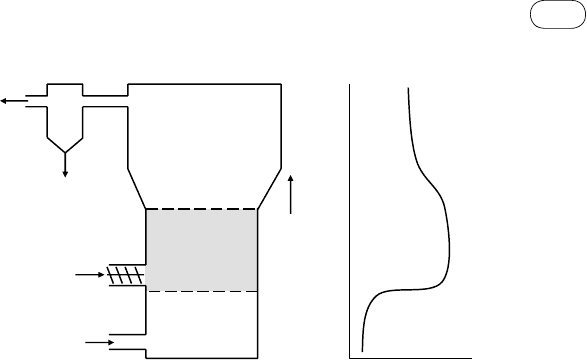
Fluidized-Bed Gasifier
In a typical fluidized bed (bubbling or circulating) air enters from the bottom,
but is fuel fed from the side or top. In either case, the fuel is immediately mixed
throughout the bed owing to its exceptionally high degree of mixing (Figure
4.6). Thus, the fresh oxygen (in air) entering the grid comes into immediate
contact with fresh biomass particles undergoing pyrolysis as well as with spent
char particles from the biomass, which has been in the bed for some time.
Oxygen’s contact with the fresh biomass burns the tar released, while its contact
with the spent char particles causes the char to burn.
Though the solids are back-mixed, the gases flow upward in plug-flow
mode. This means that further up in the bed neither older char particles nor
fresh pyrolyzing biomass particles come in contact with the oxygen. Any tar
released moves up in the bed and leaves along with the product gas. For this
reason, tar generation in a fluidized-bed gasifier is between the two extremes
represented by updraft and downdraft gasifiers, averaging about 10
mg/Nm
3
.
Entrained-Flow Gasifier
Tar production is negligible, as whatever is released passes through a very-
high-temperature (>1000 °C) zone and is therefore nearly all converted into
gases.
Freeboard
Fluid bed
Plenum
Biomass
Air/steam
Cyclone
Ash
Temperature
800–900 °C
Gas, tar
Tar
fIGurE 4.6 Bubbling fluidized-bed gasifier. The tar is not produced at any specific location and
so it passes through the average-temperature zone.

112
chapter
|
4 Tar Production and Destruction
Design Modifications for Tar Removal
Modification of a reactor design for tar removal involves the following:
Secondary air injection
Separation of the pyrolysis zone from the char gasification zone
Passage of pyrolysis products through the char
Char is an effective means of tar decomposition. A moving-bed two-stage
gasifier that uses the first stage for pyrolysis and the second stage for conversion
of tar in a bed of char succeeds in reducing the tar by 40 times (Bui et
al.,
1994). Air addition in the second stage increases the temperature and thereby
reduces the tar (Knoef, 2005, p. 170).
A large commercial unit (70-MW fuel power) uses this concept, where
biomass dries and pyrolyzes in a horizontal moving bed, heated by waste heat
from a diesel engine. The tar concentration of the product gas is about 50
g/
Nm
3
. This gas passes through the neck of a vertical chamber, where injection
of preheated gas raises the temperature above 1100 °C, reducing the tar amounts
to 0.5
g/Nm
3
. It then passes through a fixed bed of char or carbon being gasified.
Tar in the gas leaving the gasifier is very low (<0.025
g/Nm
3
). It is further
cleaned to 0.005
g/Nm
3
in a bag filter (Knoef, 2005, p. 159).
Another design involves twin fluidized beds. Biomass fed into the first bed
is pyrolyzed. The char then travels to a parallel fast fluidized-bed combustor
that burns part of it. A commercial unit (8-MW fuel power) operates on this
principle, where gas leaving the gasifier contains 1.5 to 4.5
g/Nm
3
tar. A fabric
filter that separates dust and some tar reduces its concentration to 0.75
g/Nm
3
,
which is finally reduced to 0.010 to 0.04
g/Nm
3
in a scrubber.
4.3.2 Post-Gasification—secondary reduction of tar
As indicated earlier, the level of cleaning needed for the product gas depends
greatly on its end use. For example, combustion in an engine or a gas turbine
needs substantially cleaner product gas than that required by a boiler. Most
commercial plants use particulate filters or scrubbers to attain the required level
of cleanliness. A substantial amount of tar can be removed from the gas in a
post-gasification cleanup section. It can be either catalytically converted into
useful gases like hydrogen or simply captured and scrubbed away. The two
basic post-gasification methods are physical removal and cracking (catalytic or
thermal).
Physical Tar Removal
Physical cleaning is similar to the removal of dust particles from a gas. It
requires the tar to be condensed before separation. The energy content of the
tar is often lost in this process such that it remains as mist or drops on suspended
particles in gas. Physical tar removal can be accomplished by cyclones, barrier

113
4.3 Tar Reduction
filters, wet electrostatic precipitators, wet scrubbers, or alkali salts. The choice
depends on the following:
Inlet concentration of particulate and tar
Inlet particle size distribution (PSD)
Particulate tolerance of the downstream application of the gas
The size distribution of the inlet particulates is difficult to measure, espe-
cially for finer particulates, but its measurement is important in choosing the
right collection devices. For example, submicron (<1 micron) particulates need
a wet electrostatic precipitator, but this device is significantly more expensive
than others. A fabric filter may work for fines, but it may fail if there is any
chance of condensation.
Cyclones
Cyclones are not very practical for tar removal because of the tar’s stickiness
and because cyclones cannot remove small (<1 micron) tar droplets (Knoef,
2005, p. 196). A fabric filter has been used with the help of a precoat, which is
removed along with the dust cake formed on the filter.
Barrier Filters
Barrier filters present a physical barrier in the path of tar and particulates while
allowing the clean gas to pass through. One of their special features is that they
allow coating of their surface with appropriate catalytic agents to facilitate tar
cracking. These filters are of two types: candle and fabric.
Candle filters are porous, ceramic, or metallic. The porosity of the material
is chosen such that the finest particles do not pass through. Particles failing to
pass through the filter barrier deposit on the wall (Figure 4.7), forming a layer
Gas
Gas, tar
Filter material
Filter cake
f
IGurE
4.7
Operation of a barrier filter.

114
chapter
|
4 Tar Production and Destruction
of solids called a “filter cake.” Gas passes through the porous layer as well as
through the filter. One major problem with the filter cake is that as it grows in
thickness, the pressure drop across the filter increases. Thus, provision is made
for its occasional removal. A popular means of removal is pressure pulse in
opposite directions.
Besides their high-pressure drop, barrier filters also suffer from the problem
that if a filter is broken or cracked, dust and tar-laden gas preferentially flow
through that passage, adversely affecting downstream equipment. The conden-
sation of tar on the filter elements can block the filter, and this is a major
concern. Ceramic filters can be designed to operate in temperatures as high as
800 to 900 °C.
Fabric filters are made of woven fabric as opposed to porous materials as
in candle filters. Unlike candle filters, they can operate only in lower tempera-
tures (<350 °C). Here the filter cake is removed by either back-flushing as with
a candle filter, or shaking. Condensation of tar on the fabric is a problem here
if the gas is cooled excessively.
Wet Electrostatic Precipitators
Wet electrostatic precipitators (ESPs) are used in some gasification plants.
The gas is passed through a strong electric field with electrodes. High
voltage charges the solid and liquid particles. As the flue gas passes through a
chamber containing anode plates or rods with a potential of 30 to 75
kV, the
particles in the flue gas pick up the charge and are collected downstream by
positively charged cathode collector plates. Grounded plates or walls also
attract the charged particles and are often used for design simplicity. Although
collection efficiency does not decrease as particles build up on the plates,
periodic mechanical wrapping is required to clean the plates to prevent the
impediment of the gas flow or the short-circuiting of the electrodes through the
built-up ash.
The collected solid particles are cleaned by mechanical means, but a liquid
like tar needs cleaning by a thin film of water. Wet electrostatic precipitators
have very high (> 90%) collection efficiency over the entire range of particle
size down to about 0.5 micron, and they have very low pressure drop (few
inches water gauge). Sparking due to high voltage is a concern with an ESP,
especially when it is used to clean highly combustible syngas. Thus, the savings
from lower fan power due to low pressure drop is offset by a higher safety cost.
Additionally, the capital cost for ESP is three to four times higher than that for
a wet scrubber.
Wet Scrubbers
Here, water or an appropriate scrubbing liquid is sprayed on the gas. Solid
particles and tar droplets collide with the drops, forming larger droplets because
of coalescence. These larger droplets are easily separated from the gas by a

115
4.3 Tar Reduction
demisterlike cyclone. The gas needs to be cooled until it is below 100 °C before
cleaning. The tar-laden scrubbing liquid may be fed back into the gasifier
or its combustion section. Alternatively, it may be regenerated by stripping the
tar away.
Some commercial methods, such as the OLGA and TARWTC technologies,
use proprietary oil as the scrubbing liquid. The tar liquid is then reinjected into
the gasifier for further conversion (Knoef, 2005, p. 196). Scrubbers have a high
(>90%) collection efficiency, but the efficiency drops sharply below 1-micron-
sized particles. They consume a large amount of fan power owing to the large
(~50-inch water gauge) pressure drop across the scrubber. While their operating
cost is high, their capital cost is much less than that for ESPs.
A system with a tar removal scrubber produces cleaned gas with a lower
outlet temperature and a higher energy content, but it contains tars that are more
difficult to remove. The main challenge of tar removal is the formation of “tar
balls,” which are long-chained hydrocarbons that have a tendency to agglomer-
ate and stick together, fouling equipment in the initial stages of tar condensing
and collecting.
The tar-laden stripper gas, if fed into the gasifier, lowers its dewpoint well
below that of water. This allows condensation of the tar, while flue gas contain-
ing tar vapor can be recycled back to the combustion section of the gasifier for
combustion.
Alkali Remover
Compared to fossil fuels, biomass is rich in alkali salts that typically vaporize
at high gasifier temperatures but condense downstream below 600 °C. Because
condensation of alkali salts causes serious corrosion problems, efforts are made
to strip the gas of alkali. If the gas can be cooled to below 600 °C, the alkali
will condense onto fine solid particles (<5 microns) that can be captured in a
cyclone, ESPs, or filters. Some applications do not permit cooling of the gas.
In such cases, the hot gas may be passed through a bed of active bauxite main-
tained at 650 to 725 °C.
Disposal of Collected Tar
Tar removal processes produce liquid wastes with higher organic compound
concentrations, which increase the complexity of water treatment. Wastewater
contaminants include dissolved organics, inorganic acids, NH
3
, and metals.
Collected tars are classified as hazardous waste, especially if they are formed
at high temperatures (Stevens, 2001). Several technologies are available for
treatment of these contaminants before their final disposal. Hasler et
al. (1997)
presented a description of the available technologies that comprise extraction
with organic solvent, distillation, adsorption on activated carbon, wet oxidation,
oxidation with hydrogen peroxide (H
2
O
2
), oxidation with ozone (O
3
), incinera-
tion, and biological treatment.
