Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.


The complete oxidation of muscle glycogen to CO
2
substantially increases the energy yield, but this aerobic process is a
good deal slower than anaerobic glycolysis. However, as the distance of a run increases, aerobic respiration, or oxidative
phosphorylation, becomes increasingly important. For instance, part of the ATP consumed in a 1000-meter run must
come from oxidative phosphorylation. Because ATP is produced more slowly by oxidative phosphorylation than by
glycolysis (see Table 30.3), the pace is necessarily slower than in a 100-meter sprint. The championship velocity for the
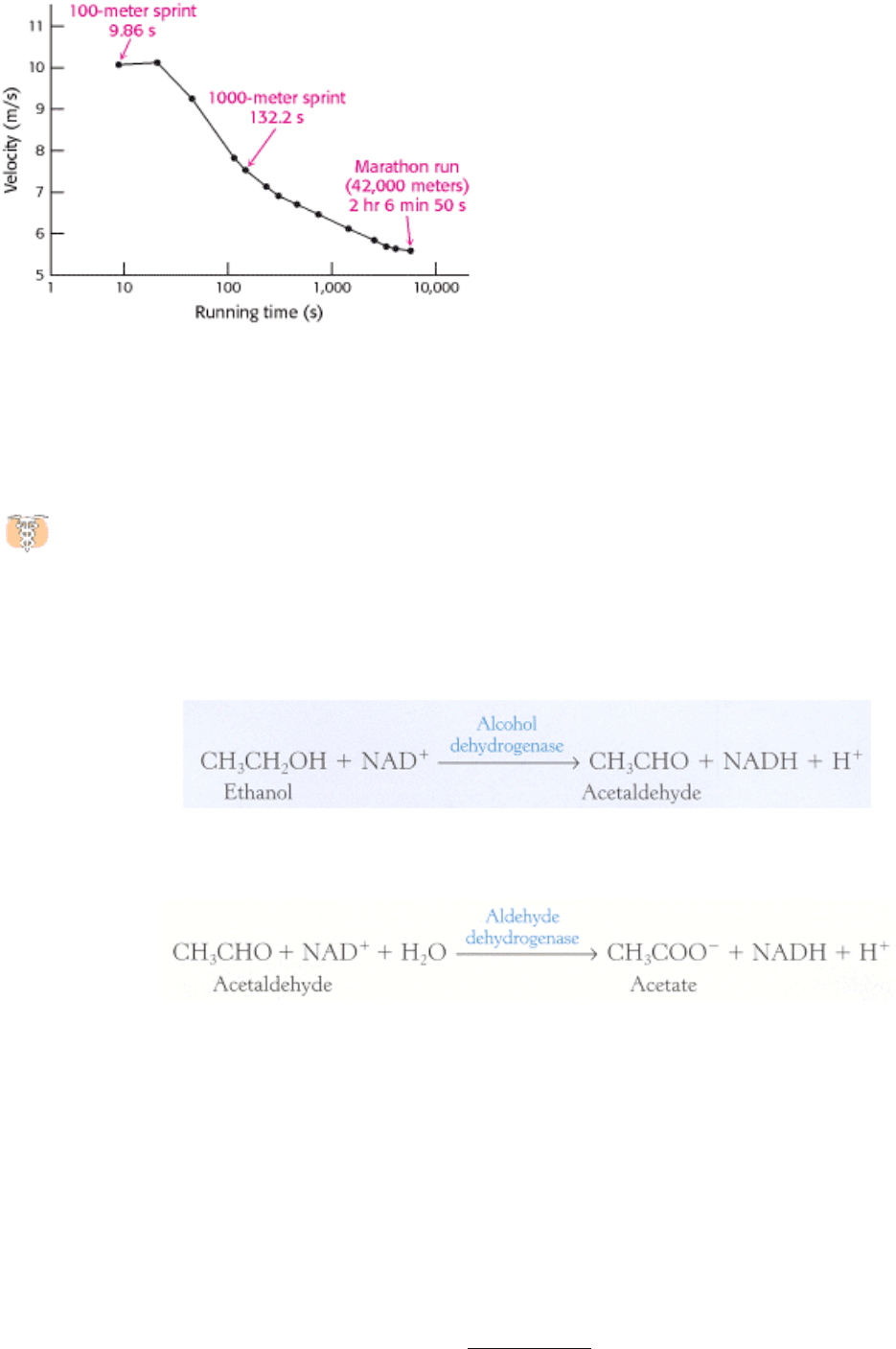
1000-meter run is about 7.6 m/s, compared with approximately 10.2 m/s for the 100-meter event (Figure 30.19).
The running of a marathon (26 miles 385 yards, or 42,200 meters), requires a different selection of fuels and is
characterized by cooperation between muscle, liver, and adipose tissue. Liver glycogen complements muscle glycogen as
an energy store that can be tapped. However, the total body glycogen stores (103 mol of ATP at best) are insufficient to
provide the 150 mol of ATP needed for this grueling ~2-hour event. Much larger quantities of ATP can be obtained by
the oxidation of fatty acids derived from the breakdown of fat in adipose tissue, but the maximal rate of ATP generation
is slower yet than that of glycogen oxidation and is more than tenfold slower than that with creatine phosphate. Thus,
ATP is generated much more slowly from high-capacity stores than from limited ones, accounting for the different
velocities of anaerobic and aerobic events.
ATP generation from fatty acids is essential for distance running. However, a marathon would take about 6 hours to run
if all the ATP came from fatty acid oxidation, because it is much slower than glycogen oxidation. Elite runners consume
about equal amounts of glycogen and fatty acids during a marathon to achieve a mean velocity of 5.5 m/s, about half that
of a 100-meter sprint. How is an optimal mix of these fuels achieved? A low blood-sugar level leads to a high glucagon/
insulin ratio, which in turn mobilizes fatty acids from adipose tissue. Fatty acids readily enter muscle, where they are
degraded by β oxidation to acetyl CoA and then to CO
2
. The elevated acetyl CoA level decreases the activity of the
pyruvate dehydrogenase complex to block the conversion of pyruvate into acetyl CoA. Hence, fatty acid oxidation
decreases the funneling of sugar into the citric acid cycle and oxidative phosphorylation. Glucose is spared so that just
enough remains available at the end of the marathon. The simultaneous use of both fuels gives a higher mean velocity
than would be attained if glycogen were totally consumed before the start of fatty acid oxidation.
III. Synthesizing the Molecules of Life 30. The Integration of Metabolism 30.4. Fuel Choice During Exercise Is Determined by Intensity and Duration of Activity
Table 30.3. Fuel sources for muscle contraction
Fuel source Maximal rate of ATP production (mmol/s) Total ~P available (mmol)
Muscle ATP 223
Creatine phosphate 73.3 446
Conversion of muscle glycogen into lactate 39.1 6,700
Conversion of muscle glycogen into CO
2
16.7 84,000
Conversion of liver glycogen into CO
2
6.2 19,000
Conversion of adipose-tissue fatty acids into
CO
2
6.7 4,000,000
Note: Fuels stored are estimated for a 70-kg person having a muscle mass of 28 kg.
Source: After E. Hultman and R. C. Harris. In Principles of Exercise Biochemistry, J. R. Poortmans (Ed.). (Karger, 1988), pp.
78
119.
III. Synthesizing the Molecules of Life 30. The Integration of Metabolism 30.4. Fuel Choice During Exercise Is Determined by Intensity and Duration of Activity
Figure 30.19. Dependence of the Velocity of Running on the Duration of the Race. The values shown are world track
records .
III. Synthesizing the Molecules of Life 30. The Integration of Metabolism
30.5. Ethanol Alters Energy Metabolism in the Liver
Ethanol has been a part of the human diet for centuries. However, its consumption in excess can result in a number
of health problems, most notably liver damage. What is the biochemical basis of these health problems?
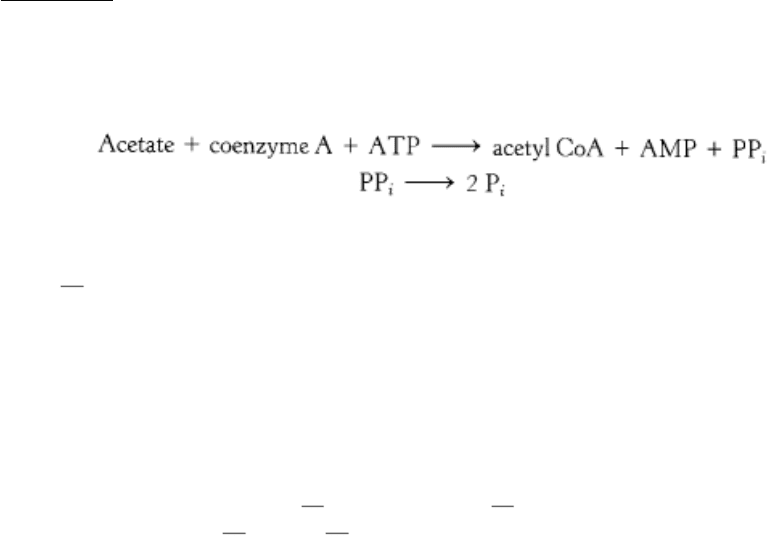
Ethanol cannot be excreted and must be metabolized, primarily by the liver. This metabolism occurs by two pathways.
The first pathway comprises two steps. The first step, catalyzed by the enzyme alcohol dehydrogenase, takes place in the
cytoplasm:
The second step, catalyzed by aldehyde dehydrogenase, takes place in mitochondria:
Note that ethanol consumption leads to an accumulation of NADH. This high concentration of NADH inhibits
gluconeogenesis by preventing the oxidation of lactate to pyruvate. In fact, the high concentration of NADH will cause
the reverse reaction to predominate, and lactate will accumulate. The consequences may be hypoglycemia and lactic
acidosis.
The NADH glut also inhibits fatty acid oxidation. The metabolic purpose of fatty acid oxidation is to generate NADH for
ATP generation by oxidative phosphorylation, but an alcohol consumer's NADH needs are met by ethanol metabolism.
In fact, the excess NADH signals that conditions are right for fatty acid synthesis. Hence, triacylglycerols accumulate in
the liver, leading to a condition known as "fatty liver."
The second pathway for ethanol metabolism is called the ethanolinducible microsomal ethanol-oxidizing system
(MEOS). This cytochrome P450-dependent pathway (Section 26.4.2) generates acetaldehyde and subsequently acetate
while oxidizing biosynthetic reducing power, NADPH, to NADP
+
. Because it uses oxygen, this pathway generates free
radicals that damage tissues. Moreover, because the system consumes NADPH, the antioxidant glutathione cannot be
regenerated (Section 20.5), exacerbating the oxidative stress.
What are the effects of the other metabolites of ethanol? Liver mitochondria can convert acetate into acetyl CoA in a
reaction requiring ATP. The enzyme is the thiokinase that normally activates short-chain fatty acids.
However, further processing of the acetyl CoA by the citric acid cycle is blocked, because NADH inhibits two important
regulatory enzymes
isocitrate dehydrogenase and α-ketoglutarate dehydrogenase. The accumulation of acetyl CoA
has several consequences. First, ketone bodies will form and be released into the blood, exacerbating the acidic condition
already resulting from the high lactate concentration. The processing of the acetate in the liver becomes inefficient,
leading to a buildup of acetaldehyde. This very reactive compound forms covalent bonds with many important functional
groups in proteins, impairing protein function. If ethanol is consistently consumed at high levels, the acetaldehyde can
significantly damage the liver, eventually leading to cell death.
Liver damage from excessive ethanol consumption occurs in three stages. The first stage is the aforementioned
development of fatty liver. In the second stage
alcoholic hepatitis groups of cells die and inflammation results. This
stage can itself be fatal. In stage three cirrhosis fibrous structure and scar tissue are produced around the dead cells.
Cirrhosis impairs many of the liver's biochemical functions. The cirrhotic liver is unable to convert ammonia into urea,
and blood levels of ammonia rise. Ammonia is toxic to the nervous system and can cause coma and death. Cirrhosis of
the liver arises in about 25% of alcoholics, and about 75% of all cases of liver cirrhosis are the result of alcoholism. Viral
hepatitis is a nonalcoholic cause of liver cirrhosis.
III. Synthesizing the Molecules of Life 30. The Integration of Metabolism
Summary
Metabolism Consists of Highly Interconnected Pathways
The basic strategy of metabolism is simple: form ATP, reducing power, and building blocks for biosyntheses. This
complex network of reactions is controlled by allosteric interactions and reversible covalent modifications of enzymes
and changes in their amounts, by compartmentation, and by interactions between metabolically distinct organs. The
enzyme catalyzing the committed step in a pathway is usually the most important control site. Opposing pathways such
as gluconeogenesis and glycolysis are reciprocally regulated so that one pathway is usually quiescent when the other is
highly active.
Each Organ Has a Unique Metabolic Profile
The metabolic patterns of the brain, muscle, adipose tissue, kidney, and liver are very different. Glucose is essentially the
sole fuel for the brain in a well-fed person. During starvation, ketone bodies (acetoacetate and 3-hydroxybutyrate)
become the predominant fuel of the brain. Adipose tissue is specialized for the synthesis, storage, and mobilization of
triacylglycerols. The kidney produces urine and reabsorbs glucose. The diverse metabolic activities of the liver support
the other organs. The liver can rapidly mobilize glycogen and carry out gluconeogenesis to meet the glucose needs of
other organs. It plays a central role in the regulation of lipid metabolism. When fuels are abundant, fatty acids are
synthesized, esterified, and sent from the liver to adipose tissue. In the fasting state, however, fatty acids are converted
into ketone bodies by the liver.
Food Intake and Starvation Induce Metabolic Changes
Insulin signals the fed state: it stimulates the formation of glycogen and triacylglycerols and the synthesis of proteins. In
contrast, glucagon signals a low blood-glucose level: it stimulates glycogen breakdown and gluconeogenesis by the liver
and triacylglycerol hydrolysis by adipose tissue. After a meal, the rise in the blood-glucose level leads to increased
secretion of insulin and decreased secretion of glucagon. Consequently, glycogen is synthesized in muscle and the liver.
When the blood-glucose level drops several hours later, glucose is then formed by the degradation of glycogen and by
the gluconeogenic pathway, and fatty acids are released by the hydrolysis of triacylglycerols. The liver and muscle then
use fatty acids instead of glucose to meet their own energy needs so that glucose is conserved for use by the brain.
The metabolic adaptations in starvation serve to minimize protein degradation. Large amounts of ketone bodies are
formed by the liver from fatty acids and released into the blood within a few days after the onset of starvation. After
several weeks of starvation, ketone bodies become the major fuel of the brain. The diminished need for glucose
decreases the rate of muscle breakdown, and so the likelihood of survival is enhanced.
Diabetes mellitus, the most common serious metabolic disease, is due to metabolic derangements resulting in an
insufficiency of insulin and an excess of glucagon relative to the needs of the individual. The result is an elevated blood-
glucose level, the mobilization of triacylglycerols, and excessive ketone-body formation. Accelerated ketone-body
formation can lead to acidosis, coma, and death in untreated insulin-dependent diabetics.
Fuel Choice During Exercise Is Determined by Intensity and Duration of Activity
Sprinting and marathon running are powered by different fuels to maximize power output. The 100-meter sprint is
powered by stored ATP, creatine phosphate, and anaerobic glycolysis. In contrast, the oxidation of both muscle glycogen
and fatty acids derived from adipose tissue is essential in the running of a marathon, a highly aerobic process.
Ethanol Alters Energy Metabolism in the Liver
The oxidation of ethanol results in an unregulated overproduction of NADH, which has several consequences. A rise in
the blood levels of lactic acid and ketone bodies causes a fall in blood pH, or acidosis. The liver is damaged because the
excess NADH causes excessive fat formation as well as the generation of acetaldehyde, a reactive molecule. Severe liver
damage can result.
Key Terms
allosteric interaction
covalent modification
glycolysis
phosphofructokinase
citric acid cycle
oxidative phosphorylation
pentose phosphate pathway
gluconeogenesis

glycogen synthesis
glycogen degradation
glucose 6-phosphate
pyruvate
acetyl CoA
ketone body
starved-fed cycle
glucose homeostasis
insulin
glucagon
caloric homeostasis
leptin
creatine phosphate
III. Synthesizing the Molecules of Life 30. The Integration of Metabolism
Problems
1.
Distinctive organs. What are the key enzymatic differences between liver, kidney, muscle, and brain that account for
their differing utilization of metabolic fuels?
See answer
2.
Missing enzymes. Predict the major consequence of each of the following enzymatic deficiencies:
(a) Hexokinase in adipose tissue
(b) Glucose 6-phosphatase in liver
(c) Carnitine acyltransferase I in skeletal muscle
(d) Glucokinase in liver
(e) Thiolase in brain
(f) Kinase in liver that synthesizes fructose 2,6-bisphosphate
See answer

3.
Contrasting milieux. Cerebrospinal fluid has a low content of albumin and other proteins compared with plasma.
(a) What effect does this lower content have on the concentration of fatty acids in the extracellular medium of the
brain?
(b) Propose a plausible reason for the selection by the brain of glucose rather than fatty acids as the prime fuel.
(c) How does the fuel preference of muscle complement that of the brain?
See answer
4.
Metabolic energy and power. The rate of energy expenditure of a typical 70-kg person at rest is about 70 watts (W),
like a light bulb.
(a) Express this rate in kilojoules per second and in kilocalories per second.
(b) How many electrons flow through the mitochondrial electron-transport chain per second under these conditions?
(c) Estimate the corresponding rate of ATP production.
(d) The total ATP content of the body is about 50 g. Estimate how often an ATP molecule turns over in a person at
rest.
See answer
5.
Respiratory quotient (RQ). This classic metabolic index is defined as the volume of CO
2
released divided by the
volume of O
2
consumed.
(a) Calculate the RQ values for the complete oxidation of glucose and of tripalmitoylglycerol.
(b) What do RQ measurements reveal about the contributions of different energy sources during intense exercise?
(Assume that protein degradation is negligible.)
See answer
6.
Camel's hump. Compare the H
2
O yield from the complete oxidation of 1 g of glucose with that of 1 g of
tripalmitoylglycerol. Relate these values to the evolutionary selection of the contents of a camel's hump.
See answer
7.
The wages of sin. How long does one have to jog to offset the calories obtained from eating 10 macadamia nuts (18
kcal/nut)? [Assume an incremental power consumption of 400 W.]
See answer
8.
Sweet hazard. Ingesting large amounts of glucose before a marathon might seem to be a good way of increasing the
fuel stores. However, experienced runners do not ingest glucose before a race. What is the biochemical reason for
their avoidance of this potential fuel? (Hint: Consider the effect of glucose ingestion on the level of insulin.)
See answer

9.
An effect of diabetes. Insulin-dependent diabetes is often accompanied by hypertriglyceridemia, which is an excess
blood level of triacylglycerides in the form of very low density lipoproteins. Suggest a biochemical explanation.
See answer
10.
Sharing the wealth. The hormone glucagon signifies the starved state, yet it inhibits glycolysis in the liver. How
does this inhibition of an energy-production pathway benefit the organism?
See answer
11.
Compartmentation. Glycolysis takes place in the cytoplasm, whereas fatty acid degradation takes place in
mitochondria. What metabolic pathways depend on the interplay of reactions that take place in both compartments?
See answer
12.
Kwashiorkor. The most common form of malnutrition in children in the world, kwashiorkor is caused by a diet
having ample calories but little protein. The high levels of carbohydrate result in high levels of insulin. What is the
effect of high levels of insulin on
(a) lipid utilization?
(b) protein metabolism?
(c) Children suffering from kwashiorkor often have large distended bellies caused by water from the blood leaking
into extracellular spaces. Suggest a biochemical basis for this condition.
See answer
13.
Oxygen deficit. After light exercise, the oxygen consumed in recovery is approximately equal to the oxygen deficit,
which is the amount of additional oxygen that would have been consumed had oxygen consumption reached steady
state immediately. How is the oxygen consumed in recovery used?
See answer
14.
Excess post-exercise oxygen consumption. The oxygen consumed after strenuous exercise stops is significantly
greater than the oxygen deficit and is termed excess post-exercise oxygen consumption (EPOC). Why is so much
more oxygen required after intense exercise?
See answer
15.
Psychotropic effects. Ethanol is unusual in that it is freely soluble in both water and lipids. Thus, it has access to all
regions of the highly vascularized brain. Although the molecular basis of ethanol action in the brain is not clear, it
is evident that ethanol influences a number of neurotransmitter receptors and ion channels. Suggest a biochemical
explanation for the diverse effects of ethanol.
See answer
16.
Fiber type. Skeletal muscle has several distinct fiber types. Type I is used primarily for aerobic activity, whereas
type II is specialized for short, intense bursts of activity. How could you distinguish between these types of muscle
fiber if you viewed them with an electron microscope?
See answer
17.
Tour de France. Cyclists in the Tour de France (more than 2000 miles in 3 weeks) require about 200,000 kcal of
energy, or 10,000 kcal day
-1
(a resting male requires 2000 kcal day
-1
).
(a) With the assumptions that the energy yield of ATP is about 12 kcal mol
-1
and that ATP has a molecular weight
of 503 gm mol
-1
, how much ATP would be expended by a Tour de France cyclist?
(b) Pure ATP can be purchased at the cost of approximately $150 per gram. How much would it cost to power a
cyclist through the Tour de France if the ATP had to be purchased?
See answer
III. Synthesizing the Molecules of Life 30. The Integration of Metabolism
Selected Readings
Where to start
G.J. Kemp. 2000. Studying metabolic regulation in human muscle Biochem. Soc. Trans. 28: 100-103. (PubMed)
G.E. Lienhard, J.W. Slot, D.E. James, and M.M. Mueckler. 1992. How cells absorb glucose Sci. Am. 266: (1) 86-91.
(PubMed)
Books
Fell, D., 1997. Understanding the Control of Metabolism. Portland Press.
Frayn, K. N., 1996. Metabolic Regulation: A Human Perspective. Portland Press.
Hargreaves, M., and Thompson, M. (Eds.) 1999. Biochemistry of Exercise X. Human Kinetics.
Harris, R. A., and Crabb, D. W. 1997. Metabolic interrelationships. In Textbook of Biochemistry with Clinical
Correlations (pp. 525
562), edited by T. M. Devlin. Wiley-Liss.
Fuel metabolism
F. Rolland, J. Winderickx, and J.M. Thevelein. 2001. Glucosesensing mechanism in eukaryotic cells Trends Biochem.
Sci. 26: 310-317. (PubMed)
B.B. Rasmussen and R.R. Wolfe. 1999. Regulation of fatty acid oxidation in skeletal muscle Annu. Rev. Nutr. 19: 463-
484. (PubMed)
P.W. Hochachka. 2000. Oxygen, homeostasis, and metabolic regulation Adv. Exp. Med. Biol. 475: 311-335. (PubMed)
E. Holm, O. Sedlaczek, and E. Grips. 1999. Amino acid metabolism in liver disease Curr. Opin. Clin. Nutr. Metab. Care
2: 47-53. (PubMed)
A.J. Wagenmakers. 1998. Protein and amino acid metabolism in human muscle Adv. Exp. Med. Biol. 441: 307-319.
(PubMed)

Metabolic adaptations in starvation
G. Baverel, B. Ferrier, and M. Martin. 1995. Fuel selection by the kidney: Adaptation to starvation Proc. Nutr. Soc. 54:
197-212. (PubMed)
I.A. MacDonald and J. Webber. 1995. Feeding, fasting and starvation: Factors affecting fuel utilization Proc. Nutr. Soc.
54: 267-274. (PubMed)
G.F. Cahill Jr. 1976. Starvation in man Clin. Endocrinol. Metab. 5: 397-415. (PubMed)
M.C. Sugden, M.J. Holness, and T.N. Palmer. 1989. Fuel selection and carbon flux during the starved-to-fed transition
Biochem. J. 263: 313-323. (PubMed)
Diabetes mellitus
G.A. Rutter. 2000. Diabetes: The importance of the liver Curr. Biol. 10: R736-R738. (PubMed)
A.R. Saltiel. 2001. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes Cell 104: 517-529.
(PubMed)
G.I. Bell, S.J. Pilikis, I.T. Weber, and K.S. Polonsky. 1996. Glucokinase mutations, insulin secretion, and diabetes
mellitus Annu. Rev. Physiol. 58: 171-186. (PubMed)
D.J. Withers and M. White. 2000. Perspective: The insulin signaling system
a common link in the pathogenesis of type
2 diabetes Endocrinology 141: 1917-1921. (PubMed)
Taylor, S. I., 1995. Diabetes mellitus. In The Metabolic Basis of Inherited Diseases (7th ed.; pp. 935 936), edited by C.
R. Striver, A. L. Beaudet, W. S. Sly, D. Valle, J. B. Stanbury, J. B. Wyngaarden, and D. S. Fredrickson. McGraw-Hill.
Exercise metabolism
R.G. Shulman and D.L. Rothman. 2001. The "glycogen shunt" in exercising muscle: A role for glycogen in muscle
energetics and fatigue Proc. Natl. Acad. Sci. USA 98: 457-461. (PubMed) (Full Text in PMC)
T. Gleason. 1996. Post-exercise lactate metabolism: A comparative review of sites, pathways, and regulation Annu. Rev.
Physiol. 58: 556-581.
J.O. Holloszy and W.M. Kohrt. 1996. Regulation of carbohydrate and fat metabolism during and after exercise Annu.
Rev. Nutr. 16: 121-138. (PubMed)
P.W. Hochachka and G.B. McClelland. 1997. Cellular metabolic homeostasis during large-scale change in ATP turnover
rates in muscles J. Exp. Biol. 200: 381-386. (PubMed)
J.F. Horowitz and S. Klein. 2000. Lipid metabolism during endurance exercise Am. J. Clin. Nutr. 72: 558S-563S.
(PubMed)
A.J. Wagenmakers. 1999. Muscle amino acid metabolism at rest and during exercise Diabetes Nutr. Metab. 12: 316-322.
(PubMed)
Ethanol metabolism
S. Stewart, D. Jones, and C.P. Day. 2001. Alcoholic liver disease: New insights into mechanisms and preventive
strategies Trends Mol. Med. 7: 408-413. (PubMed)
C.S. Lieber. 2000. Alcohol: Its metabolism and interaction with nutrients Annu. Rev. Nutr. 20: 395-430. (PubMed)
O. Niemela. 1999. Aldehyde-protein adducts in the liver as a result of ethanol-induced oxidative stress Front. Biosci. 1:
D506-D513.
H. Riveros-Rosas, A. Julian-Sanchez, and E. Pina. 1997. Enzymology of ethanol and acetaldehyde metabolism in
mammals Arch. Med. Res. 28: 453-471. (PubMed)
I. Diamond and A.S. Gordon. 1997. Cellular and molecular neuroscience of alcoholism Physiol. Rev. 77: 1-20.
(PubMed)
III. Synthesizing the Molecules of Life
31. The Control of Gene Expression
Bacteria are highly versatile and responsive organisms: the rate of synthesis of some proteins in bacteria may vary more
than a 1000-fold in response to the supply of nutrients or to environmental challenges. Cells of multicellular organisms
also respond to varying conditions. Such cells exposed to hormones and growth factors will change substantially in
shape, growth rate, and other characteristics. Moreover, many different cell types are present in multicellular organisms.
For example, cells from muscle and nerve tissue show strikingly different morphologies and other properties, yet they
contain exactly the same DNA. These diverse properties are the result of differences in gene expression.
Gene expression is the combined process of the transcription of a gene into mRNA, the processing of that mRNA, and its
translation into protein (for protein-encoding genes). A comparison of the gene-expression patterns of cells from the
pancreas, which secretes digestive enzymes, and the liver, the site of lipid transport and energy transduction, reveals
marked differences in the genes that are highly expressed (Table 31.1), a difference consistent with the physiological
roles of these tissues.
How is gene expression controlled? Gene activity is controlled first and foremost at the level of transcription. Much of
this control is achieved through the interplay between proteins that bind to specific DNA sequences and their DNA-
binding sites. In this chapter, we shall see how signals from the environment of a cell can alter this interplay to induce
changes in gene expression. We first consider gene-regulation mechanisms in prokaryotes and particularly in E. coli,
because these processes have been extensively investigated in this organism. We then turn to eukaryotic gene regulation.
In the chapter's final section, we explore mechanisms for regulating gene expression past the level of transcription.
III. Synthesizing the Molecules of Life 31. The Control of Gene Expression
Table 31.1. Highly expressed protein-encoding genes of the pancreas and liver (as percentage of total
mRNA pool)
Rank Pancreas % Liver %
1 Procarboxypeptidase A1 7.6 Albumin 3.5
2 Pancreatic trypsinogen 2 5.5 Apolipoprotein A-I 2.8
3 Chymotrypsinogen 4.4 Apolipoprotein C-I 2.5
4 Pancreatic trypsin 1 3.7 Apolipoprotein C-III 2.1
5 Elastase IIIB 2.4 ATPase 6/8 1.5
6 Protease E 1.9 Cytochrome oxidase 3 1.1
7 Pancreatic lipase 1.9 Cytochrome oxidase 2 1.1
8 Procarboxypeptidase B 1.7
α-1-Antitrypsin
1.0
9 Pancreatic amylase 1.7 Cytochrome oxidase 1 0.9
10 Bile salt-stimulated lipase 1.4 Apolipoprotein E 0.9
