Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Iron – Absorption, Transport, and Loss
0004 Iron is readily absorbed in the state Fe
2þ
, but most
dietary iron is in the ferric form Fe
3þ
. The gastric
secretions dissolve the iron and permit it to form
soluble complexes with ascorbic acids and other sub-
stances that aid its reduction to the Fe
2þ
form. In
humans iron-deficiency anemia is a relatively fre-
quent complication of partial gastrectomy. Heme is
also absorbed because of its solubility. Fe
2þ
contained
in heme is released into mucosal cells.
0005 Iron is absorbed from the upper part of the small
intestine, because duodenum and adjacent jejunum
contain most mucosal cells suitable for iron absorp-
tion. Various dietary factors influence iron absorption,
for example, phytic acid from cereals, which forms
iron-insoluble compounds in the intestine, as do
phosphates and oxalates.
0006 Nonheme iron binds to mucin. Cell adhesion mol-
ecule integrin transfers the iron to mobilferrin.
Mobilferrin carries iron into the cell and binds more
iron in iron-deficiency states. The mucosal cells
contain iron-binding protein, or apoferritin. Some
iron is utilized by mitochondria, and the remainder
is partitioned between apoferritin in the mucosal cells
and transferrin. Transferrin is a protein that carries
iron in the blood. Specific cell-surface receptors de-
liver the transferrin with its bound iron to endosomes.
Here the low pH induces the separation of transferrin
and the iron. Excess iron in the blood is deposited in
all cells of the body, especially in liver cells. Apoferri-
tin is also found in many other tissues, where, to-
gether with iron, it forms ferritin. Apoferritin is a
globular protein. Iron forms a micelle of ferric hydro-
xyphosphate. In ferritin 24 subunits of protein sur-
round each micelle. The ferritin micelle can contain
4500 atoms of iron. It is the iron storage form in
tissues. Molecules of ferritin may aggregate in lysoso-
mal membranes as deposits, containing up to 50%
iron. These deposits are called hemosiderin.
0007 Iron is also transported across the placenta from
mother to fetus. Maternal iron plasma concentration
is usually lower than fetal concentration. However,
the binding capacity of maternal plasma for iron is
much higher, even though iron is actively transported
into the fetal compartment. A daily transport
of about 1.8 mmol is necessary to meet total fetal
requirements.
0008 In adult humans, the amount of iron lost from the
body is relatively small. It is unregulated, and total
body stores of iron are regulated by changes in the
rate at which iron is absorbed. Men lose about 0.6 mg
day
1
, whereas women have a variable, larger loss
because of the additional iron lost in blood shed
during menstruation.
Biosynthesis and Significance of Heme
0009Evolution of higher organisms has been accompanied
by the development of oxygen transport protein. In
humans this protein – hemoglobin – is concentrated
in specialized cells – erythrocytes. Each erythrocyte
contains about 300 million hemoglobin molecules.
The organic structure of a protein is unable to direct
binding of oxygen. However, certain metals, in their
lower oxidation states (for example, Fe
2þ
) have a
strong tendency to bind oxygen. Thus, in the evolu-
tion of the hemoglobin–myoglobin family of proteins,
Fe
2þ
has been utilized in the O
2
-binding site.
0010There are a number of possibilities in which vari-
ous iron-containing proteins hold iron in the form
Fe
2þ
. Hemoglobin and myoglobin are a family of
proteins, in which iron is chelated by the tetrapyrrole
ring system – protoporphyrin IX. It is one protein in a
large class of porphyrins, which are also encountered
in chlorophyl, cytochrome proteins, and other nat-
ural pigments. The porphyrins are colored. The iron
porphyrin in hemoglobin accounts for the red color of
blood. The complex of protoporphyrin IX with Fe
2þ
is called heme. Ferrous iron is octahedrally coordin-
ated, which means it should have six ligands or bind-
ing groups attached to it. The nitrogen atoms of the
porphyrin ring account for only four ligands. Two
remaining available coordination sites lie along an
axis perpendicular to the plane of the ring. In myo-
globin one of these sites is occupied by nitrogen of
histidine (part of the protein helix).
0011When oxygen is bound, the O
2
molecule occupies
the vacant side. Hemoglobin has evolved from myo-
globin and forms a tetrameric structure. Each of the
four chains in hemoglobin has a folded structure
similar to that of myoglobin, and each carries a
heme. Hemoglobin contains four subunits: two
a-chains and two b-chains. Both chains are very simi-
lar but are distinguishable in primary structures and
folding. Each subunit has primary, secondary, and
tertiary structures. Amino acid side chains in hemo-
globin provide hydrophobic, salt bridges, and hydro-
gen bond interactions. These are necessary to stabilize
a particular quaternary structure.
0012Each hemoglobin molecule can bind four oxygen
molecules, in four myoglobin-like sites. To simplify,
we can consider hemoglobin as having two states of
quaternary structure: one characteristic for the deoxy
form and the other for the oxygenated form. The oxy
form has a higher affinity for O
2
. The transition from
the deoxy to the oxy conformation involves changes
in the interactions between the subunits. A ligand
oxygen, binding to its side, tends to pull the Fe
2þ
a
very short distance down into the heme and flattens
the heme. Consequently, a molecular rearrangement
3380 IRON/Biosynthesis and Significance of Heme (Haem)

occurs, and pulling the Fe into the heme produces a
much larger shift in the surrounding structure, par-
ticularly at the ab interfaces. Cooperative binding,
oxygen transport, and allosteric transition between
structurally different high-affinity and low-affinity
states mean that hemoglobin behaves in an allosteric
way. Several factors, such as carbon dioxide, protons,
and other substances, promote these changes. They
are called allosteric effectors.
0013 For each polypeptide chain produced by an organ-
ism there exists a corresponding gene. The nucleotide
sequence in the gene dictates, via the genetic code, the
amino acid sequence of the protein. This process is
exemplified by the b-globin gene. In the gene for
human b hemoglobin, introns (noncoding regions)
alternate with exons (regions that are expressed in
the polypeptide sequence). From this gene is pro-
duced a primary transcript (pre-mRNA), which is
spliced to yield the final mRNA. This mRNA is trans-
lated into the b-chain, which then adopts its favored
three-dimensional structure. Very primitive animals
had only a myoglobinlike, single-chain globin for
oxygen storage (transport was not necessary; most
of these animals were very small). More than 800
million years ago the primitive globin gene was dupli-
cated. The one copy evolved into the gene for
an oxygen transport protein and to hemoglobins.
The evolutionary line led to mammals carrying
both a-andb-globin genes and capable of forming
tetrametric hemoglobins. Gene duplication has also
occurred in the hemoglobin line, leading to embry-
onic and fetal forms, and accounting for the special
hemoglobin, which is adapted to promote oxygen
transfer through the placenta from the mother to
the fetus.
0014Evolution of hemoglobin genes continues. This fact
is seen in the existence of several hundred recognized
mutant hemoglobins in the human population. Each
of these mutant forms exists in only a small part of the
total human population. Many give rise to recognized
pathologies, while others are referred to as neutral
mutations.
See also: Anemia (Anaemia): Iron-deficiency Anemia;
Ascorbic Acid: Properties and Determination;
Physiology; Iron: Properties and Determination;
Physiology
Further Reading
Ganong WE (1999) Review of Medical Physiology. New
York: Appleton & Lange.
Greger R and Windhorst U (1996) Comprehensive Human
Physiology. London: Springer.
Mathews CK and Van Holde KE (1988) Biochemistry.
London: Benjamin/Cummings Publishing.
IRRADIATION OF FOODS
Contents
Basic Principles
Applications
Processing Technology
Legal and Consumer Aspects
Basic Principles
P B Roberts, Institute of Geological and Nuclear
Sciences Ltd, Lower Hutt, New Zealand
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The absorption of energy during irradiation can pro-
vide foods with desirable new benefits. Irradiation is
therefore analogous to more common processes such
as pasteurization or drying that use heat energy. The
energy absorbed during irradiation is high-energy,
ionizing radiation. (See Drying: Theory of Air-drying;
Pasteurization: Principles.)
0002Irradiation of food must not be confused with
the contamination of food by radioactive materials,
which themselves emit radiations that may harm a
consumer. Such contamination followed nuclear
weapon tests and the Chernobyl accident. Food
irradiation cannot make food radioactive since the
radiation used, though of high energy, is not powerful
enough to induce the necessary changes in atomic
nuclei. (See Radioactivity in Food.)
0003This article discusses the history and current status
of food irradiation. The radiation sources and
IRRADIATION OF FOODS/Basic Principles 3381

potential applications are outlined and the general
chemical, microbiological, and nutritional effects
summarized.
History
Before 1950
0004 Ro
¨
ntgen discovered X-radiation in 1895 and
Becquerel observed natural radioactivity in 1896.
The radiations produced were soon found to have
many biological effects, including the ability to
inactivate bacteria. In 1904 Prescott suggested
that spoilage bacteria in food could be destroyed by
radiation. Patents were obtained in the US and France
in 1921 and 1930. However, no practical source of
radiation to treat significant food volumes existed
until the 1940s when high-energy electron acceler-
ators and nuclear reactors able to produce large
quantities of artificial radioactive nuclides became
available.
1950–1983
0005 The US Army Quartermaster Corps sponsored the
first cooperative effort to develop food irradiation
as part of research into light-weight, shelf-stable
army rations. By 1966 safety studies on 21 foods
were complete and sterilization of bacon and pork,
disinfestation of wheat and its products, and
inhibition of potato sprouting were uses of radiation
approved by the US Food and Drug Administration
(FDA). After this, safety studies in the US stalled for
10 years and commercial interest waned.
0006 European interest increased, however, and in 1970
the International Project in the Field of Food Irradi-
ation (IFIP) was initiated. IFIP compiled safety data
on many foods to be considered by expert committees
that were sponsored by the United Nations organiza-
tions responsible for secure, safe food supplies and for
the peaceful uses of atomic energy.
0007 In 1980 a joint expert committee on food irradi-
ation stated that ‘the irradiation of food up to an
overall average absorbed dose of 10 kGy presents
no toxicological hazard’ and ‘introduced no special
nutritional or microbiological problems.’ This advice
prompted the Codex Alimentarius Commission,
the body responsible for world food standards, to
adopt a worldwide general standard for irradiated
food in 1983. The standard sets general conditions
for the irradiation of food. It does not imply that
irradiation is appropriate for all foods but that
irradiation should be regarded and controlled like
other physical processes such as canning, dehydra-
tion, etc.
Current Status
0008The Codex standard was issued at a time of increased
awareness of food losses in developing countries and a
growing aversion to chemical contamination of food
via chemical fumigation and preservation in developed
countries. By 1999, 41 countries had cleared one or
more foods for irradiation. There are over 50 irradi-
ation plants in nearly 30 countries irradiating food,
although the volume treated in most is small.
0009However, the 1980s saw a rise in public suspicion
about any technology associated with radiation.
Demand for ‘natural,’ minimally processed foods
has increased and official assurances on the whole-
someness of processed foods and additives are
increasingly questioned. There has been sufficient
public opposition to food irradiation to insure a
very cautious attitude to it within the food industry.
The annual volume of food irradiated is probably
only 500 000 tonnes worldwide. Foods that are
irradiated include dried herbs and spices, potatoes,
onions, garlic, dried vegetables, tropical fruits, and
chicken. Countries that regularly irradiate foods on a
commercial basis include the US, The Netherlands,
France, Belgium, Japan, South Africa, and China.
The Radiations Used
0010The changes in food or its contaminating organisms
achieved by irradiation processing are caused by
chemical reactions induced between the constituent
atoms or molecules. These reactions are initiated by
radiations that can strip electrons away from atoms
or molecules that are left positively charged (ionized).
The charged species rapidly split into fragments
called free radicals that have a free or unpaired elec-
tron. Free radicals react extremely rapidly with each
other and nearby molecules as they seek to become
more stable by gaining or losing an electron. It is these
free radical reactions that trigger the chemical effects
leading to the ultimate changes in the food.
0011There are two principal forms of ionizing radi-
ation: accelerated subatomic particles and high-
energy electromagnetic (EM) radiation.
Accelerated Particles
0012Particles travelling at speeds approaching the speed of
light can cause ionization. The only particle used for
food irradiation is the electron. An electron acceler-
ator works on similar principles to a television tube
(Figure 1). Electrons produced by heating a filament
are injected into a vacuum chamber and attracted to a
positive terminal. An electron beam is focused and
then accelerated within the chamber by the voltage
difference experienced by the electrons between the
3382 IRRADIATION OF FOODS/Basic Principles
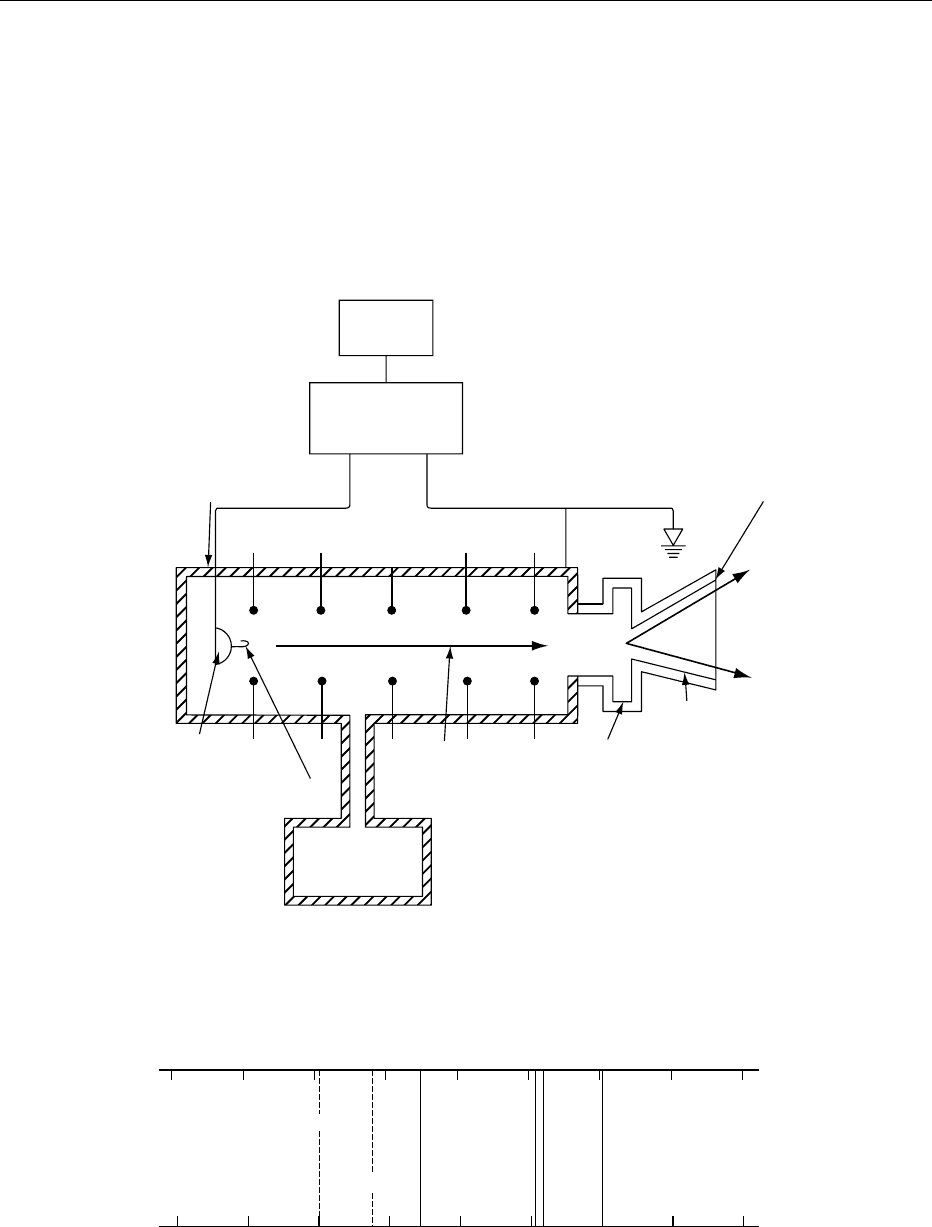
start and finish of their travel in the chamber, a
difference generated electrostatically or by a radio-
frequency field. The beam emerges through a thin
window at a speed close to that of light. It is swept
from side to side by a changing magnetic field while
irradiating large targets such as food.
001 3 An electron accelerated by a voltage difference of
1 10
6
V acquires an energy of 1MeV (megaelectron
volt). Energies above 1 MeV provide sufficient
penetration in food to be useful. An upper limit of
10 MeV is recommended by the Codex general stand-
ard to avoid inducing radioactivity in the food.
Electromagnetic Radiation
0014Figure 2 shows that there is a range of EM radiations,
each characterized by different wavelengths or energy
and with different uses. Only X- and g-rays have
sufficient energy to cause ionization.
AC
input
High-voltage
generator
and rectifiers
High-
voltage
DC power
Accelerator tube
Thin window
Scan horn
Scanning
magnet chamber
Electron
beam
High-vacuum
pumping
system
High-voltage
terminal
Filament (electron gun)
fig0001 Figure 1 Schematic diagram of an electron accelerator with the voltage difference obtained electrostatically. Reproduced from
Irradiation of Foods: Basic Principles. Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and
Sadler MJ (eds), 1993, Academic Press.
10
−10
10
−6
10
−2
10
2
10
6
10
4
10
−4
10
−8
10
−12
1
Wavelength (m)
Radiowaves
Microwaves
Visible light
Ultraviolet light
X-rays
γ-rays
Infrared
(heat waves)
Photon energy (eV)
fig0002 Figure 2 The electromagnetic spectrum. Reproduced from Irradiation of Foods: Basic Principles. Encyclopaedia of Food Science,
Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
IRRADIATION OF FOODS/Basic Principles 3383

0015 X-rays are produced when high-speed particles
strike a metal target and are rapidly decelerated. To
avoid inducing radioactivity in food, X-ray sources
must operate below 5 MeV. The efficiency of the con-
version of 5 MeV electrons to X-rays is under 10%,
making X-ray generators more costly to operate than
simple electron machines.
0016 g-Rays are emitted by many atomic nuclei under-
going radioactive decay. Sources for food irradiation
are limited to cobalt-60 and caesium-137, which emit
g-rays with energies around 1 MeV that cannot
induce radioactivity.
0017 Cobalt-60 is produced in a nuclear reactor by
neutron bombardment of the stable element. It is a
highly insoluble, high-melting-point metal. Caesium-
137 is one of the fission products produced in the
fuel rods of nuclear reactors from which it is
chemically separated as a potentially volatile, soluble
salt. Cobalt-60 dominates the g-processing industry
because of its more favorable production and
environmental features.
0018 Commercial decisions about the type and source of
radiation to use are dictated by cost, beam penetra-
tion, and the form of the food to be treated.
Most irradiation plants use cobalt-60. A few use
electron beams and the first plants using X-rays are
being constructed.
Dose and General Radiation Effects
0019 Radiation effects in food are caused by the energy
that is transferred to the food. The transferred energy
is called the absorbed dose (commonly, just dose). Its
unit is the gray (Gy) which is the absorption of one
joule per kilogram. An older unit is the rad; 1 Gy
equals 100 rad.
0020 As the absorbed dose increases, the number of
chemical reactions and the severity of the effects
caused will increase. Food irradiation usually
involves doses of 0.05–30 kGy, depending upon the
desired result, although the upper limit recommended
by the Codex Alimentarius is 10 kGy.
0021At low doses chemical reactions can disrupt bio-
chemical and hormonal processes in fresh produce.
As the dose increases, cell division is prevented as
chemical disruption of DNA becomes severe. In this
way microorganisms are inactivated and insects
sterilized. At the highest doses used, all the micro-
organisms are killed and the food is sterilized.
0022Such effects indicate that extensive chemical
changes are induced by irradiation. It may seem
surprising that any food can withstand irradiation
without being altered to an unacceptable extent. How-
ever, radiation deposits energy randomly throughout
the food while inactivation of microorganisms, for
example, requires that energy be deposited at a few
critical points in a vital molecule such as DNA or a
chromosome. Such molecules are relatively large
targets and present in very few copies. In contrast,
significant depletion or alteration of individual food
constituents (e.g., amino acids) requires energy to
be deposited in a large fraction of molecules that
are far smaller and far more numerous than DNA.
Frequently, therefore, food irradiation results in a
product that is virtually indistinguishable from
unprocessed food. However, as with conventional
processes, some foods will suffer from undesirable
changes in taste, smell, or texture.
Beneficial Uses of Irradiation
0023The major potential benefits of food irradiation are
summarized in Table 1. Each benefit requires a min-
imum dose to be effective. For a particular food the
maximum dose is limited by the onset of unacceptable
changes in quality. Before a commercial decision is
taken to irradiate a food, it is necessary to perform a
cost-versus-benefit analysis and to assess the benefit
to consumers against any potential loss in sensory
qualities.
tbl0001 Table 1 Major technical benefits of food irradiation
Benefit Doserange (kGy) Candidate foods
a
Inhibit sprouting 0.05–0.15 Potato, onion, garlic
Delay ripening 0.05–0.15 Some tropical fruit
Reduce parasites 0.1–0.3 Pork
Disinfest insects 0.1–1 Grain, rice, some fruit and vegetables
Delay spoilage (ambient) 0.5–5 Strawberry
Delay spoilage (refrigerated) 0.5–10 Meat, poultry, fish
Reduce pathogens 2–10 Meat, poultry, seafood, dried foods
Sterilization 10–30
b
Spices, herbs, special diets
a
Not a comprehensive list.
b
Doses above 10 kGy exceed a Codex recommendation and are permitted only in a few countries for special purposes.
3384 IRRADIATION OF FOODS/Basic Principles

Safety Considerations
0024 When considering whether any processed food is safe
and wholesome the chemical, microbiological, and
nutritional changes must be evaluated. Several expert
international and national committees have con-
sidered the safety of irradiated foods. The World
Health Organization (WHO) completed its latest
reviews in 1994 and 1999. All the reviews reinforce
the 1980 joint expert committee finding that,
provided good manufacturing practice is followed,
the irradiation of any food up to an overall average
absorbed dose up to 10 kGy presents no toxicological
hazard and no special microbiological or nutritional
hazards.
0025 The 1999 WHO report examined the safety of
foods irradiated above 10 kGy. It concluded that
foods treated above 10 kGy should also be considered
safe and nutritionally adequate when produced under
established good manufacturing practice.
Chemical Changes
0026 Chemical reactions induced by a 10 kGy dose lead
to about 300 mg of products per kilogram of food.
Radiation, therefore, produces a large number of com-
pounds but at very low individual concentrations. A
few products are species that were not present signifi-
cantly before irradiation. Some could conceivably be
unique to irradiated foods and of unknown toxicity.
0027 Any unique products must be present in extremely
low concentrations since modern analytical methods
can identify all the radiation products (or very similar
compounds) in other processed or unprocessed foods.
Several expert committees have considered a huge
database of toxicological experiments. Although a
small number of experiments initially raised some
questions, the committees are satisfied that any
doubts were due to inadequate experimental or
analytical methods and that any toxicological risk is
negligible.
Microbiological Changes
0028 All physical processes have the potential to mutate
microorganisms and lead to increased resistance, en-
hanced pathogenicity, or changed physiological traits
important to their identification. If the process does
not sterilize the food the surviving organisms will
be those most resistant to the process. Subsequent
regrowth can lead to microbial populations and risk
different to those originally present.
0029 However, irradiation appears little different to
other physical processes in its potential for micro-
biological change. An exception may be its inability
to destroy toxins produced by microorganisms that
were present before processing, a useful property of
heat for some toxins. In practice the risks are small.
Following codes of good manufacturing practice
developed for other processes and for storage will
insure microbiologically safe irradiated foods.
Nutritional Changes
0030Irradiation up to 10 kGy does not alter significantly
the nutritional value of proteins, carbohydrates,
minerals, or saturated fats. Oxidation reactions can
lead to the loss of essential unsaturated fatty acids.
These reactions also induce rancid flavors and some
foods with a high unsaturated fat content will not
be suitable for irradiation. (See Oxidation of Food
Components.)
0031In common with heating, freezing, dehydration,
and storage, irradiation causes vitamin loss. Vitamin
E and thiamin are the most radiation-sensitive, with
vitamins A, C, and K also quite sensitive. Losses
below 1 kGy are insignificant. Above 1 kGy the losses
are comparable to those caused by other physical
processes and may reach 10–20% for the more
sensitive vitamins. If irradiated foods are to be
further processed or cooked, the combined losses
must be taken into account. (See Ascorbic Acid:
Properties and Determination; Retinol: Properties
and Determination; Tocopherols: Properties and
Determination.)
0032The overall nutritional consequences of irradiating
a particular food will depend upon:
.
0033whether the food is a significant source of particu-
lar nutrients
.
0034whether those nutrients are radiation-sensitive
.
0035the dose
.
0036the overall proportion of the food in the diet that is
irradiated
0037Although there may be consumers with particular
dietary habits or needs who could be adversely
affected, the nutritional consequences of food irradi-
ation will be insignificant for people eating a reason-
ably balanced diet.
See also: Ascorbic Acid: Properties and Determination;
Cheeses: Manufacture of Hard and Semi-hard Varieties
of Cheese; Drying: Theory of Air-drying; Oxidation of
Food Components; Pasteurization: Principles;
Radioactivity in Food; Tocopherols: Properties and
Determination
Further Reading
Goresline HE (1982) Historical aspects of the radiation
preservation of food. In: Josephson ES and Peterson
MS (eds) Preservation of Food by Ionizing Radiation,
pp. 1–46. Boca Raton, FL: CRC Press.
IRRADIATION OF FOODS/Basic Principles 3385

McLaughlin WL, Boyd AW, Chadwick KH, Mcdonald JC
and Miller A (1989) Dosimetry for Radiation Process-
ing. London, UK: Taylor and Francis.
Murray DR (1990) Biology of Food Irradiation. New York:
Wiley.
Ramler WJ (1982) Machine sources. In: Josephson ES and
Peterson MS (eds) Preservation of Food by Ionizing
Radiation, pp. 165–188. Boca Raton, FL: CRC Press.
Satin M (1993) Food Irradiation: A Guidebook. Lancaster,
USA: Technomic.
Thayer DW (1990) Food irradiation: benefits and con-
cerns.Journal of Food Quality 13: 147–169.
World Health Organization (1988) Food Irradiation: A
Technique for Preserving and Improving the Safety of
Food. Geneva: World Health Organization.
World Health Organization (1994) Safety and Nutritional
Adequacy of Irradiated Food. Geneva: World Health
Organization.
World Health Organization (1999) High Dose Irradiation:
Wholesomeness of Food Irradiated above 10 kGy.Re-
port of a Joint FAO/IAEA/WHO Study Group. WHO
Technical Report Series 890. Geneva: World Health Or-
ganization.
Applications
P B Roberts, Institute of Geological and Nuclear
Sciences Ltd, Lower Hutt, New Zealand
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 This article discusses for various foodstuffs the prac-
tical benefits of irradiation, including reduction of
pathogens, insect disinfestation, extension of shelf-
life, and delay of ripening or senescence.
General Principles
0002 Food presented for irradiation should be of good
quality and the process must not substitute for good
manufacturing practice (GMP) at any stage in the
production of food. When food is treated to reduce
pathogen or insect numbers, appropriate packaging
should be used to prevent recontamination.
0003 The maximum dose recommended by the Codex
Alimentarius, 10 kGy, causes a temperature rise of
under 2.4
C, resulting in an unchanged appearance
and texture in many temperature-sensitive foods.
Oxidative stress does occur and not all foods with-
stand the doses required to achieve the intended bene-
fits without a loss of quality. The doses suggested here
are guides only; the optimum dose varies with
conditions and varieties of food. (See Oxidation of
Food Components.)
0004Combining irradiation with another process may
result in an overall improvement in quality. For
example, fruit is less susceptible to softening when
lower radiation doses are combined with a mild heat
treatment, and insect disinfestation is still achieved.
Combination treatments are not discussed further.
Major Applications
0005Applications of food irradiation fall into several
major categories.
Improved Food Safety
0006Foodborne pathogens are a significant and increasing
cause of ill health and economic loss. Irradiation has
great potential to control pathogen numbers in at-risk
foods. For example, it is being considered for use as
part of Hazard Analysis Critical Control Point
(HACCP) systems for control of pathogens in meat.
Irradiation is a ‘cold pasteurization’ process suitable
for solid foods, especially for foods of animal origin
and consumed raw. Concern in some countries about
the increase in the presence of Escherichia coli 0157
H7 in foods has added considerable impetus to the
use of irradiation as a decontamination measure.
Improved Food Security
0007Irradiation can control insect numbers, inhibit
sprouting of bulbs and tubers, and delay maturation,
decay, and senescence of some fruits and vegetables.
Therefore irradiation can help to combat postharvest
food losses that may range from 10 to 50% for some
crops, depending on climatic conditions and available
storage technologies.
Quarantine Security
0008Most countries require that insect species not found
within their borders do not ‘hitchhike’ on imported
foods. The available chemical fumigants are being
phased out because of health and environmental
concerns. Irradiation is being accepted by plant
protection authorities as a technically viable, broad-
spectrum alternative to fumigation.
Reduction in Chemical Residues
0009Irradiation leaves no treatment residues. Consumer
demand for foods free of chemical residues is there-
fore an opportunity for irradiation to supplant chem-
ical fumigation and preservation methods.
Increased Trade
0010The four applications above provide increasing op-
portunity for increased international trade in foods.
3386 IRRADIATION OF FOODS/Applications

Irradiation can assist in meeting the hygiene, quaran-
tine, and quality demands of food-importing coun-
tries. This is likely to become economically important
for developing countries seeking to expand food
exports to markets in developed countries.
Raw Plant Products
Fresh Fruits
0011 Insect disinfestation The doses required to guarantee
quarantine security are far lower than the Codex-
recommended maximum (Table 1) and many fruit
suffer no loss of quality (Table 2) or vitamins. Fruit
still require proper storage conditions and ozone
produced during treatment should be vented to reduce
damage to the fruit. (See Insect Pests: Insects and
Related Pests.)
0012 Irradiation up to 1 kGy guarantees that insects
cannot reproduce. However, not all insect stages will
be rapidly killed. US authorities have a regulation in
place on the use of irradiation as a phytosanitary
treatment of imported fruits and vegetables.
0013 Extension of shelf-life
Reduction of spoilage Spoilage of fruit is usually
caused by fungal or yeast infections. These organisms
are inactivated by doses above 1.75 kGy, which ap-
proaches the maximum dose tolerated by most fruit.
Above the tolerated dose, softening and texture loss
occur because of degradation of pectin and cellulose.
Spoilage may even be accelerated since infection of
the weakened tissue becomes easier or natural antibi-
otic production is inhibited. (See Spoilage: Yeasts in
Spoilage.)
0014Sweet cherries and apricots may be protected from
fungal rots by a 4 kGy dose without loss of quality but
the only fruit generally considered suitable for such
treatment is the strawberry. Grey mold rot (Botrytis
cinerara) in temperate climates and rhizopus rot (Rhi-
zopus stolonifer) in subtropical areas can be con-
trolled. Treatment with 2–2.5 kGy and refrigerated
storage can increase strawberry shelf-life by a factor
of 2–4. (See Apricots; Cherries; Strawberries.)
0015Delay in ripening Nonclimacteric fruit are picked
ripe and then steadily deteriorate. Climacteric fruit,
which are mature but unripe at harvest, undergo an
initial decrease in respiration followed by a burst of
respiration associated with ethylene production and
the start of ripening. (See Ripening of Fruit.)
0016Irradiation can suppress ethylene production and
the ripening process in preclimacteric fruit. The time
between harvest and full ripening is an important
marketing consideration and irradiation could induce
a useful extension.
0017Doses above 1 kGy are required to delay ripening
of apples and pears and result in fruit of poor quality.
In peaches, nectarines, and apricots ripening is accel-
erated, not delayed. (See Apples; Peaches and Nectar-
ines; Pears.)
0018Ripening can be delayed successfully in some sub-
tropical fruits. A delay of a week (30
C storage) or
10–12 days (20
C) is observed in bananas after a
dose of 0.4 kGy. Higher doses cause softening and
discoloration. The fruit should be treated before full
maturity – an exception to the general rule. Mangoes
in the hard green state are delayed from ripening by
about 7 days (25
C storage) after 0.25–0.75 kGy.
Higher doses cause skin darkening. Papayas have a
tbl0001 Table 1 Irradiation treatments to provide quarantine security against insect pests
a
Insect species Stage Minimum dose (kGy) Criterion
Bactrocera tryoni (Queensland fruit fly) Eggs/larvae 0.075 Nonemergence of normal adults
Tephritidae Eggs/larvae 0.15 Nonemergence of normal adults
Cydia pomonella (coding moth) All 0.25 Nonemergence of normal adults
All other All 0.3 Sterilization of any adults present, and nonemergence
of normal adults from preadult stages
a
Treatments and criteria extracted from the findings of an international task force meeting on irradiation as a quarantine treatment organized by the
International Consultative Group on Food Irradiation (IAEA) in Changmai, Thailand, February 1986.
tbl0002 Table 2 The ability of fruits to retain quality after doses up to 1 kGy
a
Quality retention Fruit
Good Apple, cherry, date, guava, mango, nectarine, papaya, peach, raspberry, strawberry, tamarillo, tomato
Reasonable Apricot, banana, cherimoya, fig, grapefruit, lychee, orange, passionfruit, pear, pineapple, plum, tangelo, tangerine
Poor Avocado, grape, lemon, lime, olive
Unknown Pomegranate, kiwifruit
a
Adapted from Kader AA (1986) Potential applications of ionising radiation in post-harvest handling fresh fruits and vegetables. Food Technology June:
117–121.
IRRADIATION OF FOODS/Applications 3387

ripening delay of 3 days at ambient temperature
induced by 0.5–0.75 kGy. Skin scald results from
higher doses. (See Bananas and Plantains; Mangoes;
Papayas.)
0019 Delay of senescence All fruit, even if not susceptible
to rots, eventually deteriorate as the carbohydrate
polymers responsible for firmness and texture break
down. Generally, irradiation accelerates breakdown
but senescence is delayed in sweet cherries, apricots
(3 kGy, 4
C storage) and papayas (0.75 kGy, 25
C
storage).
Fresh Vegetables
0020 Inhibition of sprouting in tubers and bulbs The sale-
able life of potatoes, onions, garlic, etc., is reduced
by sprouting. Low doses (0.02–0.2 kGy), allied with
cool, dry storage can inhibit sprouting for months.
The mechanism is uncertain but the likely causes are
interference with nucleic acid and protein synthesis,
chromosomal aberrations in meristematic cells, and
inhibition of the production of hormones that con-
trol dormancy and sprouting. At even lower doses
(< 0.01 kGy) sprouting may be stimulated. Irradiation
inhibits wound healing in potatoes which are there-
fore stored 3–4 weeks before processing. (See Onions
and Related Crops.)
0021 Texture and overall appearance are unaffected but
other features can be affected adversely depending
upon dose, storage conditions, and variety. A small
brown discoloration near the stem end may occur
in onions. A blue-gray discoloration that occurs on
cooking potatoes that have been stored for several
months may be increased by irradiation. Many potato
varieties display a temporary rise in sugar content
after irradiation. Some varieties are unsuitable for
processing into French fries or potato chips since the
sugar content increases permanently on storage.
0022 Inhibition of greening Greening of potatoes on ex-
posure to air is inhibited by doses that prevent
sprouting. There is conflicting evidence on whether
the production of the toxic alkaloid solanine which is
associated with greening is also prevented. Greening
of endive can be reduced by a dose of 2 kGy.
0023 Other effects Undesirable changes during storage of
mushrooms include opening and browning of the cap,
darkening of the gills, and browning and elongation
of the stem. Doses in the range of 0.05–0.5 kGy in-
hibit these changes with little effect on flavor. (See
Mushrooms and Truffles: Use of Wild Mushrooms.)
0024 Postharvest curvature of asparagus is inhibited by
0.15 kGy. Higher doses produce splitting of the ends
and a slimy, darkened appearance. (See Vegetables of
Temperate Climates: Stem and Other Vegetables.)
Cereal Grains
0025Dry cereals such as barley, wheat, rice, and maize are
subject to depredation by insects (e.g., coleoptera,
lepidoptera, and mites). Doses of 3–5 kGy are needed
to kill the insects within 24 h. However 0.5 kGy re-
duces their ability to feed and renders them sterile.
This is useful as the grains are usually stored for long
periods. Grains should be stored cool and dry to
avoid mold growth. (See Cereals: Handling of Grain
for Storage.)
Processed Plant Products
Dried Fruits and Vegetables
0026Low moisture content acts as a sufficient preserva-
tive measure in dried produce. The major benefit
of irradiation is to disinfest them of insects such as
the saw-toothed grain beetle, the fig moth, and the
Indian meal moth. Doses up to 1 kGy are required
and generally cause no detectable sensory changes.
Darkening may occur upon storage in some dried
fruits.
0027An additional benefit of irradiation is that rehydra-
tion and swelling properties tend to be improved and
cooking times reduced.
Dried Herbs, Spices, and Vegetable Seasonings
0028These useful food ingredients are usually dried and
ground, chopped, or finely divided. They may be sold
as such or used as an ingredient of other processed
foods. (See Herbs: Herbs and Their Uses.)
0029The initial microbial population is often high (over
10
6
g
1
) and irradiation at the recommended Codex
limit of 10 kGy is used as a decontamination measure.
Some countries permit treatment at 30 kGy because
these ingredients are of minor dietary importance.
0030The changes in aroma, color, and flavor caused by
irradiation are insignificant at 10 kGy and slight at
30 kGy.
Fresh Meats
Red Meats
0031Inactivation of Trichinella spiralis The infectious
cycle of the helminthic parasite Trichinella spiralis
in pork can be broken by a dose of 0.3 kGy. No
special handling procedures are required and meat
quality is retained.
Extension of Shelf-life and Pathogen Reduction
0032Meat is sterile at death but bacterial contamination
may occur during slaughter and processing. Adequate
3388 IRRADIATION OF FOODS/Applications
GMP should insure sufficient retail shelf-life, and
vacuum packaging technology can extend the shelf-
life of lamb and beef by several weeks. (See Meat:
Preservation.)
0033 If a further guarantee of product hygiene is re-
quired, irradiation may be used to reduce pathogen
numbers. This will coincidentally extend the shelf-life
by reducing spoilage organisms. The induction of off-
flavors limits the maximum dose applied. Highly
trained taste panels can detect flavor changes in
some meats at quite low doses but the threshold
dose for unacceptable changes in pork is 1.75 kGy.
Doses in the range 1–2.5 kGy are acceptable for beef
and lamb.
003 4 Such doses significantly reduce the population of
many pathogens (Table 3). The meat will not be
pathogen-free if the initial contamination is substan-
tial or if more resistant pathogens are present. Spoil-
age is usually caused by Gram-negative bacteria of
the family Enterobacteriaceae and genus Pseudomo-
nas, which are radiation-sensitive. Irradiation shifts
the population to Gram-positive Lactobacillus and
Achromobacter (Moraxella-Acinetobacter) sp. with
different spoilage characteristics.
0035 Other processes that reduce shelf-life are ‘drip,’ an
unsightly exudate, and oxidation which causes discol-
oration and flavor changes. Irradiation does not in-
hibit such processes.
Fresh Poultry
0036 The principles of decontamination and shelf-life ex-
tension are similar for poultry and red meats. Poultry
have generally been considered the more likely candi-
date for irradiation since poultry are a major vehicle
for the spread of foodborne pathogens. (See Poultry:
Chicken; Ducks and Geese.) However, concerns
about E. coli 0157 H7 have put more emphasis on
red meats.
0037Doses of 1–2.5 kGy significantly reduce pathogen
numbers and double shelf-life. Below 2.5 kGy, suffi-
cient spoilage organisms will survive to insure spoil-
age occurs prior to the possible, though unlikely,
production of botulism toxin. Other safety measures
include storage below 4
C and oxygen-permeable
packaging.
Frozen Red Meats and Poultry
0038Irradiation can provide extra protection against the
presence of pathogens. Doses in the range 3–7 kGy
are effective, require no special handling, and retain
quality.
Processed Meats
0039Processed meats are often vacuum-packaged to insure
a long shelf-life. However, they may be highly spiced
and have a high surface/volume ratio if sliced. During
long storage these factors favor pathogen growth
which can be inhibited by 2.5–3 kGy.
Seafoods
Dried or Salted and Dried Fish
0040Irradiation at 0.5 kGy disinfects the stored fish of
insects (Dermestes maculatus, Necrobia rufipes and
members of the family Sarcophagidae) but will not
inhibit spoilage due to mold growth. Sensory quality
is unaffected.
Fresh Fin Fish
0041Fresh fish has a relatively short shelf-life due to
microbial spoilage, enzyme action, and oxidation.
Irradiation (1–2.5 kGy) reduces microbial spoilage,
and the shelf-life can be extended safely by several
days. Other spoilage mechanisms are unaffected
and fish still lose quality during storage. Ideally the
fish should be irradiated on board the fishing
boat; alternatively, the fish should be put on ice
before irradiation on shore. (See Fish: Spoilage of
Seafood.)
0042Both marine and fresh-water fish can be treated
successfully. Accelerated rancidity is a greater prob-
lem in fish with a high oil content (herring, salmon,
tuna), though in some oily fish (mackerel) their strong
flavor can mask taste changes. Storage below 4
Cis
essential to prevent growth of Clostridium botulinum
and toxin production. This can also be inhibited by
oxygen-permeable wrapping which will, however,
not inhibit oxidation. Below 2.5 kGy sufficient other
microorganisms such as radiation-resistant Achromo-
bacter spp. will survive and cause spoilage before
toxin is produced.
tbl0003 Table 3 Sensitivity to ionizing radiation of some pathogenic
bacteria when present in red meat
a
Genus D
10
(kGy)
b
Campylobacter 0.08–0.16
Escherichia 0.30–0.55
Listeria 0.20–1.10
Salmonella 0.31–1.30
Staphylococcus 0.34
Streptococcus 0.69–1.20
Ye r s i n i a 0.04–0.21
a
Data adapted from Farkas J (1987) Decontamination, including parasite
control, of dried, chilled and frozen foods by irradiation. Acta Alimentaria
16: 351–384, and as published by the US Council for Agricultural Science
and Technology in Task Force Report no. 115, June 1989.
b
The dose required to reduce viable cell numbers to 10% of the original
numbers.
IRRADIATION OF FOODS/Applications 3389
