Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

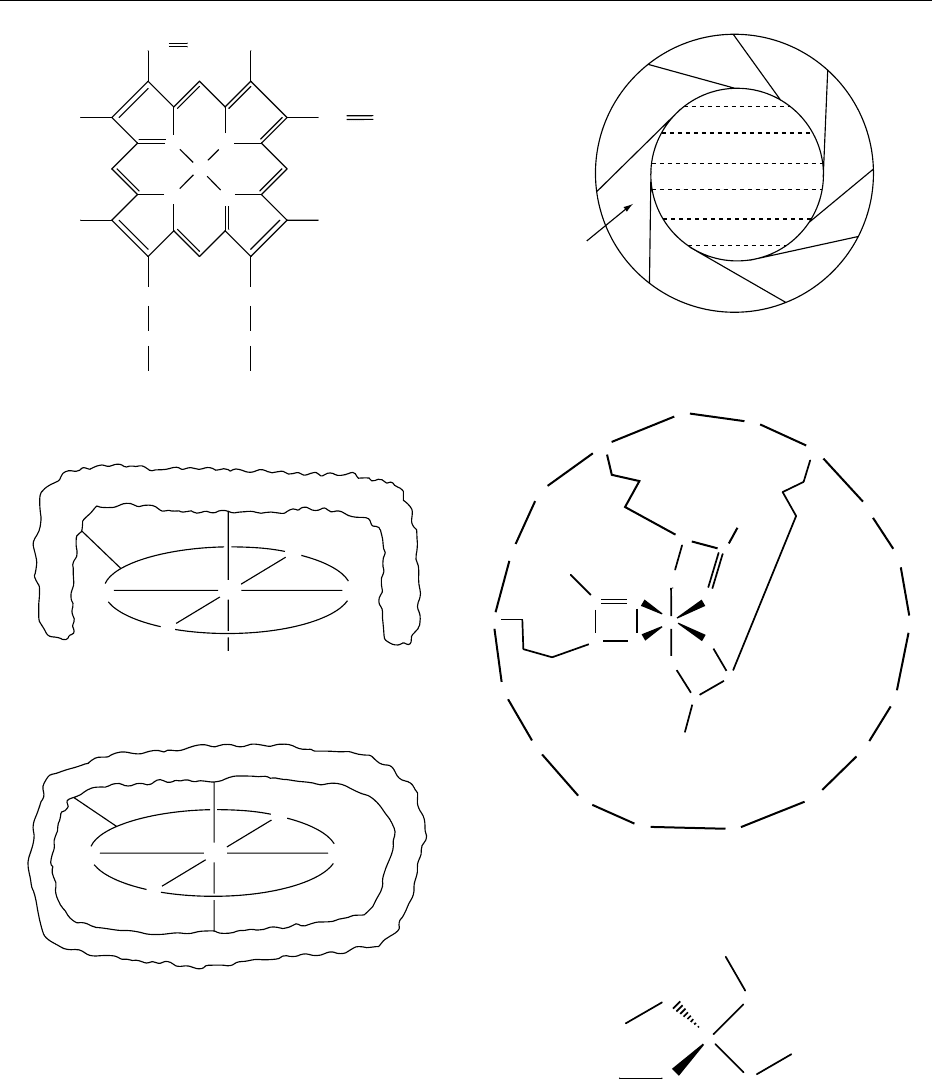
iron atoms, while the iron atoms are linked to the rest
of the protein via the sulfur atoms of cysteines. In each
of these three types of iron–sulfur protein each iron
atom is tetrahedrally surrounded by four sulfur
atoms. Compounds containing more than one iron
atom show complex redox behavior as each iron
atom can be either iron(II) or iron(III).
Environment
0012 Terrestrial The earth’s core is composed of metallic
iron, and iron compounds form 1.5% of the litho-
sphere, making iron the eighth most abundant
element. The most common iron mineral is hematite
(Fe
2
O
3
), with magnetite (Fe
3
O
4
), siderite (FeCO
3
),
limonite (FeO(OH)) and iron pyrites (Fool’s gold,
FeS
2
) also widely distributed. At neutral pH, aerial
oxygen readily oxidizes iron(II) in solution to iro-
n(III). To be stable, iron(II) compounds must, like
iron pyrites, be insoluble.
Protein
Fe
N
N
NN
fig0009 Figure 9
Protein
bundles
surrounding
core
Mineral
core
fig0010Figure 10
Fe
O
O
O
O
O
C
N
O
N
C
CH
3
C
N
H
3
C
H
3
C
HN
HC
O
C
C
O
N
H
H
N
CH
CO
NH
CH
2
CH
2
H
2
C
CO
NH
OC
OC
HN
HC
fig0011Figure 11
Protein
Fe
O
2
N
NN
N
fig0008 Figure 8
Fe
N
N
N
N
CH
3
CH
3
CH CH
2
CH CH
2
H
3
C
H
3
C
CH
2
CH
2
CO
2
−
CH
2
CH
2
CO
2
−
fig0007 Figure 7
S
Fe
S
SS
Cys
Cys
Cys
Cys
fig0012Figure 12
3370 IRON/Properties and Determination
0013 Aquatic Sea water contains low concentrations of
iron (0.002–0.02 ppm) complexed by chloride ions.
Fresh water, with pH around 7, can contain suspen-
sions of hydrated iron(III) oxide, or iron complexed
to organic material.
Losses/Gains during Food Processing
(Including Changes in Speciation)
0014 Iron compounds in foods are not appreciably volatile
so iron cannot be lost by vaporization during normal
processing. As iron compounds occur naturally asso-
ciated with proteins, the iron compounds found in
foods are generally changed on heating, so that the
tertiary structure of the protein is destroyed. This will
cause the iron compound to lose its functionality.
Thus, after heating, myoglobin will no longer revers-
ibly bind oxygen – the iron(II) is irreversibly oxidized
to iron(III). Normal cooking will not break the pep-
tide links in the protein, or free iron from its coordin-
ation shell.
0015 In principle, foods cooked or processed in iron or
steel vessels could potentially pick up significant
amounts of iron. However, as commercial processing
is now largely carried out in equipment constructed
of stainless steel, this is not a major problem. The
most likely situation where significant iron pick-up
may occur is with acidic foods or those containing
high levels of chloride ions. In domestic situations
where cast iron pots are used, iron uptake may
be appreciable, in relation to the amounts naturally
present.
0016The levels of iron resulting from contamination are
unlikely to be significant from a toxicological (or
even nutritional) point of view. However, they may
lead to metallic taints in some foods and they
may accelerate formation of rancidity by acting as a
catalyst of lipid oxidation.
Iron Fortification of Foods
0017Iron compounds used for fortification of foods are
varied. These additives are still widely referred to by
their older (ferrous/ferric) names. Iron(II) (ferrous)
sulfate is the cheapest iron source, and iron intro-
duced this way is well absorbed. Iron absorption
depends on the nature of the food. Sources of ascorbic
acid enhance iron absorption from additives, while
tea inhibits absorption. Choice of fortificant is a
balance between using iron in a form which is well
absorbed, while not causing unacceptable color or
taste changes in the food. Many countries add iron
compounds to flour and cereals to counteract iron-
deficiency anemia. Flour is fortified to iron levels of
between 16.5 ppm (UK) and 44 ppm (US).
0018Dependent on the liquidity of the product, one
can use soluble compounds with good iron bioavail-
ability (iron(II) (ferrous) sulfate, iron(II) (ferrous)
gluconate, iron(II) (ferrous) lactate, iron(III) (ferric)
saccharate, iron(III) (ferric) ammonium citrate,
soluble iron(III) (ferric) pyrophosphate). Care has to
be taken to avoid unpleasant taste, undesirable
coloring and catalysis of fat oxidation. Less soluble
iron sources (iron(II) (ferrous) fumarate, iron(II) (fer-
rous) succinate, iron(III) (ferric) pyrophosphate,
elemental iron, sodium iron(III) pyrophosphate, iro-
n(III) (ferric) orthophosphate) may be better with
more solid foods.
Analysis: Isolation and Extraction
0019It is relatively easy to analyze food samples for their
iron content. The method of analysis depends on the
nature both of the samples, and of the detection tech-
nique selected. It is usual to remove the organic part
of foods from the inorganic constituents before analy-
sis. This can be done by wet digestion or dry ashing
methods.
Wet digestion
0020Many wet analytical techniques require the iron to be
present as uncomplexed iron(II) or iron(III) ions in
the analyte with absence of organic residues. A uni-
versal method to achieve this is to heat the sample
with a digestion mixture containing concentrated
nitric, perchloric and sulfuric acids in a Kjeldahl
flask for some hours. All organic matter is oxidized
S
Fe
Fe
S
SFe
Fe
S
Cys
S
S
Cys
S
Cys
S
Cys
fig0014 Figure 14
Fe
S
Fe
S
S
S
Cys
Cys
S
Cys
Cys
S
fig0013 Figure 13
IRON/Properties and Determination 3371

to water and carbon dioxide, while metal ions are left
behind as uncomplexed ions (the anions from these
acids are poor ligands). While not necessary for
oxidation, the presence of sulfuric acid insures that
samples do not dry out to an explosive perchlorate.
After digestion, the sample is diluted appropriately.
Dry Ashing
0021 The simplest way to remove organic matter from a
sample prior to analysis is by aerial oxidation at red
heat in a silica, porcelain, or platinum crucible.
Samples (1–10 g) are normally dried carefully at
100–110
C before heating in a muffle furnace. All
iron is oxidized to Fe
2
O
3
, and organic matter present
is oxidized to carbon dioxide and water vapor. At
high temperatures, iron porphyrin compounds and
others may volatilize before oxidation and iron may
be lost, so ashing temperatures must be kept as low as
possible – temperatures of 500–550
C are generally
used. Dry ashing methods must be tested for iron
recovery before routine use. After ashing, the cooled
ash is dissolved in dilute hydrochloric acid.
Analysis: Spectroscopic and
Electrochemical Methods
0022 The simplest way to assay the iron content of the
solutions resulting from ashing is to reduce all iron
to iron(II) using metallic zinc, and then to titrate the
iron(II) with potassium permanganate.
Spectroscopic Methods
0023 Instrumental methods, mainly spectroscopic, are now
widely used.
0024 UV/Visible Absorbance Both iron(II) and iron(III)
form colored complexes, which can be used to assay
iron. Samples from ashing normally contain iron as
iron(III). Addition of thiocyanate produces a blood-
red color of [Fe(H
2
O)
5
SCN]
2þ
, which can be spectro-
photometrically measured at 480 nm. Normally
samples are reduced to iron(II) with a reductant
such as ascorbic acid, or hydroxylamine hydro-
chloride, the complexing ligands 1,10-phenanthro-
line (phen) or 2,2
0
-dipyridyl (dipy) added, and
absorbances of the colored complex ions
[Fe(phen)
3
]
2þ
or [Fe(dipy)
3
]
2þ
measured at 505–
510 nm.
0025 Atomic Absorbance Spectrophotometry Atomic ab-
sorption spectrophotometry (AAS) has been widely
used to assay for iron. Solutions containing iron(II) or
iron(III) are introduced as an aerosol into the flame
where atomization takes place. The method has a
detection limit of 0.003 ppm, and is easily automated
for the analysis of many samples. Interference from
other metals in the sample is minimal. Normally only
a single element, e.g. iron, can be analyzed for at a
time on each sample.
0026Inductively Coupled Plasma Optical Emission Spec-
trophotometry (ICPOES) Solutions containing iro-
n(II) or iron(III) are introduced into a plasma as an
aerosol, and the emissions from atomic transitions
specific to iron are measured in a suitable spectropho-
tometer. This technique has a similar sensitivity to
AAS, but is more convenient for multielement assays.
As atomic transitions are detected, the technique is
sometimes referred to as Inductively Coupled Plasma
Atomic Emission Spectrophotometry (ICPAES).
0027Spark Emission Spectroscopy This works in a simi-
lar way to ICPOES. The liquid sample is placed so
that sparks are formed between the solution and an
inert electrode. In the sparks, iron atoms are excited
to high energy levels, and emissions at wavelengths
specific to iron are measured.
Electrochemical Methods
0028Spectroscopic methods are now widely used for iron
analysis. Electrochemical methods, though effective,
are of secondary importance. Iron-containing solu-
tions may be reduced to convert all iron to iron(II)
which can be titrated with ceric sulfate, and the end-
point detected potentiometrically. Low concentra-
tions of iron may be determined by polarography,
but advanced polarographic techniques such as
differential pulse polarography generally give better
results.
See also: Iron: Properties and Determination;
Spectroscopy: Atomic Emission and Absorption; Visible
Spectroscopy and Colorimetry
Further Reading
Clydesdale FM and Weimer KL (1985) Iron Fortification of
Foods. Orlando, FL: Academic Press.
Cotton FA, Wilkinson G, Murillo CA and Bochmann M
(1999) Advanced Inorganic Chemistry, 6th edn. New
York: Wiley-Interscience.
Cunniff P (1995) Official Methods of Analysis, 16th edn.
Arlington: Association of Official Analytical Chemists.
Greenwood NN and Earnshaw A (1997) Chemistry of the
Elements, 2nd edn. Boston: Butterworth-Heinemann.
Holland B, Welch AA, Unwin ID, Bun DH and Paul AA
(1991) McCance and Widdowson’s The Composition of
Foods, 5th edn. Cambridge: Royal Society of Chemistry.
Hughes MN (1987) Coordination compounds in biology.
In Wilkinson G, Gillard RD and McLeverty JA (eds)
Comprehensive Coordination Chemistry, vol. 6.
Oxford: Pergamon.
3372 IRON/Properties and Determination
Kaim W and Schwederski B (1994) Bioinorganic Chemis-
try: Inorganic Elements in the Chemistry of Life. Wiley:
Chichester.
Neilson SS (1998) Food Analysis, 2nd edn. Aspen: Gaithers-
berg.
Pare
´
JRJ and Be
´
langer JMR (1997) Instrumental Methods
in Food Analysis. Amsterdam: Elsevier.
Reilly C (1991) Metal Contamination of Food, 2nd edn.
London: Elsevier.
Sigel A and Sigel H (1998) Metal Ions in Biological Systems,
vol. 35. Iron Transport and Storage in Microorganisms,
Plants and Animals. New York: M Dekker.
Physiology
S R Lynch, Eastern Virginia Medical School, Norfolk,
Virginia, VA, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Iron plays a central role in metabolic processes in-
volving oxygen transport and storage as well as oxi-
dative metabolism and cellular growth. The fact that
it readily serves as an electron donor or acceptor
accounts both for its critical metabolic role and its
potential toxicity. Iron-containing compounds func-
tion as carriers for oxygen and electrons and as cata-
lysts for oxidation and hydroxylation reactions. Ionic
iron can also participate in reactions that produce
toxic free radicals. Free radicals may in turn damage
cellular constituents. It is therefore not surprising that
body iron content is controlled within narrow limits,
and that the metal itself is transported and stored as a
component of specific iron-binding proteins rather
than as the free cation.
0002 This article reviews the functional role of iron in
the body, the physiological processes responsible for
the control of internal exchange and balance, and the
consequences of iron deficiency and excess. Some
aspects of the physiology of iron that are specifically
related to iron-deficiency anemia are discussed later.
(See Anemia (Anaemia): Iron deficiency Anemia.)
Body Iron Distribution
0003 The normal human body contains 3–4 g of iron
(40–50 mg per kg of body weight; Table 1), 75%
(approximately 36 mg kg
1
) of which is present in
metabolically active compounds. The remainder is
contained in a storage pool (approximately 10 mg
kg
1
in men and 5 mg kg
1
in menstruating women)
which is readily available if metabolically active iron
is depleted for any reason.
Functional Iron Compartment
Heme Proteins
0004Most of the functional iron in the body is present in
the form of heme proteins, i.e., proteins with an iron
protoporphyrin IX prosthetic group.
0005Hemoglobin, which is made up of four globin
chains, each with an attached heme group, transports
oxygen to the tissues. It is quantitatively the most
important heme protein and contains 80% of all
functional iron.
0006Myoglobin is found in the sarcoplasm of muscles.
It has a structure similar to hemoglobin but contains
only one globin chain attached to a single heme group
and accounts for a further 10% of functional iron.
Myoglobin acts as an oxygen store, insuring an
adequate oxygen supply during muscle contraction.
(See Exercise: Muscle.)
0007Despite its vital metabolic role, all other tissue iron
represents only a small fraction of total body iron.
The cytochromes are a group of heme-containing
electron transport enzymes that are essential for the
oxidative metabolism necessary to generate adeno-
sine triphosphate (ATP) as well as for the oxidative
degradation of drugs and endogenous substrates.
Catalase and peroxidase are involved in the reduction
of endogenously generated hydrogen peroxide.
Nonheme Tissue Iron
0008In mitochondria, nonheme compounds account for
more iron than do those containing heme. This
group of enzymes includes the metalloflavoproteins,
the iron sulfur proteins, and ribonucleotide reductase.
In addition, iron is necessary in a loosely bound form
for the activity of other enzymes, such as those re-
sponsible for the hydroxylation of proline and lysine
in protocollagen, during the synthesis of collagen.
(See Coenzymes.)
0009All functional iron compounds are constantly being
degraded and replaced by newly synthesized material.
tbl0001Table 1 Distribution of iron in adult human beings
Men: total
body content
Women
a
:total
bodycontent
mg mg kg
1
mg mg kg
1
Functional
Hemoglobin 2300 31 1700 28
Myoglobin 320 4 180 3
Heme enzymes 80 1 60 1
Nonheme enzymes 100 1 76 1
Storage
Ferritin 540 7 200 3
Hemosiderin 235 3 100 2
a
Age range 18–44 years.
IRON/Physiology 3373

Internal iron exchange therefore plays a crucial
role in preserving normal iron-dependent metabolic
processes.
Iron Transport and Storage
0010 Iron entering the plasma is rapidly bound to the
specific iron transport protein, transferrin. The
iron-free protein apotransferrin is a single-chain
glycoprotein (mol wt 79 570) with two nonidentical
iron-binding sites that have a high affinity for ferric
iron under physiological conditions (effective stabil-
ity constant, 10
24
mol l
1
). Plasma apotransferrin is
synthesized predominantly in the liver. It exists in the
plasma in the iron-free form or as monoferric or
diferric transferrin since iron loading at each binding
site is a random process.
0011 Iron delivery from plasma transferrin to the tissues
is mediated by a specific transferrin receptor which
is a transmembrane glycoprotein dimer composed
of two identical subunits (each with mol wt 94 000)
linked by a disulfide bond. Transferrin receptors are
expressed on the surfaces of all cells in proportion to
their iron requirements. Large numbers are present in
tissues with high requirements, e.g., developing red
blood cells and placenta.
0012 At the pH of plasma and extravascular fluid
bathing cell surfaces, receptors have very little affinity
for apotransferrin and the highest affinity for diferric
transferrin (2–710
9
mol l
1
). Once bound, the
transferrin–transferrin receptor complex, together
with its attached iron, is internalized by the cell in a
clathrin-coated pit that closes to form an endosome.
The endosome then fuses with an acidic vesicle
(pH < 5.5). The fall in pH results in release of iron
from the transferrin. The iron is transferred across the
endosomal membrane into the cell. Natural resistance-
associated macrophage protein 2 (Nramp 2, now also
called divalent metal transporter 1, DMT-1) has re-
cently been identified as the putative transmembrane
iron transport protein that transfers iron out of the
endosome. The rat isoform of Nramp 2 is a divalent
cation transporter (DCT-1) with a broad substrate
range that includes ferrous iron. The transferrin re-
ceptor remains intact. Its affinity for apotransferrin
increases, becoming equal to that for diferric trans-
ferrin, because of the lower pH. The complex is
transported back to the cell surface, where the apo-
transferrin is released back into the circulating
plasma.
0013 Any iron entering a cell that is not immediately
used for the synthesis of metabolically active com-
pounds is stored in the form of ferritin. Apoferritin
is a hollow, spherical protein shell composed of 24
subunits that may be of two types, differing slightly
in molecular weight – L (mol wt 19 700) and H
(mol wt 21 100). Each complete apoferritin molecule
can store as many as 4500 iron atoms within its
central core as ferric hydroxyphosphate. Iron enters
and leaves the intact protein through channels in the
shell.
0014Catabolism of cellular ferritin may result in the
formation of a second type of iron storage protein,
hemosiderin, which is water-insoluble. It has both
a higher iron content and a slower turnover than
ferritin.
0015The acquisition and storage of iron by cells is regu-
lated by the translational control of the synthesis of
transferrin receptors and of apoferritin. Two iron-
regulatory proteins (IRP-1 and IRP-2) that both
sense and adjust cellular iron supply have been iden-
tified. They are cytoplasmic RNA-binding proteins
that modulate the expression of messenger RNA
(mRNA) for transferrin receptor and apoferritin by
binding to iron-responsive elements (IREs) on the 3
0
and 5
0
untranslated regions of the mRNAs for trans-
ferrin receptors and the H- and L-chains of ferritin
respectively. Low cellular iron levels favor increased
binding of the IRPs to the IREs, repressing the syn-
thesis of ferritin, but stabilizing transferrin receptor
mRNA against cellular ribonucleases, thereby in-
creasing transferrin receptor expression and cellular
iron uptake. High cellular iron leads to decreased IRP
binding with a decrease in iron uptake and increased
ferritin synthesis and iron storage.
0016The two IRPs are functionally similar, but are regu-
lated in different ways. In iron-replete cells IRP-1 has
been identified as cytosolic aconitase, an iron-sulfur
protein that mediates the enzymatic interconversion
of citrate to isocitrate in the tricarbocylic acid cycle.
Low cellular iron results in reversible conversion of
enzymatically active aconitase to a form with high
RNA-binding affinity and no enzymatic activity.
Transferrin receptor synthesis is enhanced, apoferri-
tin formation is suppressed, and functional iron
homeostasis is restored. IRP-2 is functionally similar
to IRP-1, but lacks aconitase activity. Unlike IRP-1,
the binding of IRP-2 to the IREs is regulated by
degradation of the protein when cells are iron-replete.
0017The above description characterizes iron transport
and storage in most cells of the human body. How-
ever, erythroid cells have very high iron requirements
and appear to possess mechanisms for regulating iron
uptake at a transcriptional level that can override
posttranscriptional control. Iron transport and stor-
age by the macrophages of the spleen, bone marrow,
and liver are also different. These are cells primarily
involved in processing hemoglobin derived from
senescent red blood cells. At the end of their life
span, erythrocytes are phagocytosed by macrophages,
3374 IRON/Physiology
predominantly in the spleen. The heme is separated
from the globin and rapidly catabolized by the
enzyme, heme oxygenase. Iron is released and either
returned to the plasma within a few hours or incorp-
orated into the storage compartment of the cell with a
more gradual return to the plasma (half-life about 7
days). Normally, two-thirds of the iron is released
immediately, but this fraction may be either increased
when demands are high or reduced when less iron is
required.
Internal Iron Exchange
0018 The development of radioiron tracers made it possible
to quantify the processes involved in the internal iron
exchange described above. Since 80% of the body’s
functional iron is in the hemoglobin of the circulating
red blood cells, measurements of internal iron ex-
change are dominated by the requirements of this
compartment (Figure 1). Complete exchange of the
iron in the circulating red blood cell compartment
occurs every 4 months. This involves rapid transfer
of iron by plasma transferrin. Only 3–4 mg iron is
found in the plasma at any one time, but 35 mg is
transported through this compartment each day.
Most of it comes from hemoglobin catabolism in
macrophages. Two-thirds (24 mg day
1
) is delivered
to erythroid precursors in the bone marrow for the
synthesis of new hemoglobin. While there is some
iron loss owing to ineffective red cell production or
the removal of iron not used for hemoglobin synthesis
from red cell precursors, most of the iron (70%) is
returned to the circulation as hemoglobin in erythro-
cytes. Erythrocytes have a life span of about 120 days.
The senescent cells are catabolized in the macro-
phages of the liver, spleen, and bone marrow, com-
pleting the cycle.
0019Most of the iron lost during red cell production and
the iron derived from senescent red cells is processed
by macrophages. Thus a quantity of iron equivalent
to about 1% of the functional hemoglobin iron in the
circulating red cell compartment (22 mg iron) enters
the macrophages each day with a corresponding
quantity being released to the plasma.
0020A smaller but quantitatively significant daily ex-
change occurs between the plasma iron and storage
iron in hepatocytes. The rate of exchange and direc-
tion of net flow is dependent on serum iron concen-
tration and transferrin saturation. A high serum iron
concentration favors the accumulation of iron in the
liver.
0021Finally, a minor fraction of the daily iron turnover
(about 3 mg day
1
) is transferred between the plasma
and the extravascular transferrin compartment and
approximately two-thirds of this (2 mg day
1
) ex-
changes with the tissues, supplying iron to heme and
nonheme iron-dependent enzymes.
Iron Absorption and Excretion
0022The total body iron content of healthy human beings
is held within narrow limits. This is primarily a con-
sequence of the fact that most of the iron is located
in tightly regulated functional compartments. Iron
stores usually account for only 15–30% of total
body iron. The size of the storage compartment may
vary 25-fold without any apparent physiological
impairment.
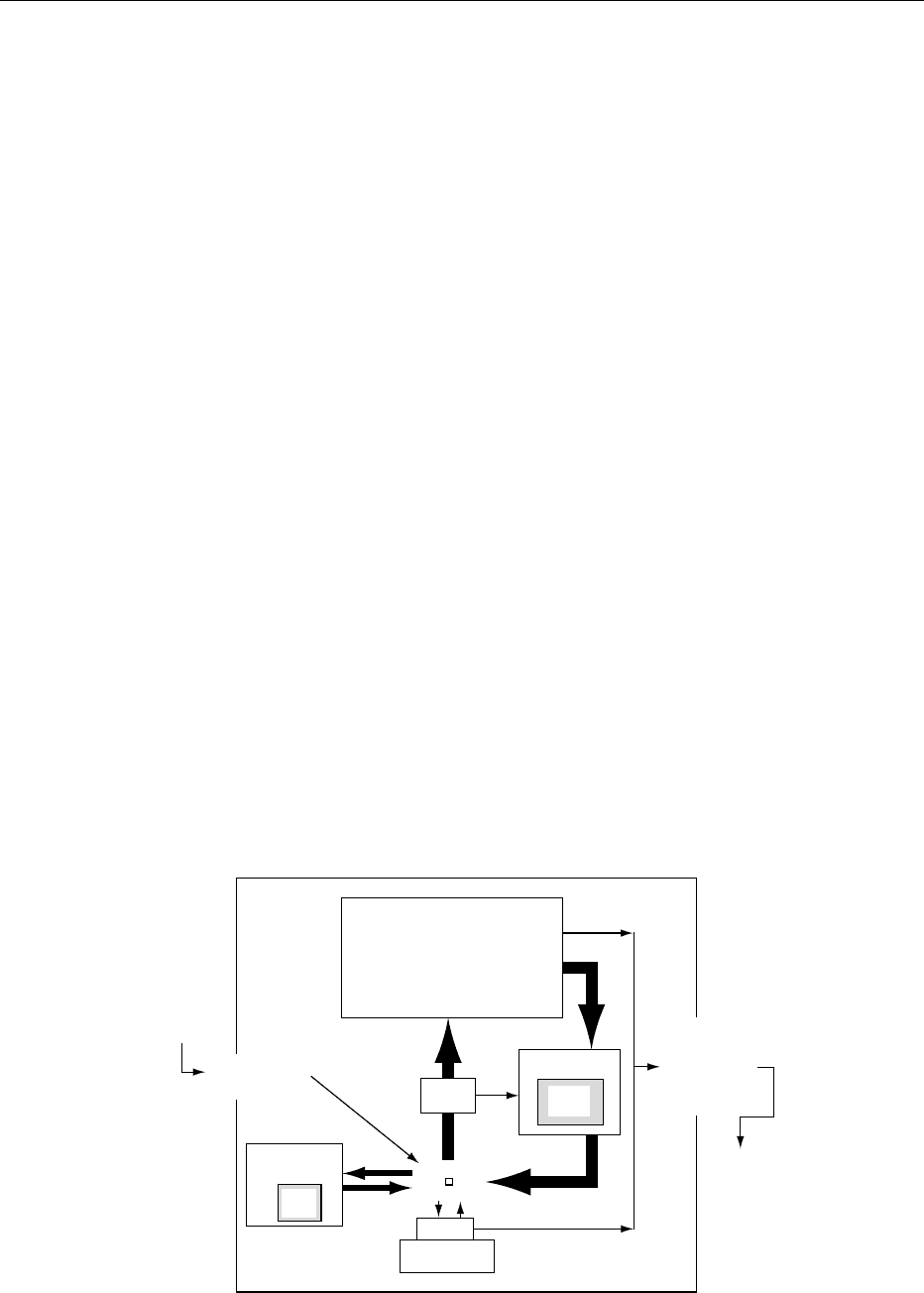
Red blood cell
hemoglobin
Bone
marrow
Macrophage
Iron
store
Iron
store
Plasma
Transferrin
Tissues
Myoglobin
Liver
(hepatocytes)
Gastrointestinal
tract
Absorption
Uterus
Gastrointestinal
tract
Skin, sweat
Kidney, urine
Iron loss
fig0001 Figure 1 Body iron exchange.
IRON/Physiology 3375

Iron Loss
0023 In contrast to the dynamic transfer of iron between
body compartments, exchange with the external en-
vironment is minimal. No adjustable excretory mech-
anism exists, but a small obligatory loss occurs with
the physiological turnover of skin epithelium and
the cells of the gastrointestinal and urinary tracts
(Figure 1). Low concentrations of iron are also pre-
sent in sweat, bile, and urine. In addition, the feces
contain small quantities of blood. Finally, menstru-
ation accounts for a significant proportion of the iron
lost by women of child-bearing age.
0024 In normal men the loss of iron from the body is
about 1 mg day
1
. This is balanced by an equivalent
absorption. Complete iron exchange with the envir-
onment in a normal man would therefore be expected
to take about 10 years. In women menstrual losses
account for an extra 0.5 mg day
1
. The higher iron
loss is matched by a higher rate of absorption.
Iron Absorption
0025 Typical western diets contain approximately 1.5 mg
iron MJ
1
. From the point of view of absorption,
food iron may be considered to exist in one of three
forms. Ten percent or less is present as heme derived
chiefly from hemoglobin and myoglobin in meat.
Heme iron is readily absorbed and little affected by
dietary factors. This small fraction may supply a third
of the iron requirements of individuals eating a mixed
meat-containing diet. The remainder of the iron
(90% or more in western diets and often virtually
100% in the diets of developing countries) must be
solubilized for absorption. If solubilized, nonheme
iron derived from all dietary sources enters a common
pool in the lumen of the upper small intestine before
absorption. A variable proportion of nonheme iron is
insoluble and unavailable for absorption. This iron
is generally regarded as contaminant iron. Much of it
enters food products during storage or processing,
particularly in developing countries. (See Meat:
Nutritional Value.)
0026 Absorption of soluble nonheme iron from the
common pool is quite variable. It is governed both
by the body’s requirement and the balance between
enhancing and inhibiting ligands in the diet that either
promote uptake by mucosal cells (increase bioavail-
ability) or render the iron in the pool unavailable for
absorption. Enhancing factors include ascorbic acid,
other organic acids, and meat and fish tissue. Low
gastric luminal pH resulting from hydrochloric acid
secretion by the stomach or from the ingestion of
acidic foods also promotes nonheme iron absorption.
The most powerful inhibitors are found in vegetable
foods and include phytates, polyphenols, and vege-
table proteins. (See Bioavailability of Nutrients.)
0027Absorption of both heme and nonheme iron is
maximal in the duodenum, partly because the luminal
conditions (particularly lower pH) favor solubiliza-
tion and absorption of nonheme iron, but also be-
cause cells in his region show the greatest ability to
respond to changes in body iron needs.
0028Absorption may be considered to occur in three
phases: first, uptake across the mucosal cell brush
border; second, a phase that involves either rapid
transfer of iron through the mucosal cells or its reten-
tion in the cellular ferritin store; and third, transfer
from the mucosal cell to plasma transferrin. Most of
the iron retained in the cell is lost when the cell exfoli-
ates. The absorption of both heme and nonheme iron
is regulated by the size of the body iron stores.
0029Heme iron enters the mucosal cell through a path-
way different from that for nonheme iron. It is taken
up as the intact heme moiety. Once within the cell,
iron is released from heme by the enzyme, heme
oxygenase. It joins an absorption pathway common
to both heme and nonheme iron.
0030Percentage absorption from the small heme pool
varies about twofold. The primary adaptation to
changes in iron requirements occurs in the adjustment
of absorption from the larger nonheme pool. Percent-
age absorption is inversely correlated with the size of
the body iron stores and may vary as much as 20-fold.
Regulation occurs both during mucosal iron uptake
by the enterocyte and during release of iron from the
cell to circulating transferrin. Mucosal uptake is the
rate-limiting step.
0031Despite years of research, the precise molecular
mechanisms involved in the uptake of elemental iron
into mucosal cells and its transfer to the plasma
remain a subject of controversy. However, it was
recently discovered that two isoforms of Nramp 2
(DMT-1) are expressed at the brush borders of the
apical poles of enterocytes in the duodenum, the site
of maximal iron absorption. Dietary iron starvation
leads to a marked upregulation of Nramp 2 isoform
1 in proximal duodenal enterocytes. Cells in the rest
of the small intestine are not affected. Nramp 2
mRNA has an IRE in its 3
0
untranslated region.
0032It has been postulated that the iron status of duo-
denal enterocytes is determined by their iron uptake
from the circulation during development in the duo-
denal crypts. They only become functional iron-
absorbing cells 48 h later when they reach the
duodenal villous tips. According to the theory the
cells are programmed to regulate iron absorption
during this period of time by an IRP–IRE mechanism.
In the face of iron deficiency, enterocytes acquire less
iron from the plasma during their early development.
3376 IRON/Physiology

DMT-1 is upregulated and apoferritin synthesis
limited. The cells absorb and transfer dietary iron
through the basolateral membrane to circulating
plasma transferrin efficiently. If the individual is
iron-sufficient, the enterocytes acquire more iron
during early development. They will express less
DMT-1, take up less iron from the duodenal lumen,
and store some of the absorbed iron intracellularly as
ferritin. Very little of the iron stored as ferritin reen-
ters the absorptive pathway at a later stage. Most of it
is lost when the cell exfoliates.
Iron Deficiency
0033 In western countries iron deficiency occurs most often
when there is a relatively sudden increase in iron
requirements or iron losses, e.g., during pregnancy
or in association with pathological blood loss.
0034 The physiological importance of both the storage
iron compartment and the capacity for the rapid trans-
fer of iron through the plasma in preventing overt
functional iron deficiency is illustrated by the effect
of blood loss. In response to significant anemia caused
by bleeding, individuals with an iron store of 1000 mg
can mobilize 40 mg day
1
, allowing rapid restoration
of the functional deficit. On the other hand, an indi-
vidual who lacks storage iron and depends on absorp-
tion from an average diet for the additional iron will
increase delivery to the bone marrow by only 2–
4 mg day
1
. Red cell production increases by a small
margin. Thus, loss of iron from the major functional
compartments is rapidly corrected when stores are
adequate, but very slowly replaced by absorption,
even when dietary iron bioavailability is relatively
high. If meal iron bioavailability is low, positive bal-
ance may not be achieved, leading to chronic iron-
deficiency anemia. Low bioavailability diets also
lead to iron deficiency when requirements are in-
creased by rapid growth or menstruation.
0035 Negative iron balance leads to a reduction in the
iron content of all functional compartments. Anemia
caused by reduced hemoglobin synthesis is the most
easily documented. Nevertheless, the availability of
iron to support metabolic systems in the tissues is
reduced concurrently. The physiological conse-
quences of iron deficiency therefore result both from
impaired oxygen delivery and functional abnormal-
ities in the tissues. However it is noteworthy that
clinically significant tissue iron deficiency does not
appear to occur in the absence of anemia.
Effects of Iron Deficiency
0036 The clinical manifestations of moderate to severe
anemia are well known and include pallor, fatigue,
weakness, dizziness, and reduced maximal work cap-
acity. The consequences of anemia per se and the
adverse effects of iron-deficiency anemia on the out-
come of pregnancy are dealt with in the section on
iron-deficiency anemia. (See Anemia (Anaemia):
Iron-deficiency Anemia.)
0037Several additional consequences are thought to
result primarily from the associated tissue iron defi-
ciency. From the epidemiological point of view, the
most important are developmental delay and cogni-
tive impairment in childhood and impaired work
performance at all ages.
Mental and Motor Development
0038Iron-deficiency anemia is associated with impaired
mental development and physical coordination in
children under the age of 2 years, difficulty with
visual discrimination tests, and the ability to maintain
selective attention in preschool children and poor
school achievement in later childhood. Short-term
memory and emotional health may also be affected.
These functional abnormalities are thought to be the
result of the effects of iron deficiency on brain neuro-
transmitters, but their biochemical basis is poorly
understood. In older children they appear to be re-
versible by successful iron supplementation.
Skeletal Muscle Dysfunction
0039Significant limitation of the ability to perform endur-
ance physical activity has emerged as an important
consequence of chronic iron deficiency. Animal stud-
ies conducted by Finch and coworkers demonstrated
that iron-deficient rats show marked impairment of
running ability which is unrelated to hemoglobin
level. It results from impaired oxidative metabolism
in iron-depleted muscles. Field studies from many
developing countries suggest that a similar disability
reduces an iron-deficient individual’s ability to carry
out prolonged physical work.
Immunity and Infection
0040Several laboratory tests of immune function are
abnormal in patients with iron-deficiency anemia.
Lymphocyte proliferation in response to the mito-
gens, phytohemagglutinin, and concanavalin A is
reduced, demonstrating defective T-cell immunity.
Intracellular bacterial killing by polymorphonuclear
leukocytes is impaired. The clinical importance of
these laboratory observations is uncertain, although
some studies have suggested that the administration
of iron to iron-deficient children may reduce the
prevalence of enteritis and influenza-like illnesses.
Iron deficiency also appears to be a predisposing
factor in chronic mucocutaneous candidiasis.
IRON/Physiology 3377

Miscellaneous Liabilities
0041 Angular stomatitis, glossitis, postcricoid webbing
of the esophagus associated with painful dysphagia
(Patterson–Kelly or Plummer–Vinson syndrome),
atrophic gastritis, and koilonychia (spoon-shaped
finger nails) have all been attributed to tissue iron
deficiency. These clinical findings appear to be less
common in recent years and a marked geographic
variation in prevalence has frequently been noted,
suggesting that factors other than iron deficiency
may play an important role.
0042 An intriguing sensory disturbance encountered in
both children and adults who are iron-deficient is the
perversion of taste leading to the consumption of
nonfood items (pica) or compulsive ice eating (pago-
phagia). The specificity of pagophagia as a symptom
of iron deficiency has been confirmed by the study of
patients in whom iron deficiency was induced by
phlebotomy alone, making the contribution of con-
founding nutritional and social factors unlikely. It is
corrected by iron repletion.
0043 Iron deficiency may also lead to impaired thermo-
genesis as well as abnormalities in thyroid metab-
olism and catecholamine turnover. Finally, lead
absorption may be enhanced, increasing the risk of
lead toxicity, particularly in children.
Iron Toxicity
Acute Iron Toxicity
0044 The ingestion of large quantities of elemental iron can
cause acute iron poisoning. This occurs most often in
young children who may eat iron tablets as ‘candy.’
The pathological consequences include a severe nec-
rotizing gastroenteritis as well as disseminated intra-
vascular coagulation, liver and cardiac injury. Death
may ensue if the dose is large or treatment is not
instituted immediately.
Chronic Iron Toxicity
0045 More important from the nutritional point of view is
the gradual accumulation that occurs when the quan-
tity of iron entering the body exceeds requirements by
even a small margin. The body has no means of
increasing iron excretion significantly, making a posi-
tive iron balance inevitable if regulation of absorption
is impaired, or if the diet contains a quantity of avail-
able iron that overwhelms the absorptive control
mechanisms. Excess iron can also be introduced
through the parenteral route (blood transfusion, par-
enteral iron administration).
0046 Impaired regulation of absorption Hereditary he-
mochromatosis is the commonest cause of iron
overload resulting from the impaired regulation of
iron absorption, and the commonest form of iron
overload in the USA, western Europe, and Australia.
It is an autosomal recessive disorder with a gene
frequency reported to be as high as 1 in 10 in people
of northern European descent. One in 300 are homo-
zygous and at risk for developing severe iron overload
with its associated pathological consequences. A
single G-to-A mutation resulting in a cysteine-
to-tyrosine substitution (C282Y) in the HFE gene
has recently been shown to be responsible for the
majority of cases of human leukocyte antigen
(HLA)-linked hereditary hemochromatosis. The HFE
gene is located on the short arm of chromosome 6 and
is tightly linked to the HLA locus. It has been identi-
fied in between 60% and 100% of patients depending
on whether their ethnic origin was southern or north-
ern Europe. A second mutation, H63D, has been
linked to an additional 1–10% of cases, especially
when present with C282Y in the compound hetero-
zygous form. The mechanisms by which mutations in
the HFE gene lead to tissue iron overload have not yet
been established.
0047HFE-associated hemochromatosis is characterized
by a rate of iron absorption that is inappropriately
high for the size of body iron stores. Downregulation
of nonheme iron absorption in the face of rising stor-
age iron is impaired and heme iron absorption
virtually unregulated. Equally important to the
pathogenesis of the condition is the presence of an
abnormally high serum iron and transferrin satur-
ation associated with disordered iron distribution
within the body, favoring iron accumulation in the
parenchymal cells of organs such as the liver, heart,
and pancreas. Comparatively less iron is located in
the normal macrophage iron store.
0048Although the defective control of absorption is an
inborn error of metabolism manifest from birth, the
prevalence of the clinical syndrome is highest in men
over the age of 40 years. Excess iron is accumulated
slowly because maximal absorption from the average
western diet is only 3–5 mg day
1
. Once the total
body iron load has increased to 15 g or more (a pro-
cess which takes 15–20 years), organ damage be-
comes evident.
0049The exact mechanism by which the iron injures
tissues has not been established, but lipid peroxida-
tion in membranes and subcellular organelles, as well
as iron-induced lysosomal disruption, are probably
involved. The clinical consequences include increased
skin pigmentation, cirrhosis of the liver associated
with an increased risk of developing liver cancer,
congestive cardiac failure and cardiac arrhythmias,
diabetes mellitus, and hypogonadism owing both to
end organ and pituitary dysfunction. In addition,
3378 IRON/Physiology

patients with hemochromatosis often suffer from an
arthropathy characterized by chondrocalcinosis and
a predilection for involvement of the second and
third metacarpophalangeal joints of the hand.
0050 If HFE-associated hemochromatosis is identified
before significant iron overload has occurred, all of
the clinical findings may be averted by iron removal.
Therapeutic phlebotomy is usually employed. Un-
checked progressive iron overload is associated with
severe irreversible morbidity and a significantly
shortened life span.
0051 Excessive iron absorption from a normal diet may
also occur in association with certain iron-loading
anemias, chronic liver disease, and porphyria cutanea
tarda.
0052 Dietary iron overload is a form of chronic iron
toxicity unique to the indigenous population of sev-
eral countries in southern Africa (subSaharan iron
overload). Traditional acidic fermented beverages
brewed in containers made from iron become
contaminated with iron at concentrations of 15–
40 mg l
1
. The beer has a low alcohol content and
the large volumes consumed may supply 50–100 mg
of available dietary iron per day. Total body iron
burdens comparable to those encountered in heredi-
tary hemochromatosis are encountered. Organ
damage with progression to cirrhosis and the devel-
opment of diabetes mellitus occur. However in the
earlier stages cellular iron distribution tends to be
different from that seen in HFE-associated hereditary
hemochromatosis with greater accumulation in the
macrophages of the spleen, liver, bone marrow, and
muscle. Two other clinical conditions are commonly
associated with the presence of excess iron – ascorbic
acid deficiency and osteoporosis. Ascorbic acid defi-
ciency results from accelerated catabolism in patients
with severe iron overload, often in association with a
low dietary intake. The factors responsible for the
osteoporosis remain poorly understood.
0053 SubSaharan dietary iron overload was originally
believed to be solely a consequence of excessive iron
consumption. Recent investigations have challenged
this belief. Family studies suggest that iron loading
occurs only when iron intake is excessive and a puta-
tive non-HLA-linked iron-loading gene is present.
See also: Anemia (Anaemia): Iron-deficiency Anemia;
Bioavailability of Nutrients; Coenzymes; Exercise:
Muscle; Immunology of Food; Meat: Nutritional Value
Further Reading
Brock JH, Halliday JW, Pippard MJ and Powell LW (1994)
Iron Metabolism in Health and Disease. London: WB
Saunders.
Canonne-Hergaux F, Gruenheid S, Ponka P and Gros P
(1999) Cellular and subcellular localization of the
Nramp 2 iron transporter in the intestinal brush
border and regulation by dietary iron. Blood 93:
4406–4417.
Dallman PR (1982) Manifestations of iron deficiency. Sem-
inars in Hematology 19: 19–30.
Finch CA and Huebers H (1982) Perspectives in iron
metabolism. New England Journal of Medicine 306:
1520–1528.
Klausner RD, Rouault TA and Harford JB (1993) Regulat-
ing the fate of mRNA: the control of cellular iron me-
tabolism. Cell 72: 19–28.
Ponka P (1997) Tissue-specific regulation of iron metabol-
ism and heme synthesis: distinct control mechanisms in
erythroid cells. Blood 89: 1–25.
Biosynthesis and Significance
of Heme (Haem)
J J Klawe, The Ludwik-Rydygier Medical University,
Bydgoszcz, Poland
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Iron – Biological Significance
0001Iron is important for the synthesis of hemoglobin,
myoglobin, and as a cofactor of numerous enzymes,
including cytochromes, cytochrome oxidase, per-
oxidase, and catalase.
0002The total quantity of iron in the human body aver-
ages about 4.5 g, 65–70% of which is present in the
form of hemoglobin. Several forms of iron ‘biological
container’ are presented in Table 1.
0003The maintenance of iron balance is essential and
necessary for health. Iron deficiency causes anemia.
Prolonged iron overload causes an accumulation of
hemosiderin in the tissues, clinically manifested as
hemosiderosis, and its severe form with damage of
tissues – hemochromatosis.
tbl0001Table 1 Forms of iron ‘biological containers’
Biologicaliron storage % of total
iron quantity
Hemoglobin 70
Myoglobin 3–4
Various heme enzymes controlling intracellular
oxidation
1
Transferrin in the blood plasma 0.1
Ferritin or hemosiderin 15
Other forms 10–15
IRON/Biosynthesis and Significance of Heme (Haem) 3379
