Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


0004 Lactose may appear in different crystal poly-
morphs, depending on the crystallization conditions.
Each polymorph has its specific properties. As an
example, a-lactose (which crystallizes as a hydrate
containing equimolar amounts of lactose and water)
has very hard crystals and is not hygroscopic, as with
b-lactose. However, a hydrous b-lactose (which crys-
tallizes at temperatures above 93.5
C) forms small
crystals with a solubility up to 10 times higher than
that of a-lactose. Because of this higher solubility,
b-lactose is experienced sweeter than a-lactose.
0005 The solubility of a-lactose is more temperature-
dependent than that of b-lactose, and the solubility
curves intersect at 93.5
C. A solution at 60
Ccon-
tains approximately 59 g of lactose per 100 g of water.
Therefore, if a 50% solution of lactose (approx. 30 g
of b- and 20 g of a-) at 60
C is cooled to 15
C, then
at this temperature, the solution can contain only 7 g
of a-lactose or a total of 18.2 g per 100 g of water at
equilibrium. Therefore, a-lactose will crystallize very
slowly out of solution as irregularly sized crystals,
which give rise to a sandy, gritty texture.
0006 a-andb-lactose differ considerably in solubility
and in the temperature dependence of solubility. If
a-lactose is brought in water, much less dissolves at
the outset than later. This is because of mutarotation:
a-lactose is converted to b, hence the a-concentration
diminishes, and more a can dissolve. If b-lactose is
brought in water, more dissolves at the outset than
later (at least below 70
C): on mutarotation, more
a-lactose forms than can stay dissolved, and a-lactose
starts to crystallize.
0007 The solution of lactose is temperature-dependent,
and solutions are capable of being highly supersatur-
ated before spontaneous crystallization occurs, and
even then, crystallization may be slow. In general,
supersolubility at any temperature equals the satur-
ation (solubility) value at a temperature of 30
C
higher. The low solubility of lactose, coupled with
its capacity to form supersaturated solutions, is of
considerable practical importance in the manufacture
of concentrated milk products. This is of prime im-
portance in sweetened condensed milk, and crystal
size must be controlled if a product with a desirable
texture is to be produced. (See Carbohydrates: Clas-
sification and Properties.)
0008 Usually, a-lactose crystallizes as a hydrate contain-
ing one molecule water of crystallization. The crystals
are very hard, slightly hygroscopic, often fairly large,
and dissolve slowly. The water of crystallization
is very strongly bound. Above 93.5
C, anhydrous
b-lactose crystallizes from an aqueous solution.
b-lactose is not very hydroscopic, and it dissolves
quickly; its solubility is good. Therefore, dehydrating
a-hydrate is difficult. It may cause problems when
determining the dry matter of milk and milk prod-
ucts; this determination implies evaporation of water
at elevated temperatures. Maintaining the tempera-
ture > 93.5
C during the assay is paramount to pre-
vent the formation of a-lactose hydrate crystals.
0009Amorphous lactose is formed during rapid drying,
as in a spray drier. It is present in the glassy state,
which means that many properties, including hard-
ness, density, and specific heat, are similar to those of
crystalline sugar but that the packing of the molecules
does not show perfect order. Amorphous lactose con-
tains at least a few percent of water and can quickly
dissolve on addition of water. But then, a-lactose
hydrate may start to crystallize. If the water content
of the amorphous lactose is low, say 5%, crystalliza-
tion is postponed. However, the product attracts
water from moist air, and when moisture content
rises about 8%, a-lactose hydrate starts to crystallize
(at room temperature). The postponed crystallization
is an important factor in relation to spray-dried
powders made from skim milk or whey because it
leads to hard lumps in the powder, and eventually,
the whole mass of powder turns into one solid cake.
0010This caking is caused by the crystallization of lac-
tose, as it causes the powder particles, largely consist-
ing of lactose, to grow together (to sinter). Since
water is needed for crystallization of a-lactose, caking
does not occur at a low a
w
, below 0.4. At a higher
temperature, crystallization can occur far more read-
ily, a
w
being higher; moreover, the viscosity of the
highly concentrated lactose solution (essentially the
continuous phase of the powder particles) is lower,
leading to a more rapid nucleation, hence crystalliza-
tion. The susceptibility of caking, especially higher in
whey powder, is considerably reduced if most of the
lactose is crystallized before the drying (in the concen-
trate). Such precrystallized powder is usually called
‘nonhygroscopic,’ which may be a misnomer because
the powder concerned does not attract less water (this
is determined by its a
w
in relation to that of the air),
but the effects differ.
Sensory Properties and Digestibility
0011Lactose is a disaccharide that yields d-glucose and d-
galactose on hydrolysis. Lactose occurs in both a- and
b-forms, which differ in the steric configuration on
the H and OH around the C-1 glucose. Lactose is not
as sweet as sucrose or fructose. For example, 1.08,
2.2, and 4.91% sucrose are of equivalent in sweetness
to 3.75, 7.5, and 15% equilibrium lactose solutions.
b-lactose is 1.05–1.22 times as sweet as a-lactose, but
this small difference in sweetness is of no practical
value because equilibration of the anomers occurs
rapidly in solution.
LACTOSE 3473

0012 The low sweetness of lactose compared with other
saccharides may be due to a weaker binding of the
molecule to the tongue papillae when compared with
fructose, sucrose, or glucose. To take advantage of the
fact that the b-form is sweeter than the a-form,
methods have been developed to convert the mono-
hydrate a-form to the b-form by treatment with
methanol and sodium hydroxide. However, when
the b-form is in solution, mutarotation occurs
quickly, and the solution becomes a mixture of a-
and b-anomers and must be utilized in quick dissolv-
ing applications if only the b form is specified.
0013 The sweetness of lactose syrups is increased with
partial hydrolysis to form a solution of galactose,
glucose, and lactose. The three sugars have a syner-
gistic effect on sweetness when mixed in solution.
0014 Hydrolysis of lactose to glucose/galactose mark-
edly changes the two properties of greatest commer-
cial importance in sugar, i.e., sweetness and solubility.
The hydrolyzed lactose syrup is expected to find
applications in nonalcoholic beverages, ice cream,
yogurt, salad dressings, jams, pectin jellies, toffees,
fudge, and boiled sweets. The technology exists for
the commercial conversion of glucose (from starch
hydrolysis) into fructose via immobilized glucose
isomerase. Thus, using two linked immobilized
enzyme reactors, it is possible to produce sweet
glucose–fructose–galactose syrup from lactose:
Lactose
β-galactosidase
glucose + galactose
glucose isomerase
glucose + fructose + galactose
0015 Low levels of intestinal lactase activity occur in
many otherwise healthy adults and children in vari-
ous population groups. Current evidence indicates
that these low levels are normal for most populations
of the world, notable exceptions being peoples of
northern European extraction: approximately two-
thirds of the world’s adult population are believed to
be lactose malabsorbers. Individuals who have genet-
ically acquired low lactase levels as adults were able
to drink milk without any ill effects as infants.
0016 Clinically identifiable lactose malabsorption
evolves over time and may be classified into three
distinct periods: the initial period is characterized by
a decline in lactase activity through childhood; the
second phase in this continuum is noted when the
decline in lactase activity is sufficient to result in a
level of undigested lactose quantitatively high enough
to cause symptoms associated with the ingestion of a
lactose load; and the third phase occurs when the
level of lactase declines to the point that it is no longer
sufficient to digest even the modest levels of lactose
usually consumed.
0017 Diarrhea, intestinal cramps, and abdominal swell-
ing are the symptoms experienced by individuals with
lactose malabsorption when foods containing milk
products are consumed. The unhydrolyzed lactose
passes to the large intestine and is fermented to lactic
acid by microflora, contributing to diarrhea. People
experiencing lactose intolerance should limit their
consumption of milk and milk products or select
lactose-modified milk. (See Food Intolerance: Lactose
Intolerance.)
0018Lactose-hydrolyzed milk is a viable product of
enzyme technology, but its manufacture must be con-
ducted optimally in terms of adaption to milk pro-
cessing and lactase efficiency. Possibilities for the
widespread production of lactose-modified dairy
products have been opened up by the development
of commercial sources of the enzyme lactase. Lactose-
modified nonfat dry milk may be manufactured for
the use as a food ingredient, and cultured products
such as yogurt may be prepared successfully from
lactase treated milk. Lactose-modified whey may be
condensed to high-solid noncrystallizing syrups for
use in soft drinks, confections, and ice cream.
0019A rare, and possibly fatal, condition of lactose
intolerance is galactosemia, in which an infant is
found to have a deficiency of galactose-1-phosphate
uridyltransferase, which converts galactose to glucose
for absorption. Infants with this condition must be
diagnosed early and given substitute milk products
that have fructose or sucrose added for energy and
sweetening properties.
Isolation and Purification
0020The principal raw material for lactose production is
cheese whey, although casein may be used. With the
application of new membrane processing techniques
in dairy technology, ultrafiltration permeate from the
production of cheese and whey protein is now becom-
ing a major dairy waste in the USA, Canada, Europe,
and New Zealand. In membrane processing oper-
ations, the problem of waste is reduced almost
entirely to milk salts and lactose. Expansion in the
use of ultrafiltration will depend largely on the prof-
itable use of permeate. A general solution to the prob-
lem of utilizing permeates rests with the human food
industry.
0021The whey protein byproduct of lactose serves as a
functional ingredient for other foods. The recovery of
lactose and protein from whey resolves two prob-
lems: (1) an economical source of a functional ingre-
dient and (2) the utilization of a product (whey) in
abundance from cheese production that otherwise is a
disposal problem. (See Whey and Whey Powders:
Protein Concentrates and Fractions.)
0022The manufacture of lactose is similar to that
of sucrose. It is basically a five-step process:
3474 LACTOSE

concentration, coagulation and removal of whey, fur-
ther concentration, crystallization, and recovery of
crystals (usually by centrifugation). The crystals are
usually washed and may be dissolved and recrystal-
lized to yield a product of high purity. The normal
form of commercial lactose is a-monohydrate, but
there is a limited market for b-lactose that is pro-
duced by crystallizing above 93.5
C. Commercial
lactose is available in a number of grades.
0023 The addition of b-galactosidase to milk for cheese
manufacture shortens the time needed to produce
cheese. The hydrolysis of lactose by the enzyme
stimulates the action of the microorganisms by con-
verting the monosaccharide to glucose and galactose,
which are more readily available for fermentation.
However, this limits the suitability of whey for lactose
recovery and poses a problem of disposal of whey that
cannot be used for lactose recovery. (See Cheeses:
Chemistry and Microbiology of Maturation.)
Food Applications
0024 One of the oldest uses of milk sugar is as a constituent
of baby food to adapt cow’s milk to the composition
of human milk. Due to its flavor-enhancing properties
and low sweetening powers, lactose is primarily used
now in numerous foodstuffs. Particularly worth
mentioning here are: baby food, cakes and biscuits,
chocolate and chocolate products, sugar confection-
ery, soups, and sauces.
0025 The presence of lactose can affect a number of prop-
erties of confectionery systems that can have implica-
tions for the processibility of the system and for the
overall product characteristics, such as flavor, mouth
feel, texture, color, and stability. The changes observed
when lactose is used in confectionery formulations
would be expected to be a function of (1) the concen-
tration, type and physical characteristics of the lactose,
(2) the function and physical characteristics of the in-
gredient that is substituted by lactose, and (3) the pro-
cessing parameters used in the products’ manufacture.
0026 Typically, at least 10% lactose can be used in most
systems as a part-substitute for sucrose or skimmed
milk powder without altering the textural acceptabil-
ity of a product to an unsatisfactory degree, whereas
up to 20% substitution is possible in certain cases. The
tendency for lactose to crystallize and grain the con-
fection appears to be a factor limiting the use of higher
levels in many cases, whereas the difference in viscosity
behavior compared with sucrose also inhibits the
preparation of textural attributes similar to products
containing sucrose. These observations, however, sug-
gest that modifications to the manufacturing proced-
ure to account for these properties could enable even
higher levels to be incorporated successfully.
0027Human milk contains *7.0% of lactose (bovine
˜
sim5.0%) and 1.0% of protein (bovine
˜
sim3.5%).
Most baby formulas are essentially ‘humanized’
cow’s milk, i.e., the lactose content is increased and
the protein content reduced. Most of the lactose used
in baby food preparations is added in the form of
demineralized whey powder. Lactose may be used as
an additive in powdered foods as a free-flowing
agent. The hygroscopicity of lactose glass may be
exploited to advantage as a means of absorbing free
moisture from low moisture foods and fixing it as
water of crystallization of lactose. For example,
food particles may be coated in a solution of flavoring
and/or coloring materials and then the coated par-
ticles tumbled in a powdered lactose glass that
absorbs surface moisture and forms a capsule around
the food particles. Lactose glass is used in ‘instantiz-
ing’ or increasing the dispersibility of certain foods.
Products are prepared containing 5–50% lactose,
spray-dried, and then ‘instantized’ by moistening
and redrying. Alternatively, powdered lactose glass
is added to the powdered food, which is then wetted
slightly, causing the lactose to crystallize and form
agglomerates that entrap the other food components.
Such products are free-flowing and capable of disper-
sing rapidly, similar to instant milk powders.
0028Lactose is used extensively in the formulation of
various types of candy, fudges, and caramels. It has
certain advantages in these products, e.g., less sweet-
ness, better color binding and better mouth-feel par-
ticles, but it also has the disadvantage of tending to
crystallize and causing grittiness. Lactose, along with
sucrose, may be used to sugar-coat chocolate buttons
and hazelnuts: the lactose suppresses sucrose crystal-
lization, thereby allowing the coating to be effected at
relatively low temperatures, and reduces the sweet-
ness of the coating.
0029Lactose is difficult to crystallize and can be a dis-
advantage in food applications. a-Lactose has a very
low solubility and cannot be used in partially pre-
pared food products that are rehydrated without
heating such as instant puddings. Large crystals may
form in the manufacture of ice cream when the mix is
cooled to a low temperature. The result is a sandy
texture or crystals large enough to be felt by the
tongue and palate. The problem can be overcome by
inhibiting crystal formation by the addition of gums
or by seeding with additional crystal nuclei to keep
the crystal size small. (See Ice cream: Methods of
Manufacture.)
0030The concentration of lactose in sweetened con-
densed milk is critical in protecting the texture of
the milk product. Condensed milk has 60% of the
water removed and sucrose added to produce a prod-
uct with a total carbohydrate concentration of 56%.
LACTOSE 3475
Too much lactose results in a product that is grainy,
and too little lactose produces a product that is slimy
in texture. (See Condensed Milk.)
0031 Lactose is used in the bakery and confectionary
industries, where it reacts with protein amino groups
to give Maillard browning, for color and flavor.
Lactose enhances the emulsifying and creaming
properties of shortenings, which improves product
quality, facilitates baking operations, gives increased
loaf volume and external appearance score, and
extends the shelf-life freshness by 50–100% com-
pared with the shelf-life of standard formulations.
Lactose may also be used in sugar coating and as a
humectant in bakery products. If proper precautions
are taken, lactose is a very suitable icing sugar for
many purposes: it gives a reduced sweetness and
better body with less chipping and cracking.
0032 The vulnerability of lactose in the Maillard
browning reaction is a problem in the manufacture
and storage of dried milk and dried whey powder.
Both products brown when stored at ambient tem-
perature, making them undesirable in terms of solu-
bility for rehydration and nutritional value. Dried
milk that has browned is difficult to reconstitute,
and browned milk is deficient in available lysine, the
e-amino group of which takes part in the Maillard
reaction. Dried milk and whey solids should be stored
under dry, cool conditions and rotated when in stock
to avoid browning during storage. (See Browning:
Nonenzymatic.)
See also: Browning: Nonenzymatic; Carbohydrates:
Classification and Properties; Cheeses: Chemistry and
Microbiology of Maturation; Condensed Milk; Ice Cream:
Methods of Manufacture; Whey and Whey Powders:
Protein Concentrates and Fractions
Further Reading
Aquilar CA, Hollender R and Ziegler GR (1994) Sensory
characteristics of milk chocolate with lactose from
spray-dried milk powder. Journal of Food Science 59:
1239–1243.
Aquilar CA and Ziegler GR (1995) Viscosity of malted milk
chocolate with lactose from spray-dried whole milk
powders. Journal of Food Science 60: 120–124.
Arvanitoyannis I and Blanshard JMV (1994) Rates of crys-
tallization of dried lactose–sucrose mixtures. Journal of
Food Science 59: 197–205.
Delaney RAM, Donnelly JK and O’Sullivan AC (1973)
Lactose and reverse osmosis. In: Birch GG and Green
LF (eds) Molecular Structure and Function of Food
Carbohydrate, pp. 155–179. New York: Wiley.
Fox PF and McSweeney PLH (1998) Dairy Chemistry and
Biochemistry, pp. 21–66. London: Blackie Academic
and Professional.
Hartel RW and Shastry AV (1991) Sugar crystallization in
food products. Critical Reviews in Food Science and
Nutrition 1: 49–112.
Kedword CJ, MacNaughton W, Blanshard JMV and
Mitchell JR (1998) Crystallization kinetics of
lactose and sucrose based on isothermal differential
scanning calorimeter. Journal of Food Science 63:
192–197.
Morrissey PA (1985) Lactose: chemical and physico-
chemical properties. In: Fox PF (ed.) Developments in
Dairy Chemistry – 3, pp. 1–35. London: Elsevier
Applied Science.
Nickerson TA (1977) Lactose sources and recovery. In:
Birch GG and Shallenberger RS (eds) Developments in
Food Carbohydrate, 1st edn, pp. 77–90. London:
Applied Science.
Thelwall LAW (1980) Lactose. In: Lee CL (ed.) Develop-
ments in Food Carbohydrate, 2nd edn, pp. 275–326.
London: Applied Science.
Walstra P, Guerts TJ, Noomen A, Jellema A and van Boekel
MAJS (1999) Dairy Technology: Principles of Milk
Properties and Processes, pp. 33–36. New York: Marcel
Dekker.
Whitaker R (1993) Some factors influencing the crystal-
lization of lactose in ice cream. Journal of Dairy Science
16: 177.
Yang ST and Silva EM (1995) Novel products and new
technologies for use of a familiar carbohydrate, milk
lactose. Journal of Dairy Science 78: 2541–2562.
Lactose Intolerance See Food Intolerance: Types; Food Allergies; Milk Allergy; Lactose Intolerance;
Elimination Diets
Lager See Beers: History and Types; Raw Materials; Wort Production; Biochemistry of Fermentation;
Chemistry of Brewing; Microbreweries
Lamb See Sheep: Meat; Milk
3476 LACTOSE
LEAD
Contents
Properties and Determination
Toxicology
Properties and Determination
A R Flegal and G M Scelfo, University of California at
Santa Cruz, Santa Cruz, CA, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Lead (Pb) is a highly toxic trace element with no
recognized biological requirement in organisms
and no established threshold concentration for sub-
lethal toxicity. Moreover, lead concentrations in the
biosphere have been substantially elevated by indus-
trial releases of lead over the past five millennia.
These contaminant lead emissions have been difficult
to quantify accurately, and many measurements of
lead in the biosphere have been biased by undetected
contamination during sampling, storage, and an-
alysis.
Occurrence in Foods
0002The relative magnitude of environmental lead con-
tamination is unparalleled among the elements.
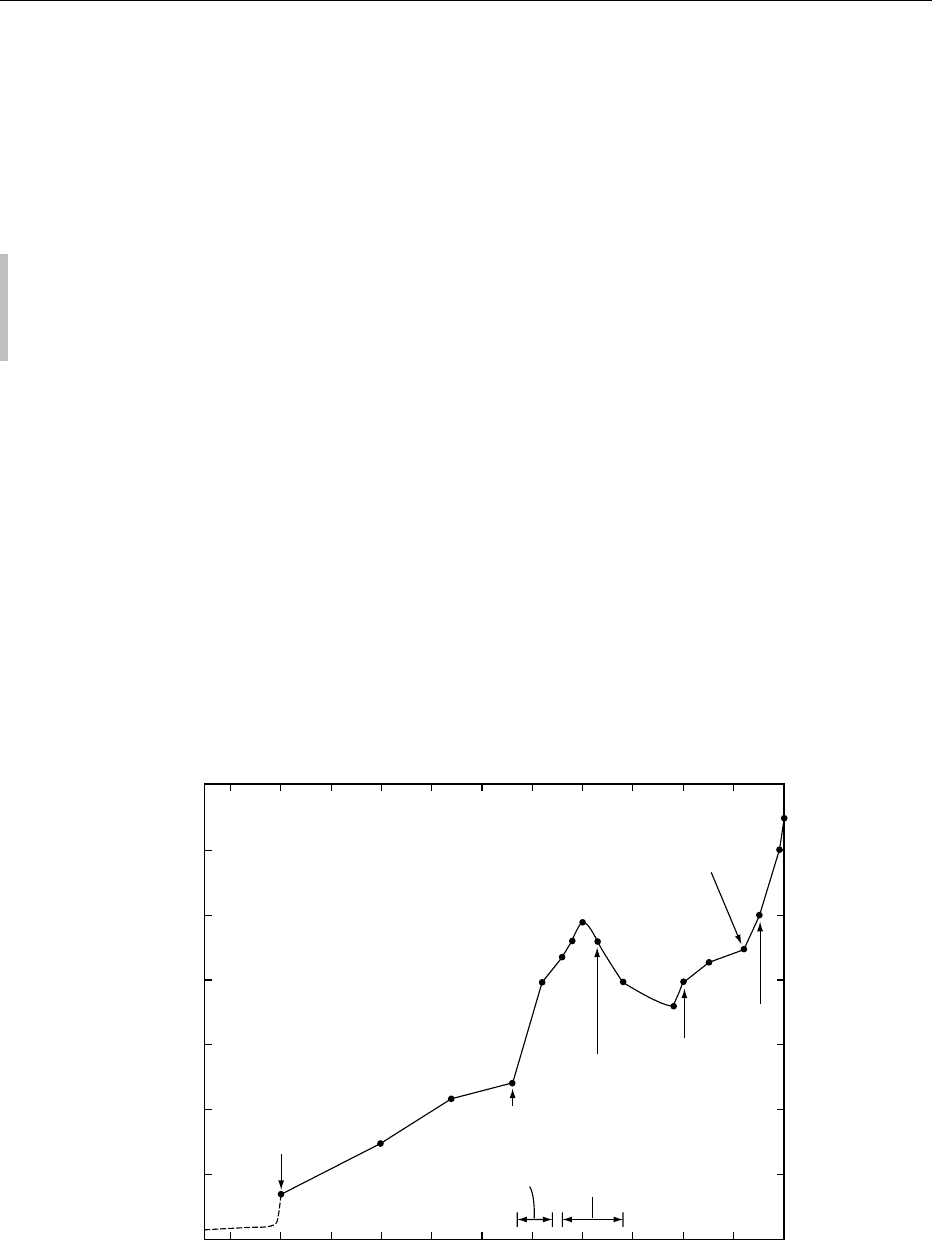
Approximately 350 million tonnes of lead have
been produced since the advent of cupellation (a
smelting process that separates precious metals
from lead ores) five millennia ago (Figure 1), and
most (> 50%) of it has been dispersed as an environ-
mental contaminant on a global scale. Production
rates of *3.4 million tonnes of lead per year were
estimated to release *1.6 million tonnes of con-
taminant lead into the environment annually during
the preceding decade. These inputs included atmos-
pheric emissions of industrial lead aerosols
(3 10
8
kg year
1
), which were nearly two orders of
magnitude (10
2
) greater than the sum of all natural
atmospheric emissions of lead (4.5 10
5
kg year
1
).
These industrial emissions increased average lead
concentrations in the biosphere by approximately
5500
10
0
10
1
10
2
10
3
10
4
10
5
10
6
5000
Discovery of
cupellation
Rise and fall
of Athens
Roman Republic
and Empire
Introduction
of coinage
Exhaustion
of Roman
lead mines
Silver
production
in Germany
Industrial
Revolution
Spanish production
of silver
in New World
4500 4000 3500 3000
Years before present
Production worldwide (tonne year
−1
)
2500 2000 1500 1000 500 0
fig0001 Figure 1 Industrial production of lead over the past five millennia. From Settle DM and Patterson CC (1980) Lead in albacore: Guide
to lead pollution in Americans. Science 207: 1167–1176, with permission.
LEAD/Properties and Determination 3477
one order of magnitude above natural levels. How-
ever, there have been measurable reductions in envir-
onmental lead concentrations that closely correlate
with reductions in industrial lead emissions over the
past three decades.
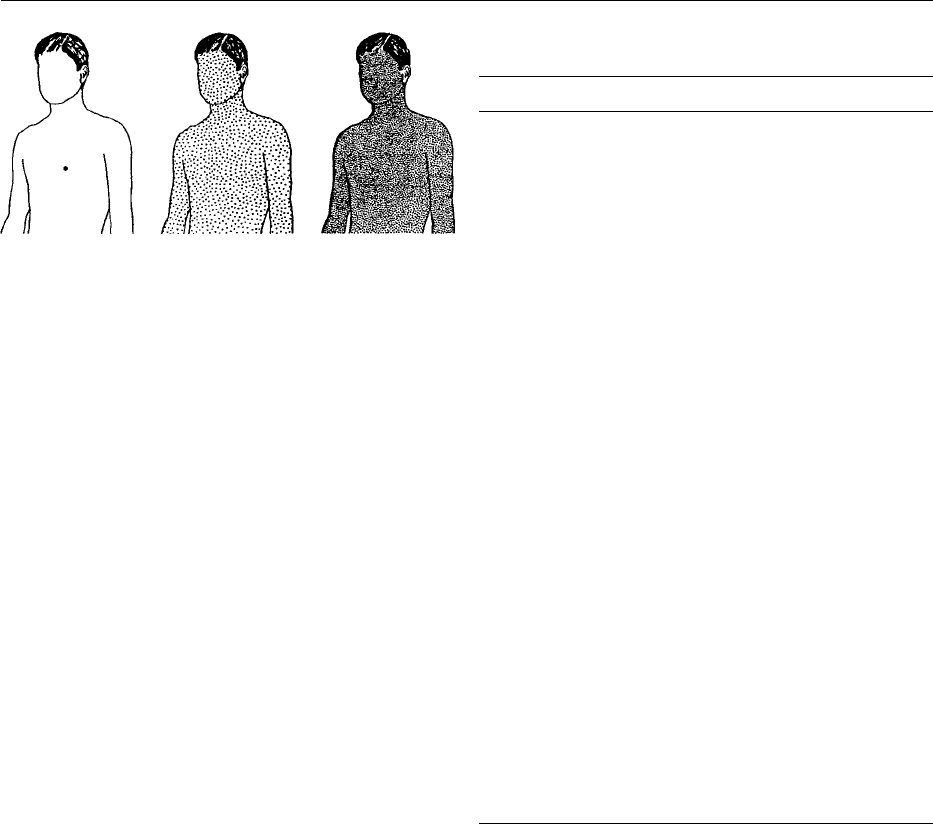
0003 One consequence of industrial lead emissions into
the biosphere has been a 500–1000-fold increase in
the lead body burdens of older (> 40 years) adults
of industrialized countries, since most (> 90%) of
that lead is in bone, where its biological residence
time is relatively long (Figure 2). Contemporary
baseline lead concentrations of those individuals are
estimated to be still approximately 40 000 mgofPb
per 70-kg person, which is *1000-fold (10
3
) higher
than natural levels in prehistoric individuals (40 mg
of Pb per 70-kg person), and only fourfold lower
than levels in individuals with overt, clinical lead
poisoning (160 000 mg Pb per 70-kg person). The
body burdens of younger adults and children are
now estimated to be much lower (*10 000 mgofPb
per 70-kg person), since they have had less exposure
to industrial lead.
0004 Presumably, there have been comparable decreases
in the lead concentrations of other components of the
biosphere, including foods in human diets. Yet, con-
taminated foods are now a principal source of ele-
vated lead concentrations in humans living in regions
where the use of leaded gasolines has sharply declined
(Table 1). The dietary intake of lead by adults in those
areas is estimated to account for approximately
two-thirds of their baseline daily consumption of
lead (*50 mgday
1
).
0005Background lead concentrations in basic food
crops and meats range from 2 to 45 p.p.b. (ng g
1
)
fresh weight (FW) (Table 2). Most (50 to > 99%) of
that lead is derived from direct and indirect atmos-
pheric inputs of industrial lead aerosols. The rest (< 1
to 50%) is derived from soils (primary minerals,
humic substances, and soil moisture), which have
natural lead concentrations of < 8 to 25 p.p.m.
(pg g
1
). Consequently, contemporary background
lead concentrations in foods are two- to 100-fold
above natural concentrations, which are estimated
to range from 0.1 to 20 ng g
1
(FW).
0006Leafy crops are most susceptible to contamination
from atmospheric deposition of industrial lead
(Table 2). These crops include wheat, rice, spinach,
fig0002 Figure 2 Relative amounts of lead contamination in adult
humans. Each dot represents 40 mg Pb per 70-kg person. The
figure on the left with one dot represents the natural concen-
tration of lead in humans (40 mg per 70 kg). The figure in the
center represents the current average lead concentration of
adults in urban environments (40 000 mg per 70 kg) in previous
decades; recent studies of ambient blood lead concentrations
indicate that average lead concentrations in children and young
adults are now substantially lower (Pirkle et al., 1998). The figure
on the right represents the minimum concentration that will actu-
ate classical lead poisoning in a significant fraction of the popu-
lation (160 000 mg per 70 kg). From Patterson CC (1980) An
alternative perspective – lead pollution in the human environ-
ment: Origin, extent and significance. In: National Research
Council. Lead in the Human Environment. Washington, DC: National
Academy of Sciences.
tbl0001Table 1 Recent trends in total baseline human exposure to
lead (units in mg day
1
)
Age/sex category 1982 1983 1984 1985
2-year-old children
Food and beverage Pb 28.4 21.6 21.7 13.1
Air Pb 2.4 1.8 1.7 1.1
Dust Pb 20.1 20.1 20.1 20.1
Total food, air, and dust Pb 50.9 43.5 43.5 34.3
Teenage females (14^16 years)
Food and beverage Pb 37.6 25.7 27.6 14.7
Air Pb 4.8 3.5 3.4 2.2
Dust Pb 4.5 4.5 4.5 4.5
Total food, air, and dust Pb 46.9 33.7 35.5 21.4
Teenage males (14^16 years)
Food and beverage Pb 53.8 36.1 41.1 20.0
Air Pb 4.8 3.5 3.4 2.2
Dust Pb 4.5 4.5 4.5 4.5
Total food, air, and dust Pb 63.1 44.1 49.0 26.7
Adult females (25^30 years)
Food and beverage Pb 37.9 27.2 28.0 14.5
Air Pb 4.8 3.5 3.4 2.2
Dust Pb 4.5 4.5 4.5 4.5
Total food, air, and dust Pb 47.2 35.2 35.9 21.2
Adult males (25^30 years)
Food and beverage Pb 55.2 36.3 42.5 19.8
Air Pb 4.8 3.5 3.4 2.2
Dust Pb 4.5 4.5 4.5 4.5
Total food, air, and dust Pb 64.5 44.3 50.4 26.5
Senior adult females (60^65 years)
Food and beverage Pb 36.7 28.6 28.3 15.5
Air Pb 4.8 3.5 3.4 2.2
Dust Pb 4.5 4.5 4.5 4.5
Total food, air, and dust Pb 46.0 36.6 36.2 22.2
Senior adult males (60^65 years)
Food and beverage Pb 45.8 34.4 36.1 18.8
Air Pb 4.8 3.5 3.4 2.2
Dust Pb 4.5 4.5 4.5 4.5
Total food, air, and dust Pb 55.1 42.4 44.0 25.5
From Flegal AR et al. (1990) Lead contamination in food. In: Nriagu JO and
Simmons MS (eds) Environmental Food Contamination, Advances in
Environmental Science and Technology. New York: John Wiley.
3478 LEAD/Properties and Determination
and lettuce, whose contemporary baseline lead con-
centrations are five- to 100-fold above natural
concentrations. Contaminant lead concentrations in
foods not directly exposed to atmospheric depos-
itions (e.g., root crops, nuts, beans, and some fruits)
are approximately twofold lower. Atmospheric in-
dustrial leads are also a primary (> 90%) source of
lead contamination of some livestock and poultry.
This occurs initially through the ingestion of contam-
inated forage, feed, and soils, and subsequently
during the processing of meat products.
0007 Current baseline lead concentrations of foods are
several orders of magnitude lower than lead concen-
trations of foods in some industrial areas (Table 3).
For example, lead levels in some plants are at sub-
part-per-million concentrations in remote, rural
environments (0.2 mgg
1
) and part-per-thousand
concentrations in some highly contaminated urban
areas (950 mgg
1
) as a consequence of local emissions
of industrial lead aerosols (e.g., automobile exhausts
of leaded fuels). Lead concentrations of consumer
organisms (herbivores, omnivores, and carnivores)
may also be increased by one to two orders of magni-
tude above baseline concentrations by the ingestion
of contaminated foods and soils.
0008 Numerous other sources of lead contribute to the
lead ingested by humans. For example, lead solder
may contribute approximately 40% of the dietary
lead accumulated by adults through the contamin-
ation of both drinking water and foods. It is estimated
that 42 million people in the USA are exposed to
drinking water with elevated lead levels (> 20 mgl
1
)
due to the lead solder in pipes. Lead solder also ac-
counts for > 99% of the lead in some foods packaged
in lead-soldered cans. Lead-glazed ceramics may
increase lead concentrations in foods to parts-per-
million concentrations, and are a notable source of
acute lead toxicity. Leaded crystal, pewter, potions,
and sealants are other sources of lead contamination
to foods. Finally, dirt may be ingested inadvertently as
a food contaminant or directly through pica. A child
that ingests as little as 1 g of soil and 200 mg of lead
paint chips a day may be consuming 2600 mgof
lead per day and absorbing 550 mg of lead per day.
Chemical Properties
0009Lead has the most stable divalent (þ2) oxidation state
of the group IVB elements, though it also occurs
in a tetravalent (þ4) oxidation state. Coordination
numbers for its divalent compounds range from 2 to
7. Those for its tetravalent compounds range from 4
to 8. The stereochemistry of lead is usually octahedral
or tetrahedral. The divalent oxidation state domin-
ates the inorganic chemistry of lead, while its organic
chemistry is dominated by the tetravalent (þ4) oxida-
tion state.
0010Divalent lead, Pb
2þ
, is a B-type or soft sphere metal
cation. Lead’s classification, which is based on the
number of electrons in its outer shell, indicates that
Pb
2þ
ions tend to form covalent bonds, in sharp con-
trast to the mainly ionic binding of hard sphere ions,
such as Ca
2þ
. This is evidenced by the tendency of
Pb
2þ
to form strong bonds with groups containing
nitrogen and sulfur (e.g., CN, R-S, -SH, and imidazol)
in biological systems. It is also apparent in mineral
deposits, where galena (PbS) is the most abundant
lead ore and where anglesite (PbSO
4
) and cerrusite
(PbCO
3
) are less common minerals.
0011Despite differences in their chemistries, lead is
introduced and cycled in biological systems as an
analog of calcium. Most (70 to > 90%) of the lead
tbl0002 Table 2 Background lead concentrations in basic food crops
and meat (units in mgg
1
fw)
Crop Natural Indirect
atmospheric
Direct
atmospheric
To t a l
Wheat 0.0015 0.0015 0.034 0.037
Potatoes 0.0045 0.0045 0.009
Field corn 0.0015 0.0015 0.019 0.022
Sweet corn 0.0015 0.0015 0.003
Soyabeans 0.021 0.021 0.042
Peanuts 0.005 0.005 0.010
Onions 0.0023 0.0023 0.0046
Rice 0.0015 0.0015 0.004 0.007
Carrots 0.0045 0.0045 0.009
Tomatoes 0.001 0.001 0.002
Spinach 0.0015 0.0015 0.042 0.045
Lettuce 0.0015 0.0015 0.010 0.013
Beef (muscle) 0.0002 0.002 0.02 0.02
Pork (muscle) 0.0002 0.002 0.06 0.06
From US Environmental Protection Agency (1986) Air quality criteria for
lead, vol. II. US EPA Environmental Criteria and Assessment Office,
Research Triangle Park, NC. EPA/600/8-83/0286F.
tbl0003Table 3 Environmental lead concentrations in remote/rural
and urban areas (units in mgg
1
, except those for ‘Air,’ which
are mgm
3
)
Remote/rural Urban
a
Air 0.05 2.3
Fresh water 1.7 10
5
0.005–0.030
Soil 10–30 150–300
Plants 0.18
b
950
c
Herbivores 2.0
c
38
c
Omnivores 1.3
c
67
c
Carnivores 1.4
c
193
c
a
Values can be highly variable, depending on organism and habitat
location.
b
Fresh-weight basis.
c
Dry-weight basis.
From Flegal AR et al. (1990) Lead contamination in food. In: Nriagu JO and
Simmons MS (eds) Environmental Food Contamination, Advances in
Environmental Science and Technology. New York: John Wiley.
LEAD/Properties and Determination 3479

in vertebrates is associated with calcareous tissues,
where lead can substitute for calcium in apatite matri-
ces. In addition, lead competes with Ca
2þ
for sites in
cellular and subcellular systems. These include synap-
tosomes, mitochonrdria, membrane vesicles, protein
kinase C, calmodulin and other Ca
2þ
-binding pro-
teins. This ionic substitution is associated with some
of the primary mechanisms of lead toxicity, which are
attributed to the alteration of calcium-mediated cel-
lular processes and the mimicry of Ca
2þ
binding to
regulatory proteins.
0012 Since lead is a biogeochemical analog of calcium,
lead concentrations are normalized to calcium con-
centrations (atomic ratios) to quantify distributions
and fluxes of lead within organisms and food chains.
This normalization indicates a systematic decrease in
the atomic ratio of lead to calcium at higher trophic
levels, a process referred to as biopurification. The
biopurification of calcium relative to lead is attrib-
uted to the selective transport of Ca
2þ
across cell
membranes, the selective retention of Ca
2þ
in meta-
bolic processes, and the selective removal of Pb
2þ
in
detoxifying processes. These biopurification pro-
cesses may be circumvented by the direct assimilation
of contaminant lead by organisms, which occurs in
addition to the ingestion of lead within food. As a
result, atomic ratios of lead to calcium in humans
may exceed the ratios in their diets.
Isotopic Composition
0013 Lead (atomic number 82) has an atomic weight of
207.19, based on the relative abundance of its four
stable isotopes:
204
Pb (1.5%),
206
Pb (23.6%),
207
Pb
(22.6%), and
208
Pb (52.3%). The latter three isotopes
(
206
Pb,
207
Pb, and
208
Pb) are formed by the radio-
active decay of the long-lived natural isotopes
238
U,
235
U, and
232
Th, respectively, while
204
Pb has no
radioactive progenitor. Since each parent isotope has
a different half-life, the relative natural abundance of
stable lead isotopes in a geological deposit varies
systematically with the age and evolution of the for-
mation. Different geological formations may, thus, be
distinguished by their characteristic (but not necessar-
ily unique) stable lead isotopic ratios.
0014 Ratios of stable lead isotopes may also be utilized
to identify and trace different sources of lead in the
environment. Natural differences in stable isotopic
compositions of lead, which are characteristic of dif-
ferent geological formations, persist in the environ-
ment because there is no measurable physical,
chemical, or biological fractionation of lead isotopes
within the biosphere. This allows sources of indus-
trial lead inputs into the atmosphere, water, soils, and
organisms to be distinguished by their isotopic
composition. Additionally, stable lead isotopic tracer
techniques may be utilized to investigate lead expos-
ure and uptake by organisms, as well as the cycling
and therapeutic manipulation of accumulated lead.
0015However, most lead tracer studies, to date, have
used man-made radiolead isotopes. One radioisotope
of lead,
210
Pb (with a half-life (t
½
) of 22 years), is
commonly used to characterize and date relatively
short (< 100 years) geological events. It is also
employed as a radiotracer in biological systems, as
are three other radioisotopes with shorter half-lives:
211
Pb (t
½
= 36.1 min),
212
Pb (t
½
= 10.64 h), and
213
Pb
(t
½
= 26.8 min). The applicability of of those radioiso-
topes is constrained by their half-lives, which often
are not suitable for analyses of many metabolic pro-
cesses, the relative insensitivity of radiometric meas-
urements, and increasing controls on the use of
radioactivity in human health studies. Conversely,
the applicability of stable lead isotopic tracers has
been rapidly increasing with the development of
high-resolution inductively coupled plasma mass
spectrometers.
Analysis – Isolation and Concentration
0016Analyses of lead concentrations in environmental
matrices have been plagued by problems of contamin-
ation during sampling, storage, and analysis. Unrec-
ognized or poorly documented contamination have
invalidated most environmental lead data reported
prior to 1980 and indicated that many subsequent
data are highly questionable. This has been demon-
strated by numerous comparisons of data from
traditional laboratories and those using ultraclean
analytical techniques. Perhaps the most celebrated
of those comparisons were analyses of lead in tuna,
which demonstrated that the lead concentration of
fresh tuna (0.3 mgg
1
FW) was three orders of magni-
tude lower than the concentration (400 mgg
1
FW)
measured with previously accepted techniques.
0017Numerous procedures have been developed to
isolate and concentrate lead for analysis. One of
the most common procedures is liquid/liquid extrac-
tion, which typically involves a water-immiscible
solvent, an aqueous solution, and a complexing
agent that forms a nonionic or neutral chelate with
lead. Another common procedure is an ion-exchange
extraction, which sequesters lead ions on a chelating
functional group immobilized on a solid substrate. A
third is electrodeposition, which concentrates lead on
to an electrode for direct electrochemical analyses or
to preconcentrate it for other instrumental analyses.
0018Total lead concentration measurements usually re-
quire a rigorous acid digestion prior to these isolation
and concentration procedures. They are required for
3480 LEAD/Properties and Determination

most of the following analytical methods, because
they do not have the requisite selectivity, sensitivity,
or freedom from matrix interferences to accurately
and precisely measure trace concentrations of lead in
complex matrices. Measurements of lead concentra-
tions in different fractions or phases typically have the
same constraints.
Colorimetric Methods
0019 Historically, lead concentrations were measured by
colorimetric methods, including spectrometry. The
primary spectrometric method for measuring lead
now is with the reagent dithizone (diphenylthiocar-
bazone). It is a relatively simple and inexpensive tech-
nique, with a relatively low (pg) level of sensitivity.
The accuracy and precision of this method is highly
dependent upon the skills of the analyst and the
utilization of rigorous trace metal clean techniques.
The same qualification applies to the following tech-
niques, which involve much more elaborate instru-
mentation.
Spectrometric Methods
0020 The most common method of measuring lead concen-
trations in a variety of matrices is atomic absorption
spectrometry (AAS). This method measures lead con-
centrations by the absorbance of lead spectra emitted
from a source lamp by vaporized lead atoms. While
flame AAS techniques may still be appropriate for
measurements of relatively high (pg g
1
) concentra-
tions of lead, most AAS analyses of lead are now by
graphite furnace AAS. Evolving AAS methodologies
include the development of alternatives to graphite
furnaces (e.g., heated quartz tubes) and couplings
with chromatographic separations for species com-
position measurements. (See Spectroscopy: Atomic
Emission and Absorption.)
0021 Atomic emission spectrometry (AES) is similar to
AAS, except that lead is measured by the light emitted
by excited ions rather than by the light they absorb.
The primary advantage of AES is that numerous
elemental concentrations may be measured simultan-
eously in a relatively small sample. This methodology
is being improved with the development of induct-
ively coupled plasma (ICP) systems, which increase
sensitivity and decrease analytical interferences. Cur-
rent ICP–AES systems now provide the sensitivity
required for analyses of lead in most biological
matrices.
0022 The most accurate method of measuring lead
concentrations in biological matrices is with isotope
dilution thermal ionization mass spectrometry
(TIMS). This definitive method is yield-independent,
extremely sensitive, and precise. It is, also, the most
accurate and precise method for measuring stable
lead isotopic compositions.
0023However, other rapidly evolving mass-spectromet-
ric methods provide comparable or complementary
measurements of lead concentrations and isotopic
compositions in different matrices. All of these mass
spectrometric methods involve the electromagnetic
and physical separation of lead ions within an instru-
ment flight tube. The most prominent of these is
inductively coupled plasma mass spectrometry (ICP-
MS), which includes both the quadrapole ICP-MS and
the high-resolution magnetic sector ICP-MS. The
latter’s sensitivity, accuracy, and precision now
rival those of TIMS. Additional mass-spectrometric
methods which have evidenced potential for analyses
of lead in biological matrices include secondary ion
mass spectrometry, glow-discharge mass spectro-
metry, and laser microprobe mass analysis.
0024Nuclear magnetic resonance (NMR) techniques are
being developed for diagnostic analyses of lead. These
focus on the resonance frequencies of
207
Pb, which
are influenced by the molecular environment around
the isotope. NMR provides a measure of lead speci-
ation in both environmental and clinical settings. For
example, NMR has been used to simultaneously
measure intracellular free calcium and lead concen-
trations in erythrocytes. (See Spectroscopy: Nuclear
Magnetic Resonance.)
Electrochemistry
0025Electrochemical methods are used to measure both
lead concentrations and its organic complexation in
aqueous systems. These measurements are based on
the current produced as lead is reduced or oxidized by
varying the potential of a working electrode in an
electrochemical cell containing the sample. The sensi-
tivity of total lead concentration measurements,
which are determined after digesting the sample, can
be increased by a preconcentration step. Organic
complexation is determined by titrating the sample
with lead and measuring the current produced after
each lead addition.
0026The two principal electrochemical methods for
determining lead in environmental samples are dif-
ferential pulse polarography and anodic stripping
voltammetry. The first method measures the faradaic
current produced by the reduction of Pb(II) to Pb(0),
while the potential on the working electrode is
scanned to negative potentials. In the second method,
lead is preconcentrated on a mercury electrode by the
reduction of Pb(II) to Pb(0) during a reducing step.
The potential is then scanned positive, and the oxida-
tion current is measured.
LEAD/Properties and Determination 3481

Chromatography
0027 Chromatography separation techniques can detect a
wide range of molecular species containing metals or
metalloids. This method involves isolation of the
species of interest from the sample in a sufficiently
volatile, thermally stable, and physicochemically re-
sistant form for separation by gas–liquid or high-
performance liquid chromatography. These methods
are coupled with elemental detectors, principally
flameless atomic absorption spectrometers, which
provide low (< 50 pg) detection limits. Other detect-
ors include flame atomic absorption spectrometers,
thermal conductivity detectors, flame ionization
detectors, electron-capture detectors, thermionic
specific detectors, and atomic emission spectrometers.
(See Chromatography: High-performance Liquid
Chromatography; Gas Chromatography.)
0028 There have been numerous applications of chroma-
tography methodologies to investigate the speciation
of lead in the environment, because of its widespread
dispersion, diverse chemical forms, and toxicity. Gas
chromatography has been used to detect organolead
compounds, including trialkyllead chlorides, in the
environment. Gas–liquid chromatography was ini-
tially used to measure tetraalkyl derivatives of lead
in the environment, and it has since been used to
investigate the biomethylation of inorganic and
ionic alkyl lead by microorganisms. Additional speci-
ation measurements may be obtained with high-
performance liquid chromatography coupled with
GFAAS, mass spectrometry, and NMR.
X-ray Fluorescence
0029 A rapidly evolving methodology for measuring lead
concentrations in situ is with X-ray fluorescence
(XRF). Sufficient incident radiation from an X-ray
generator or a radioactive source is provided to excite
an inner shell electron, with the resultant emission of
a fluorescent X-ray. The emitted energy is character-
istic of the element that absorbed the original X-ray,
and the amount of energy released is proportional to
the mass of element present.
0030 Two types of XRF are applicable for noninvasive
analyses of lead concentrations. L-line techniques
require 10.5 keV to remove an L-shell electron, and
K-line techniques require 88 keV to remove a K-shell
electron. These XRF techniques are most appropriate
for analyses of bone lead concentrations of individ-
uals. This is significant because bone is the major
reservoir of lead in humans and accounts for > 90%
of their total lead content.
See also: Chromatography: High-performance Liquid
Chromatography; Gas Chromatography; Mass
Spectrometry: Principles and Instrumentation;
Spectroscopy: Atomic Emission and Absorption; Visible
Spectroscopy and Colorimetry
Further Reading
Flegal AR, Smith DR and Elias RW (1990) Lead contamination
in food. In: Nriagu JO and Simmons MS (eds) Environmen-
tal Food Contamination, Advances in Environmental Sci-
ence and Technology. New York: John Wiley.
Halliday AN, Lee DC, Christensen JN et al. (1998) Appli-
cations of multiple collector-ICPMS to cosmochemistry,
geochemistry, and paleoceanography. Geochimica et
Cosmochimica Acta 62: 919–940.
Hamester M, Wiederin D, Willis J, Kerl W and Douthitt CB
(1999) Strategies for isotope ratio measurements with a
double focusing sector field ICP-MS. Fresnius Journal of
Analytical Chemistry 354: 495–497.
National Research Council (1993) Measuring Lead Expos-
ure in Infants, Children and Other Sensitive Popula-
tions. Washington, DC: National Academy of Sciences.
Patterson CC (1980) An alternative perspective – lead pol-
lution in the human environment: Origin, extent and
significance. In: National Research Council. Lead in
the Human Environment. Washington, DC: National
Academy of Sciences.
Pirkle JL, Kaufman RB, Brody DJ et al. (1998) Exposure of
the U.S. population to lead, 1991–1994. Environmental
Health Perspectives 106: 745–750.
Scelfo GM and Flegal AR (2000) Lead in calcium supple-
ments. Environmental Health Perspectives 108: 309–313.
Settle DM and Patterson CC (1980) Lead in albacore: Guide
to lead pollution in Americans. Science 207: 1167–1176.
Smith DR, Calacsan C, Woolard D et al. (1999) Succimer
and the urinary excretion of essential elements in a
primate model of childhood lead exposure. Toxicology
Science 54: 473–480.
Steding DJ, Dunlap C and Flegal AR (2000) New isotopic
evidence for chronic lead contamination in San Fran-
cisco Bay: Implications for the persistence of past indus-
trial lead emissions in the biosphere. Proceedings of the
National Academy of Sciences 97: 11181–11186.
Toxicology
A L Ehle, University of Chicago, Chicago, IL, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001Lead occurs widely throughout the environment.
Naturally occurring forms and lead contributed
from human activities constitute the potential source
for human exposure. Lead was one of the first metals
to be used by humans. Almost half of the world’s
production of lead is consumed by the storage battery
industry. Lead pigments, chemicals, and metals
3482 LEAD/Toxicology
