Goldfarb D. Biophysics DeMYSTiFied
Подождите немного. Документ загружается.


62 Biophysics De mystifieD
Comparing this with Eq. (4-2),
DU 5 q 2w
we see that Eqs. (4-8) and (4-2) are equivalent if
q = DH 2 V DP
This gives us some insight into what enthalpy is; it’s simply a way of dividing
up heat into two different terms: one term is the energy associated with a
change in pressure (V DP) and the other term is the change in enthalpy (DH).
Rearranging, we get
DH 5 q 1 V DP (4-9)
Enthalpy is just the heat flowing into the system plus the energy associated
with a change in pressure.
Fortunately, almost all biological processes occur at constant pressure. At con-
stant pressure, DP 5 0. This means that for a system at constant pressure, the
enthalpy change is exactly equal to the heat flowing into the system: q 5 DH.
still struggling
What is pressure times volume (pV)? how is V DP the energy associated with a
change in pressure? often we can understand a concept better by looking at the
units of the quantities involved. The units of P are force per area (n/m
2
). if we
multiply these units by meters per meter (which does not change the value at
all because meters per meter is always equal to 1) we see that the units of P are
nm/m
3
. But a nm is a joule, so the units of P are actually J/m
3
or energy per unit
volume. pressure is energy per unit volume, a kind of energy density. When we
multiple the pressure (J/m
3
) by the volume (m
3
), the units on PV are joules; PV is
the potential energy associated with pressure, and V DP is the change in this
energy due to a change in pressure.
?
PROBLEM 4-2
Choose example numbers to show that Eq. (4-6) gives correct results.
PROBLEM
Choose example numbers to show that Eq. (4-6) gives correct results.
PROBLEM
Choose example numbers to show that Eq. (4-6) gives correct results.

chapter 4 EnErgy and LifE 63
SOLUTION
The equation is D(PV) 5 P DV V DP
The trick in solving this problem is knowing what value to use for the
constant pressure or constant volume on the right side of the equation.
Since both pressure and volume are changing, the appropriate value to use
for the constant pressure and constant volume is the average value of the
pressure or volume. Here we will make an assumption that the average
value is equal to the midway point between the initial and final values. This
assumption holds true under certain conditions, for example, if the pres-
sure and volume change at a constant rate.
If we write each of the changes (denoted with D, the Greek letter delta)
in terms of the difference between the final values and the initial values,
we have
D(PV) 5 P
2
V
2
2 P
1
V
1
P DV 5 P(V
2
2 V
1
)
V DP 5 V(P
2
2 P
1
)
Now, to show Eq. (4-6) gives correct results, let’s choose some example
numbers. After reading this solution, you should try it yourself with your
own numbers. Any numbers will do, but remember that, while the change
in pressure or volume can be negative (meaning the pressure or volume
decreased), each value of pressure or volume must be positive.
For our example, let’s say the pressure went from 10 pascals (N/m
2
) to 7
pascals, while the volume went from 13 m
3
to 19 m
3
.
Substituting these numbers into the left side of Eq. (4-6), we get
D(PV) 5 P
2
V
2
2 P
1
V
1
5 (7 19) 2 (10 13) 5 133 2 130 5 3 Nm 5 3 J
And substituting the same numbers into the right side, we get
P DV 5 P
avg
(V
2
2 V
1
) 5 [(10 7)/2](19 2 13) 5 8.5(6) 5 51 J
V DP 5 V
avg
(P
2
2 P
1
) 5 [(13 19)/2](7 2 10) 5 16(23) 5 248 J
Then
P DV V DP 5 51 J 2 48 J 5 3 J
which is the same as D(PV).
SOLUTION
The equation is
✔
64 Biophysics DemystifieD
PROBLEM 4-3
Using the definition of pressure (force per unit area) and the definition of
work (force times distance), show that P DV from Eq. (4-7) is just the three-
dimensional work done by the system.
SOLUTION
Work, by definition, is force times distance moved:
w 5 F Dx
where Dx is the distance moved (change in position) along the same direc-
tion the force was applied.
Pressure, by definition, is force per unit area, or P 5 F/A.
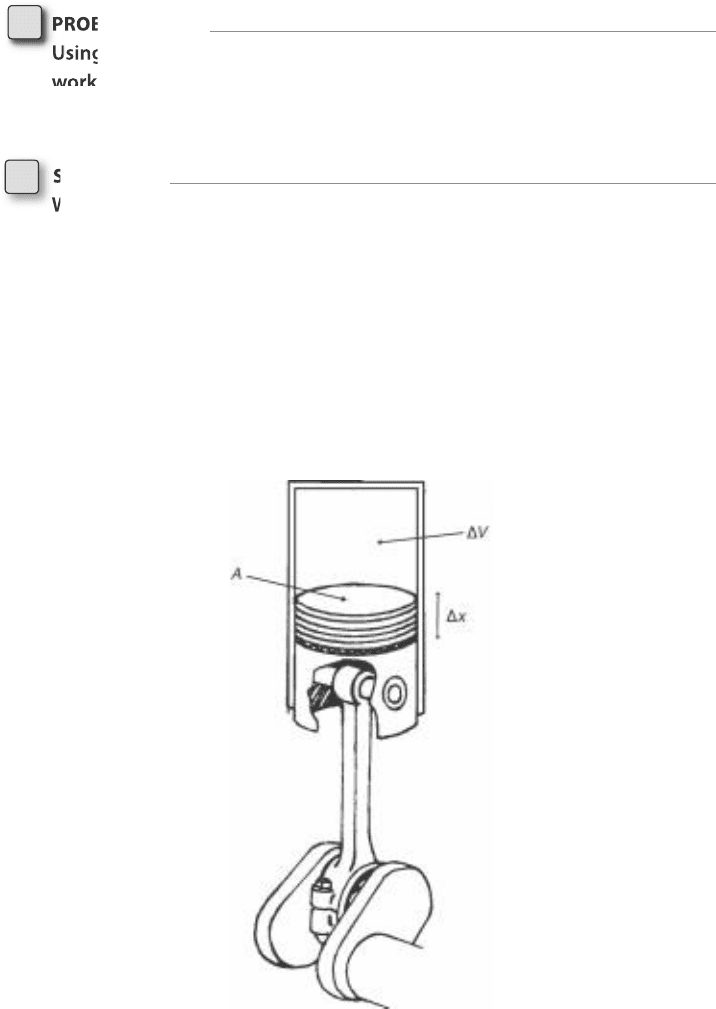
To simplify the problem, consider a cylinder closed at one end, with a
piston moving up or down inside the cylinder from the other end; see
Fig. 4-1. The volume inside the cylinder (between the piston and the
PROBLEM
Using the definition of pressure (force per unit area) and the definition of
work (force times distance), show that
PROBLEM
Using the definition of pressure (force per unit area) and the definition of
SOLUTION
Work, by definition, is force times distance moved:
✔
FigurE 4-1 • A piston in a cylinder
allows us to simplify the case of work
associated with pressure and a vol-
ume change.

chapter 4 EnErgy and LifE 65
closed end) changes as the piston moves up and down. The surface area of
the piston is constant, so the volume change is just the area times the dis-
tance the piston is moved.
DV 5 A Dx
So for the cylinder, we have
P∆∆ ∆V
F
A
V
F
A
Ax55()
The A are canceled, leaving
P DV 5 F Dx 5 w
In the case where work is done in multiple directions, we can imagine the work
done in each direction as analogous to a piston in a cylinder aligned with that direc-
tion. Take, for example, the work done in compressing or expanding a balloon. We
can imagine that at each infinitesimally small part of the balloon’s surface there is
a cylinder of constant area and the expansion or contraction of the balloon is
equivalent to moving a piston up or down inside the cylinder. The total change in
volume for the balloon is then the sum of all of these individual volume changes
given by DV = A Dx. Similarly the pressure against the surface of the balloon can
be broken down, at each infinitesimally small part of the balloon’s surface, as a force
in a direction perpendicular to the balloon’s surface at that point, divided by the
area of the infinitesimal piston for which we are considering the volume change.
The volume change for any three-dimensional shape can, in this way, be broken
down into a sum of many simple volume changes, each of which can be expressed
as a constant area times a distance. And the pressure that causes that volume change
is just, at each point on the surface, the force divided by that small constant area.
So we see that a constant pressure times a change in volume P DV is just the
three-dimensional equivalent of force times distance, or work.
PV
F
A
Ax Fx
DDD
==()
(4-10)
Entropy
The idea of entropy was developed by Rudolf Clausius in the mid-1800s. It was
well known at the time that heat engines of the day converted only a small
portion of their heat into useful work. Typically only about 1% of the heat was
66 Biophysics DemystifieD
transformed. The rest of the heat was lost, dissipated, merely warming up the
surroundings.
Clausius’s goal was to account quantitatively for this loss. He originally
called this lost energy the “equivalence value of all uncompensated transfor-
mations,” meaning that it was equivalent to the portion of the original heat
that could not be compensated for by transforming the resulting work back
into heat.
A few years later he coined the term entropy. Clausius himself said he
took the Greek word trope meaning “transformation” and put it in a form
intended to resemble the word energy. So, from trope and energy he got
entropy.
The change in entropy as the result of a process is defined as
DS 5 Q/T (4-11)
where S is the entropy and Q is the heat irreversibly lost to the surroundings at
absolute temperature T. Notice that the units on entropy are energy units
divided by temperature units, for example, joules per degree celsius.
If a process or transformation involves passing the heat through a working
body (for example, a heat engine) so that the heat is passing from a place at
temperature T
1
to a place at temperature T
2
, then the entropy change is
defined as
D
SQ
TT
=−
11
21
(4-12)
Although Clausius explained how he came up with the term entropy, he
never indicated why he chose the letter S for entropy. Some have speculated
that it was in honor of another famous early researcher of thermodynamics,
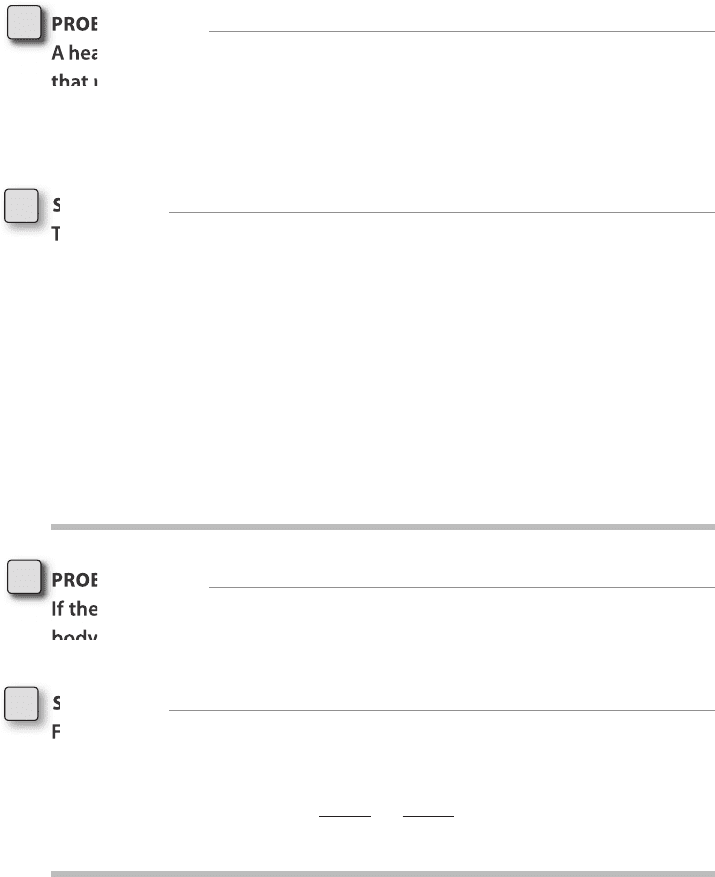
Sadi Carnot. Figure 4-2 shows a diagram by Sadi Carnot illustrating the flow of
heat and work in a heat engine.
Working
body
HOT
COLD
Q
H
T
H
T
C
Q
C
W
FigurE 4-2 • Diagram of heat engine by Sadi Carnot from
the year 1824.
chapter 4 EnErgy and LifE 67
PROBLEM 4-4
A heat engine takes 400 J of heat and converts it into 3 J of work. Assuming
that reversing the process will convert all of the work back into heat, how
much of the original heat is not compensated for by reversing the process?
What is the entropy change for this process at a temperature of 298 K?
SOLUTION
The amount of heat not compensated for by reversing the process is
Uncompensated heat 5 original heat 2 work back into heat
The original heat is 400 J. Assuming reversing the process converts all
the work back into heat, the heat compensated for by converting the work
back into heat is 3 J. Therefore the uncompensated heat is
Uncompensated heat 5 400 J 2 3 J 5 397 J
The entropy change, by definition, is
DS 5 Q/T 5 397 J/298 K 5 1.33 J/K
PROBLEM 4-5
If the heat engine from Prob. 4-4 transfers heat from a body at 350 K to a
body at 300 K, what is the entropy change for this process?
SOLUTION
From Prob. 4-5, the amount of heat lost is 397 J. The entropy change in
going from 350 K to 300 K is then
D
S525397
1
300
1
350
0 189J
KK
J/K
(
)
.
Entropy Is Related to the Number of Ways of Doing Something
It is worth mentioning here that entropy is related to order and disorder. You
may have already heard this concept. Although Clausius originally defined
entropy as a simple thermodynamic property (related to energy and work),
research over the years has shown that entropy is actually a measure of disorder
in a system.
PROBLEM
A heat engine takes 400 J of heat and converts it into 3 J of work. Assuming
that reversing the process will convert all of the work back into heat, how
PROBLEM
A heat engine takes 400 J of heat and converts it into 3 J of work. Assuming
SOLUTION
The amount of heat not compensated for by reversing the process is
✔
PROBLEM
If the heat engine from Prob. 4-4 transfers heat from a body at 350 K to a
body at 300 K, what is the entropy change for this process?
PROBLEM
If the heat engine from Prob. 4-4 transfers heat from a body at 350 K to a
SOLUTION
From Prob. 4-5, the amount of heat lost is 397 J. The entropy change in
✔
68 Biophysics DemystifieD
But what do we mean by disorder? For now, let’s just say that order and
disorder in physics are not, as common sense might imply, a matter of neat
versus messy. Rather, order and disorder relate directly to the number of different
ways of accomplishing one particular thing. The more ways there are for a system
to do one particular thing or to be in one particular state, the more disorder and
the more entropy the system has.
For example, when there are only a few ways for the molecules of a system to
arrange themselves to achieve a particular temperature, then we say that the system
is highly ordered. But when there are many different ways for the same molecules
to achieve a given temperature, then we say that the system is disordered.
gibbs Energy
About 10 years after Clausius coined the term entropy, Josiah Willard Gibbs
used Clausius’s concept of entropy to define available energy, energy available
to do useful work. Later Gibbs renamed it free energy, meaning the energy that
is free to do useful work as compared with energy that is bound to be lost
through dissipation as entropy. This latter way of thinking reflected the increas-
ing realization that some heat energy is inevitably lost and simply can never be
converted into work no matter how hard one tries. This idea that it is impos-
sible to convert all of the energy into work without some loss gave rise to the
second law of thermodynamics.
The free energy as Gibbs defined it is now called Gibbs free energy, or simply
Gibbs energy, or the Gibbs function. It is defined as
G 5 H 2 TS (4-13)
where H is enthalpy, T is the absolute temperature, and S is the entropy. You
can see clearly from this definition that Gibbs energy is what remains of the
enthalpy after subtracting out the energy loss due to entropy.
Gibbs Energy Tells Us Something about
the Spontaneity of a Process
The Gibbs energy change for a process is defined as
DG 5 DH 2 T DS (4-14)
It didn’t take long for scientists to realize that if we measure the Gibbs
energy change for a process that happens spontaneously, then the Gibbs energy
chapter 4 EnErgy and LifE 69
of the final state is always less than that of the initial state. That is, for a process
that happens spontaneously (i.e., on its own, with no outside push), Gibbs
energy always decreases; DG is negative.
This was in fact what Gibbs was striving for when he proposed a way to
measure the available energy. Gibbs knew well, from the branch of physics
called mechanics, that a force is what moves a physical object. He also knew
that there is a simple mathematical relation between force and movement
(F 5 ma). Physical chemists at that time were looking for a property analogous
to force which could be measured and which could be shown to be the force
that drives chemical reactions. Gibbs found it. Just as the force of gravity causes
a ball to roll down hill, so too, for any process that takes a system from state A
to state B, if state B has less Gibbs energy than state A, then the process will
proceed spontaneously, rolling down the Gibbs energy hill from A to B.
The Second Law of Thermodynamics
No review of thermodynamics would be complete without some discussion of
the second law of thermodynamics. In all likelihood you’ve heard of the second
law and learned something about it. And you probably know that it has
something to do with entropy increasing. Perhaps you even know there are
many ways to state the second law of thermodynamics, and as different as they
all may sound, they are all various views of the same thing. For example,
The universe is winding down.
•
Heat spontaneously flows from a place of higher temperature to a place •
of lower temperature.
When converting energy into work, some of the energy is always lost to
•
heat.
In any process, the entropy of a closed system will either increase or
•
remain constant.
In any process, the entropy of the open system and its surroundings, taken
•
together, will either increase or remain constant.
In any process, a system taken together with its surroundings will always
•
go from a more ordered state to a less ordered state.
Some statements of the second law are very precise. Others, such as “The uni-
verse is winding down,” can be ambiguous. Care must be taken not to misinterpret
70 Biophysics DemystifieD
their meaning. For example, it is not scientifically correct to make statements like
“His dorm room is proof of the second law of thermodynamics.”
When a living thing develops from an undifferentiated mass of cells to a fully
grown plant or animal, it is clear that it has gone from a less ordered state to a
more ordered state. Because of this fact and the fact that living things continue
to propagate their order by having offspring, scientists have speculated that
living things may somehow violate the second law. Or, at least, they considered
it a possibility there are physical laws we have yet to discover that are apparent
only in the case of living matter.
But living things are open systems. When taken together with their surround-
ings, the impact of the living is to increase the disorder of the environment to a
greater extent than they increase their own order. Overall the result is an increase
in disorder and the second law of thermodynamics is not violated.
The gibbs Function as the Driver of Biophysical Processes
For the most part (and this may strike you as odd) biophysics is not concerned
with the second law of thermodynamics, at least not as stated. What concerns
us more is the Gibbs function. The Gibbs function effectively has the second
law of thermodynamics built into it, so by focusing on the Gibbs function alone,
everything is taken care of.
In this context we can make the following statement, which is akin to other state-
ments of the second law: All else being equal, in any process, an increase in entropy
makes a favorable contribution to the Gibbs energy change, whereas a decrease in
entropy makes an unfavorable contribution to the Gibbs energy change. By favorable
and unfavorable we mean whether or not the process is driven forward.
So forget about the second law of thermodynamics. Well, don’t actually for-
get about it, but focus on biophysical processes in terms of the Gibbs function
being the force that drives the process.
DG 5 DH 2 T DS
We can see from the Gibbs function that there are three things that contrib-
ute to negative DG and thus to rolling down the Gibbs energy hill and to driv-
ing a process forward.
1. Releasing energy. When a system goes from a higher-energy state to a lower-
energy state, it releases energy; its enthalpy decreases. DH is negative, and
a negative DH contributes toward a decrease in Gibbs energy.
chapter 4 EnErgy and LifE 71
2. Increasing disorder. When a system goes from more ordered to less ordered,
its entropy increases, DS is positive, and so, all else being equal, its Gibbs
energy decreases.
3. Changing temperature. Note that the effect of temperature depends on
whether the entropy change is positive or negative. For a positive entropy
change, increasing temperature contributes to a favorable Gibbs energy
change.
Some processes are driven forward by simultaneously releasing energy and
increasing disorder. For other processes it may be the case that either the
enthalpy change or the entropy change are unfavorable, yet the process is still
driven forward because the overall Gibbs energy change is favorable. In such a
case if the process is driven forward primarily by releasing energy, then the
process is said to be enthalpy driven. On the other hand if the process is driven
forward primarily by increasing disorder, then the process is said to be entropy
driven.
