Gross R., Sidorenko A., Tagirov L. Nanoscale Devices - Fundamentals and Applications
Подождите немного. Документ загружается.


74 Rudolf Gross
by the heavy, whereas P
Nv²
and P
tun
are determined by the light electrons.
That means, that P
DOS
and P
Nv²
or P
tun
can be very different, since they are
determined by different types of electrons. Moreover, P
DOS
and P
Nv²
or P
tun
can have opposite sign. A typical example for this second case is Ni.
Although band structure calculations yield P
DOS
< 0 for Ni, the transport
spin polarization has opposite sign for a large energy range, i.e. P
Nv²
> 0.
Indeed, for Ni usually a positive spin polarization is derived from transport
experiments, where P
Nv²
is measured, which is however much larger than
expected from band structure calculations. This indicates that band structure
calculations underestimate the effect. This most likely is due to the fact that
band structure calculations yield a too weak localization of the d-electrons.
Classification of half-metals
In this subsection we give a classification of half-metals using the
definitions for the spin-polarization given above. Doing so, we will follow
Coey's work [151, 152]. Usually, a half-metal is defined as a material which
is a normal metal with a well-defined Fermi surface for one spin direction,
but shows a gap in the density of states around the Fermi level for the other
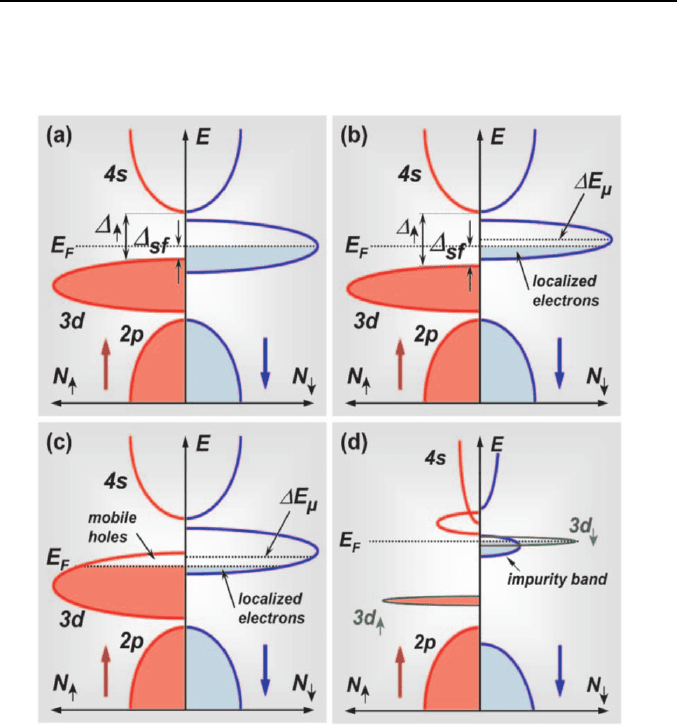
spin direction [153]. This classical definition is shown in Fig. 6a. Following
Coey we denote these half-metals as type-I half-metals. A further
classification into
I
A
and I
B
can be made depending on whether there are
less or more than five d electrons so that either the spin-up or spin-down
band is only partly filled. In either case there is a spin gap ∆
↑
or ∆
↓
and a
smaller gap ∆
sf
for spin-flip excitations (see Fig. 6a).
It is well known that ferromagnetic transition metals are no half-metals.
Although e.g. Co and Ni have a d-band split so that all 3d
↑
subbands are
filled and only the 3d
↓
subbands cross the Fermi level, the presence of an
unsplit 4s conduction band results in a finite density of states for both spin
directions at the Fermi level. A half-metallic behavior can be generated by
shifting upwards the 4s band. In oxides this is achieved by the hybridization
of the 4s metal and the 2p oxygen states pushing the 4s band up above E
F
or shifting down the Fermi level into the d band below the bottom of the 4s
band. Typical type-I half-metals are the transition metal oxides CrO
2
(I
A
)
and double perovskites such as Sr
2
FeMoO
6
(I
B
). Another well-known
example are the so called Heusler compounds. Here, heavy d elements such
as Sb tend to suppress the 3d levels below the 4s band edge by p-d
hybridization. For example, in NiMnSb [154] there are spin-up electron
with Ni e
g
character at E
F
(type I
A
). In contrast, in the compound Mn
2
VAl
[155] there are Mn t
2g
spin-down electrons at E
F
(type I
B
). It is evident that
according to the different definitions of the degree of spin polarization
Magnetic Tunnel Junctions Based on Half-Metallic Oxides 75
given above, for type-I half-metals we have P
DOS
= P
Nv
= P
Nv²
= P
tun
= 1.
That is, we have 100% spin-polarization independent of the specific
definition of the spin-polarization.
Fig. 6.
Schematic sketch of the densities of states for the spin-up and spin-down
electrons for the classification of different types of half-metals. For the type-I half-
metals, there is a finite density of states only for the majority, spin-up (not shown)
or minority, spin-down electrons (a), whereas there is a gap in the density of states
for the other spin direction. Type-II half-metals (b) are equivalent to type-I half-
metals. However, due to narrow bands the states at the Fermi level are localized
and transport is due activation across a mobility edge
∆E
µ
. Finally, type-III half-
metals (c) have mobil charge carries for one spin direction and localized ones for
the opposite.
∆
↑,↓
is the representing the gap for a particular spin direction and ∆
sf
is the gap for spin-flip processes. (d) shows a half-metal as expected from the spin-
split impurity band model [157], which describes the magnetic exchange in a high-
κ oxide (e.g. ZnO) doped with transition metal ions (e.g. Co) far below the
percolation threshold.

76 Rudolf Gross
Note that the d-bands may be very narrow. Then, in oxides the charge
carriers often form small polarons with large effective mass and small
mobility. Conduction is then by polaron hopping. Also, if the Fermi level is
close to the bottom or the top of the conduction band, disorder may cause
localization below or above a mobility edge. According Coey's
classification scheme, type-I half metals with localized charge carriers are
denoted as type-II half-metals (Fig. 6b). Again we can further subdivide
into type II
A
and II
B
depending on whether the band is less or more than
half filled. Obviously, we have P
DOS
= P
Nv
= P
Nv²
= P
tun
= 1 also for type-II
half-metals. A typical candidate for a type- II
B
half-metal is magnetite for
which the Fermi level is in the
↓
g
t
2
band of octahedral iron. The charge
carriers of this band are localized to form polarons.
The strong localization of charge carriers in oxides brings us to the
definition of type-III half-metals (Fig. 6c). In such half-metals we have a
finite density of states at the Fermi level for both spin directions. However,
whereas the charge carriers for one spin direction are mobile, those for the
other are localized and cannot contribute to transport. Then, we have a half-
metal in the sense that we have metallic behavior for one and insulating
behavior for the other spin direction, i.e. again only half of the electrons
contribute to transport. A typical example for a type- III
A
half-metal is
La
0.7
Sr
0.3
MnO
3
with mobile Mn e
g
spin-up electrons and localized Mn t
2g
spin-down electrons at E
F
. With respect to the different definitions of the
degree of spin polarization given above, for type-III half-metals we have
P
DOS
< 0, that is, the DOS spin polarization is less than 100%, it even may
be zero. However, since the charge carriers for one spin direction are
localized (e.g. v
↓
= 0), we have P
Nv
= P
Nv²
= P
tun
= 1. That is, the transport
spin polarization is 100%. This means that also type-III half-metals have
full spin-polarization for the majority of situations, where transport
properties are relevant.
As the last type we consider diluted ferromagnetic semiconductors. On
the
one hand, these materials can become ferromagnetic by a polarization
of the spins s of the conduction or valance band carriers due to the spin S of
localized ion cores by an s·S exchange. This exchange can cause a spin
splitting which is larger than the Fermi energy. Examples are (GaMn)As,
where the S = 5/2 core spin of the Mn
2+
(3d
5
) ions split the top of the
valence band producing spin-down holes [156], and EuO or EuS doped
with trivalent rare earth elements. On the other hand, ferromagnetism can
arise from the fact that the dopant impurity atoms are sufficiently close to
form a narrow impurity band, which is unstable with respect to spin
splitting. Possible candidates for this category, where none of the atoms
needs to be magnetic, are (LaCa)B
6
and ferromagnetic carbon. Another
possibility
is that the ferromagnetic exchange is mediated by charge
Magnetic Tunnel Junctions Based on Half-Metallic Oxides 77
carriers in a spin-split impurity band [157]. Such spin-split impurity band
model can account for ferromagnetism in insulating or conducting high-κ
oxides with concentrations of magnetic transition metal ions that lie far
below the percolation threshold (Fig. 6d).
Measurement of spin polarization
The analysis given above makes clear that the spin polarization of a
material reported in the literature depends on the method used to measure it.
We therefore briefly summarize the various experimental methods sketched
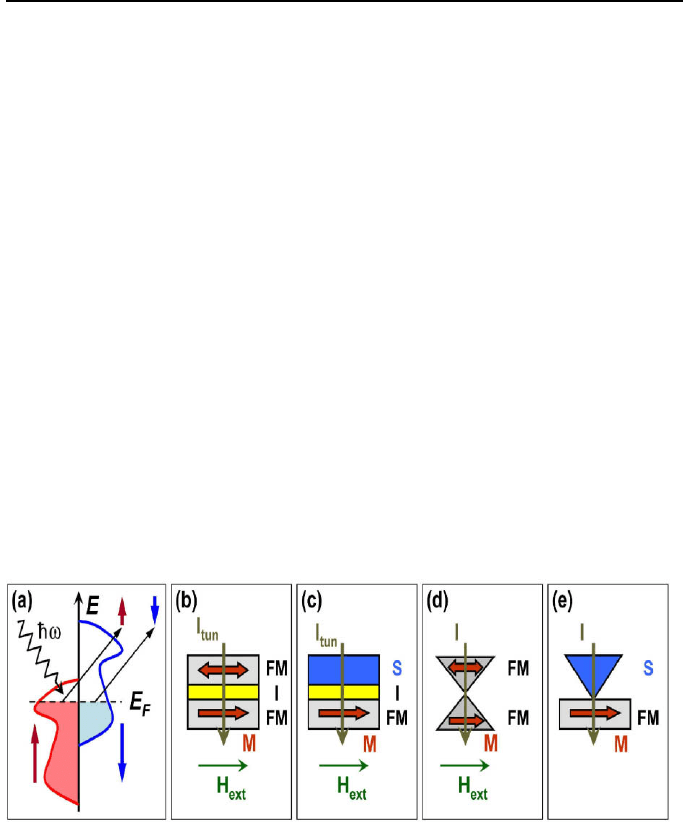
in Fig. 7 and discuss the kind of spin polarization that is achieved.
Photoelectron spectroscopy
As illustrated in Fig. 7a, in spin-resolved photoemission experiments [158]
the surface of a material is irradiated by photons of energy ħω and the
photoelectrons emitted from the surface are analyzed using a spin-sensitive
detector (Mott analyzer) [159]. This method directly measures the DOS
spin polarization P
DOS
. However, it has several drawbacks. First, it is a
surface
sensitive method, since the emission depth of the photoelectrons is
Fig. 7. Illustration of different measuring methods for the spin polarization of
ferromagnets. (a) Spin-polarized photoemission from a ferromagnet upon
irradiation by photons of energy ħω (b) spin-polarized tunneling in magnetic tunnel
junctions, (c) spin-polarized tunneling in superconductor/insulator/ ferromagnet
(S/I/FM) tunnel junctions, (d) spin-polarized transport in point contacts between
two ferromagnets and (e) spin-polarized transport between a superconductor and a
ferromagnet involving Andreev reflection. H
ext
denotes the applied magnetic field,
M the magnetization in the ferromagnet, I
tun
the tunneling current and I the ballistic
or diffusive transport current in the point contacts.

78 Rudolf Gross
of the order of 10Ǻ. Therefore, the derived values of the spin polarization
are susceptible to surface states, reconstructions, contamination etc. Second,
the energy resolution is limited. Finally, in photoemission experiments the
spin polarization is usually measured only in a few k-directions and does
therefore not represent a Fermi surface average.
Superconducting tunnel junctions
The measurement of the spin polarization using a tunnel junction consisting
of a ferromagnetic and a superconducting electrode (see Fig. 7c) was
pioneered by Meservey and Tedrow [40, 44]. The tunneling conductance of
such junction is determined by the convolution of the density of states of
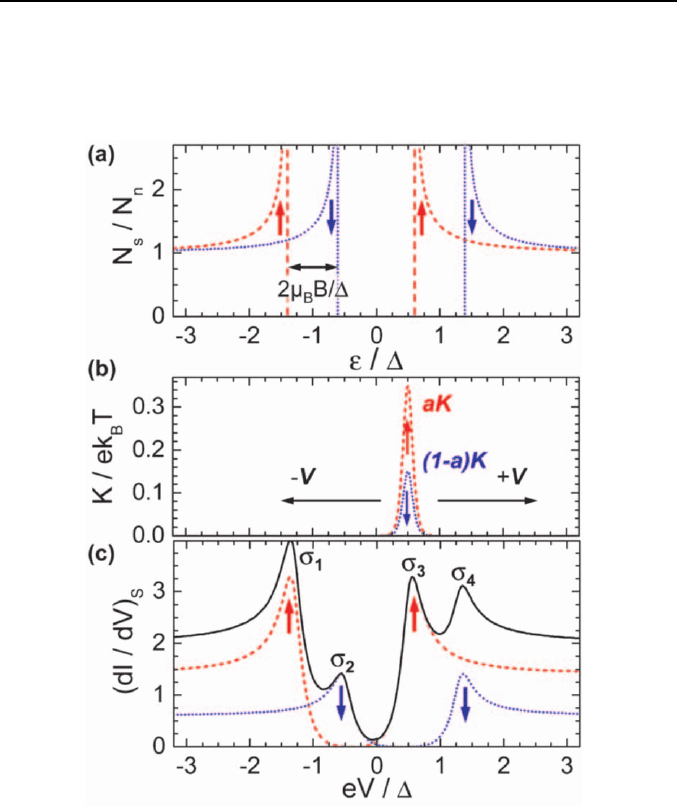
the ferromagnet and the superconductor. As illustrated in Fig. 8, the DOS of
the superconductor has a sharp peak at the gap energy ∆ and is symmetric
for spin-up and spin-down electrons. Applying a magnetic field parallel to
the junction electrodes this peak is spin-split by
±
µ
B
B. The tunneling
conductance for the two spin direction is obtained by convoluting this split
DOS by the function aK and (1-a)K, where
)/(
↓↑↑
+
≡
NNNa
and
)/()(1
↓↑↓
+≡− NNNa
is the fraction of the spin-up and spin-down
electrons at the Fermi level in the ferromagnet and K is the derivative of the
Fermi function with respect to energy. Due to the finite spin polarization of
the ferromagnet we have a ≠ (1-a) and the conductance versus voltage
curves become asymmetric with four peaks at eV = ∆
±
µ
B
B and eV = -∆
±
µ
B
B as shown in Fig. 8. In a first approximation the spin polarization can be
evaluated by measuring the conductance values
σ
1
to
σ
4
at these peak
positions giving [44, 161]
,12=
)()(
)()(
=
3124
3124
−
−+−
−
−
−
aP
σσσσ
σ
σ
σ
σ
(44)
where the second equals sign is only correct, if the tunneling probability is
the same for the spin-up and spin-down electrons. In a more detailed
evaluation the measured conductance spectra have to be fitted to the
theoretical prediction using the spin polarization as fitting parameter and
taking into account spin-orbit and spin-flip scattering [161]. Note that spin-
orbit scattering is smearing out the Zeeman-split density of states while
magnetic impurities act as pair breakers and result in a reduction of the
energy gap.
In practice, usually Al is used for the superconducting
material,
since it has small spin-orbit scattering and its native oxide forms
a very good tunneling barrier. The drawback of this method is that it
requires very low temperatures (typically below 1 K) so that the spin-
Magnetic Tunnel Junctions Based on Half-Metallic Oxides 79
splitting 2µ
B
B of the superconducting DOS peak is larger than k
B
T.
Therefore, it cannot be used to measure the temperature dependence of the
spin polarization. Recent experimental values are listed in Table 1.
Fig. 8. (a) Zeeman splitting of the DOS in a superconductor into spin-up (dashed)
and spin-down part (dotted) due to an applied magnetic field. (b) Energy derivative
K of the Fermi function for a specific voltage multiplied by the fraction a and (1-a)
of the spin-up and spin-down electrons at the Fermi level. (c) Spin-up (dashed) and
spin-down (dotted) conductivity vs voltage as well as the total conductivity (solid
line).
Although early experimental results have been interpreted in terms of
the DOS of the ferromagnetic electrodes, of course this method rather
yields the tunneling spin polarization P
tun
than P
DOS
. Indeed the
experimental values listed in Table 1 show that the spin polarization
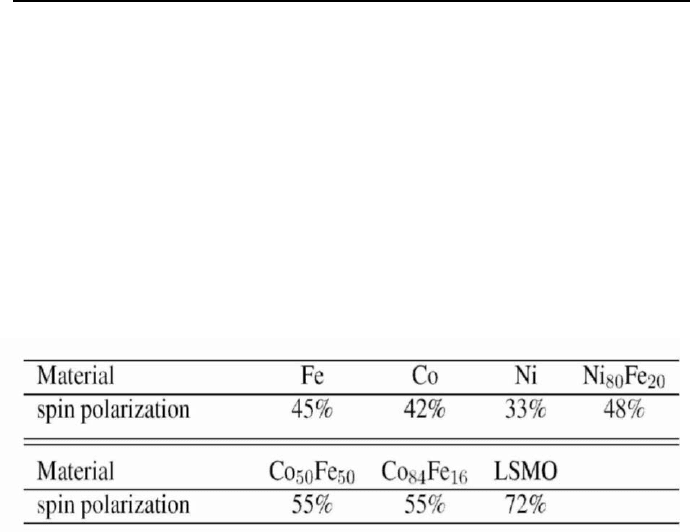
80 Rudolf Gross
determined according to eq. (44) is always positive for 3d transition metals
and alloys in obvious contradiction to band structure calculations [162],
which yield negative values for P
DOS
of Co and Ni. This contradiction was
first addressed by Stearns [74], who pointed out that for 3d transition metals
the tunneling current is dominated by the 4s electrons due to their small
effective mass and in turn large tunneling probability, whereas the DOS is
dominated by the 3d electrons. Taking this into account, a positive
tunneling spin polarization is obtained for Co and Ni in agreement with the
experiment.
with Al/AlO
x
/FM tunnel or Al/SrTiO
3
/FM (for FM = La
2/3
Sr
1/3
MnO
3
(LSMO))
junctions using equation (44) (data taken from Refs. [21, 44, 160, 161]).
Ferromagnetic point contacts. In point contacts or nano-constrictions
between two ferromagnets usually a contact area is formed that allows for
ballistic transport. Therefore, with such configuration the quantity P
Nv
can
be measured over a wide temperature range [163]. The drawback of this
technique is that in most cases there is a finite barrier strength Z or
equivalently a transmission probability T
s
< 1 of the contact. Furthermore,
usually both Z and the relative orientation of the two ferromagnets are not
precisely known. In most experiments this is circumvented by repeated
measurements with many contacts to obtain average results.
Point contact Andreev-reflection. If one part of the point contact is
replaced by a superconductor, the spin polarization of the ferromagnet can
be obtained by point contact Andreev spectroscopy [113, 114]. Andreev
scattering at a normal metal to superconductor (N/S) interface is caused by
the fact that electrons with energies smaller than the superconducting
energy gap cannot enter the superconductor due to the lack of available
states (see Fig. 9a). Therefore, an electron incident on the NS interface from
the N side is retro-reflected as a hole, while at the same time a singlet
Cooper pair formed by two electrons with opposite spin and wave vector is
Point contacts
Table 1. Spin polarization obtained from spin dependent tunneling experiments

Magnetic Tunnel Junctions Based on Half-Metallic Oxides 81
transferred to the superconductor [164]. That is, in total a charge of 2e is
transferred across the NS interface. As a result, the conductance is twice to
that of an N/N interface at bias within the gap. Andreev scattering is a
phase coherent scattering process and is responsible for the proximity effect
at N/S interfaces, where phase correlations are induced in the N material
[165, 166]. The situation changes for a ferromagnetic metal with finite spin
polarization. If P = 1, the Andreev reflection described above is completely
blocked. If an electron with wave vector k and spin-up is incident on the
FM/S interface, it requires an electron with wave vector -k and spin-down
to form a singlet Cooper pair and to enter the superconductor (formally, this
is equivalent to the retro-reflection of a spin-down hole, see Fig. 9b).
However, since for P = 1 such electrons are not available, this process is
blocked and the conductance is zero for bias within the gap [167].
In order to understand Andreev reflection at a FM/S interface we first
consider a FM/N interface. For a clean interface (Z = 0) the conduction at
zero bias, G(0), is given by
,)(=
2
/F
↓↑
+ NN
h
e
G
NM
(45)
that is, the conduction quantum times the number of contributing channels.
For a FM/S interface all the (minority) N
↓
but only the fraction (N
↓
/N
↑
)N
↑
of
the (majority) N
↑
channels contribute to Andreev reflection and transfer the
charge 2e across the interface [113, 114]. This results in the conduction
,4=22=
22
/F
↓↑
↑
↓
↓
+ N
h
e
N
N
N
N
h
e
G
SM
(46)
which would be zero for the situation shown in Fig. 9b. Normalizing to
G
FM/N
, we see that the suppression of the conductance at V = 0 and Z = 0
scales linearly with the spin polarization P of the ferromagnet as
[108, 113, 114]
,)(12=
(0)
/F
/F
P
G
G
NM
SM
−
(47)

82 Rudolf Gross
Fig. 9. Schematic illustration of Andreev scattering at (a) a normal
metal/superconductor (N/S) interfaces and (b) a ferromagnet/superconductor
(FM/S) interface. For simplicity in (b) a half-metal is assumed. The reflection of a
spin-down hole is forbidden in (b) due to lack of available states.
where G
FM/N
is the normal state conductance and the extracted spin
polarization is P = P
Nv
. For large barrier and/or diffusive transport the
extracted spin polarization is P = P
Nv²
in the first approximation. However,
for this case in general a more detailed analysis is required for exact results
[108]. Furthermore, for finite interface scattering (finite barrier strength Z)
the analysis of the experimental data is not as simple and one has to
determine the spin polarization by fitting the experimental conductance
versus voltage curve to model predictions incorporating interface scattering
[168]. Note that point contact Andreev reflection probes
〉〈
2
z
Nv averaged
over the entire Fermi surface, whereas tunneling through a thick barrier is
restricted to a narrow tunneling cone probing
2
z
Nv only at those selected
points of the Fermi surface, where quasimomentum is perpendicular to the
interface. However, the experimental data for the spin polarization obtained
from both methods are usually very close. The reason for that is the use of
polycrystalline materials in the tunneling experiments. Apparently,
averaging over individual grains helps to bring the tunneling spin-
polarization results close to the Fermi surface averaged point contact
Andreev reflection results [169].

Magnetic Tunnel Junctions Based on Half-Metallic Oxides 83
The advantage of the Andreev reflection technique is the detection of
spin polarization for a wider range of materials due to the simple sample
preparation. A large number of experimental results has been reported [167-
176]. Certainly, a drawback of the Andreev scattering method is that it can
be applied only at temperatures lower than the transition temperature of the
superconductor and therefore does not allow to determine the temperature
dependence of the spin polarization. Furthermore, care has to be taken in
the quantitative analysis of experimental data, which should take into
account factors such as an non-spherical Fermi surface [108] as well as the
roughness and size of the point contact leading to a diffusive component of
the transport [177-179].
Ferromagnetic tunnel junctions
For planar tunnel junctions formed by two ferromagnetic electrodes
separated by a thin insulating barrier the spin polarization can be
determined by measuring the resistance for parallel and anti-parallel
magnetization direction according to eqs. (2) and (3). However, as
thickness, defect states etc.). The quantity measured in such experiment is
usually the tunneling spin polarization P
tun
one has to distinguish between junctions with well oriented electrode and
barrier materials and those based on polycrystalline materials. As discussed
probability for junctions with
thick tunneling barrier only those electrons contribute significantly to the
tunneling conductance, which have the
smallest
κ
, i.e. those with k
║
≈ 0.
Therefore, tunneling only probes certain crystallographic directions for oriented
electrode material. A Fermi surface average is only obtained for polycrystalline
electrodes due to the averaging
crystalline tunneling barriers can give rise
to a much higher tunneling spin polarization due to a highly spin-dependent
evanescent decay of the relevant wave functions [61, 62, 78]. That is, for single
crystalline or well a
oriented electrode material one has to take into account the
specific crystallographic orientation of the electrode material with respect to the
tunneling direction, whereas for polycrystalline electrodes one gets an
average over all possible orientations. In general, using planar MTJs it is
difficult to extract reliable information on the intrinsic DOS spin
polarization. However, the tunneling spin polarization obtained in
experiments based on MTJs is the quantity relevant for a large variety of
the electronic states at the interface and the choice of oxide barrier (height,
given by eq. (41). Furthermore,
discussed in the introduction the measured TMR values depend critically on
in the section on the tunneling definition of the spin polarization, due to the
dominating exponential factor in the tunneling
over many grains with different crystallo-
graphic orientation. Furthermore,
