Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

270 Charged Particle and Photon Interactions with Matter
Figure 11.4 shows the transient absorption spectrum of a
TMPA N(SO CF )
+
2 3 2
−
solution of KI
recorded just after excitation by a 230nm light pulse. The spectrum is similar to that obtained pre-
viously by the pulse radiolysis technique, indicating that an electron can be ejected from iodide into
TMPA N(SO CF )
+
2 3 2
−
by CTTS band excitation.
As mentioned above, solvated electrons have not been observed in nanosecond pulse radiolysis mea-
surements on ionic liquids based on the imidazolium cation, instead forming radical species by attach-
ment to an imidazolium cation (Behar etal., 2001; Marcinek etal., 2001), which could potentially lead to
permanent degradation (Shkrob etal., 2007; Shkrob and Wishart, 2009). For this reason, neat imidazo-
lium cation–based ionic liquids might not be suitable for the nuclear fuel cycle process application, but
they could be used in mixtures. Therefore, we investigated the reaction of solvated electrons with Bmim
+
cations in TMPA
+
N(SO CF )
2 3 2
−
in the hope that the rate constant of the reaction would give us a strategy
for designing ionic liquid systems that are stable under irradiation. The rate constant was determined to
be 5.3 × 10
8
M
−1
s
−1
, indicating that the half-life of solvated electrons in neat Bmim
+
N(SO CF )
2 3 2
−
would
be 350ps. In reality, the excess electrons may be scavenged even before they become solvated—femto-
second experiments suggest that the electrons may be scavenged in less than 5ps in imidazolium iodide
ionic liquids (Chandrasekhar etal., 2008). More detailed studies are warranted.
11.4 solvation dynamiCs and reaCtions
o
F p
re-solvated
(
dry)
e
leCtrons
The sections above have discussed radiation- and photon-induced ionization of ionic liquids and the
reactivity of solvated electrons; however, from the radiation chemistry standpoint, one of the major
differences between ionic liquids and conventional solvents is the fact that dynamic timescales are hun-
dreds to thousands of times longer in ionic liquids (Giraud etal., 2003; Holbrey etal., 2004; Castriota
etal., 2005; Shirota and Castner, 2005; Shirota etal., 2005, 2007; Arzhantsev etal., 2007; Jin etal.,
2007). Moreover, the response of an ionic liquid to the introduction of an excess electron is subject to
static and dynamic heterogeneities that depend on its composition. Although the initial stages of solvent
response involve inertial motions that operate on very short timescales, even in ionic liquids, the slower
response components scale with the viscosity of the medium. Consequently, it takes much longer to sol-
vate an excess electron in an ionic liquid as compared to normal liquids, and the mobility and reactivity
of pre-solvated electron states become much larger determinants of radiolysis product yields, as a result.
10
8
6
4
2
0
400 600 800
230 nm exc.
2.1 mM
Kl in TMPA-TFSI
1000
Wavelength (nm)
Absorbance (10
–3
)
1200 1400 1600
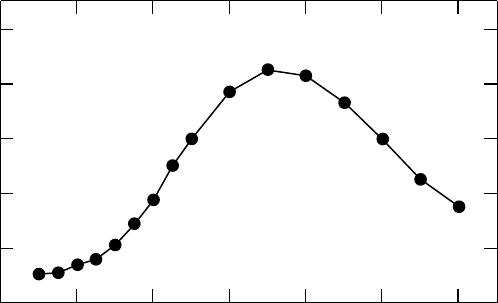
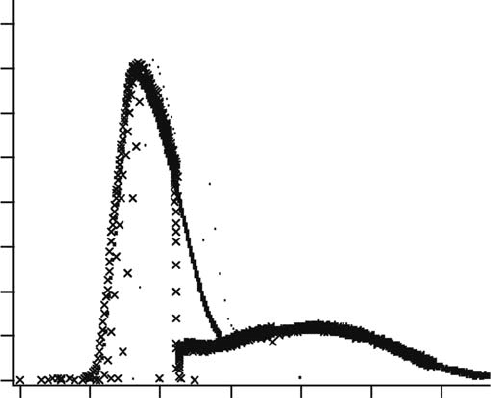
Figure 11.4 Transient absorption spectrum of the solvated electron in
TMPA N(SO CF )
+
2 3 2
−
by electron
photodetachment.
(Reproduced from Katoh, R. etal., J. Phys. Chem. B, 111, 4770, 2007. With permission.)
Radiation Chemistry and Photochemistry of Ionic Liquids 271
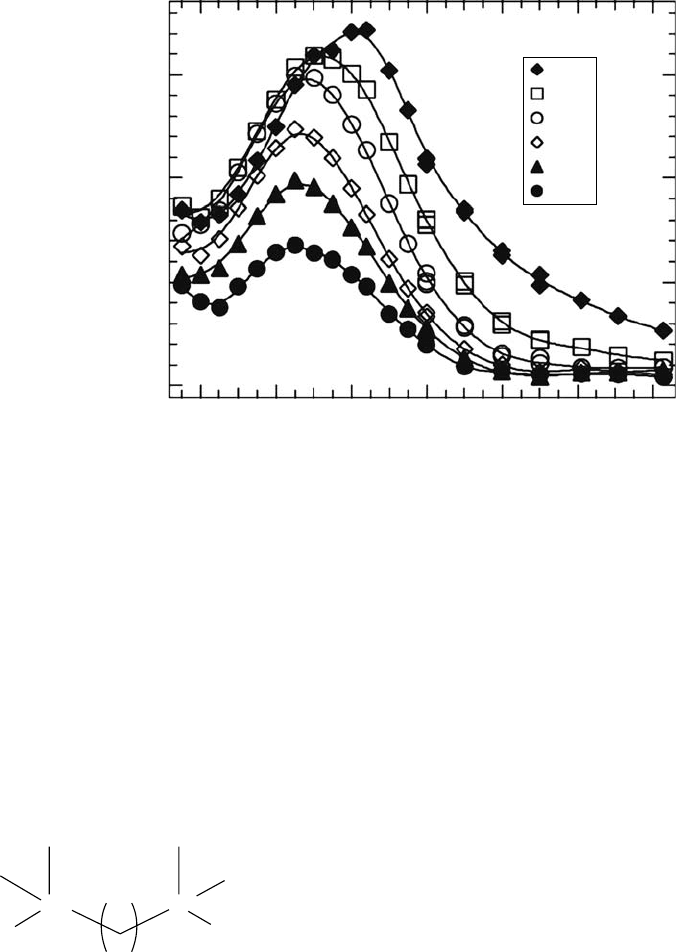
An extreme example of the slow response of ionic liquids is depicted in Figure 11.5, where
the transient spectra of the excess electron in the highly viscous alcohol-functionalized ionic liq-
uid
SH8 (N(SO CF ) )
2+
2 3 2 2
−
at different times following the electron pulse are shown (Wishart etal.,
2005). The molecular structures of the alcohol-functionalized ionic liquids in question are shown
in Figure 11.6. The spectrum at 2ns has a shoulder in the range of 1200nm characteristic of an
electron solvated in an alkane ionic liquid environment. Over the course of tens of nanoseconds,
the shoulder disappears and the remaining spectrum peaks at 650nm, characteristic of an electron
solvated by alcohol functionalities. The average electron solvation time constants in methanol, etha-
nol, and ethylene glycol are about 2–9 ps and that of viscous 1,2,6-trihydroxyhexane (2490 mPa s)
is ∼1 ns, while in the viscous ionic liquid alcohols
SH6 (N(S O CF ) )
2+
2 3 2 2
−
and SH8 (N(SO C F ) )
2+
2 3 2 2
−
(viscosities 6860 and 4570mPa s, respectively), the observed time constants for absorption decay
are 25ns (SH8
2+
) to 40ns (SH6
2+
). In the case of these ionic liquid
alcohols, dynamic hindrance of hydroxypropyl side chain reorienta-
tion imposed by the electrostatic constraints of the ionic lattice plays
the
greatest role in slowing the electron solvation process.
A
more typical example involves the popular ionic liquid N-butyl-
N-methylpyrrolidiniumbis(triuoromethanesulfonyl)imide(bmpyrr
+
N(SO CF )
2 3 2
−
), which has a relatively low viscosity (94mPa s at
20°C) and a wide electrochemical window. Picosecond pulse-probe
transient absorption spectroscopy was performed at Brookhaven
National Laboratory’s (BNL) Laser-Electron Accelerator Facility
(Wishart etal., 2004) to observe the temporal blueshift of the excess
electron spectrum associated with the solvation process in bmpyrr
+
N(SO CF )
2 3 2
−
. The spectral data, measured from 600 to 1600nm
with a time resolution of 15ps, extending to 10 ns, showed a strong
absorption around 1400 nm at short time intervals that shifted to the
equilibrated spectrum of the solvated electron peaking at 1100nm.
Factor analysis of the data showed a multiexponential or distributed
15
10
5
0
400 600 800 1000
2 ns
SH8 (NTf
2
)
2
15 ns
50 ns
200 ns
400 ns
800 ns
Wavelength (nm)
Absorbance×10
3
1200 1400 1600
Figure 11.5 Transient spectra of solvated electron in an alcohol-functionalized ionic liquid
SH8 (N(SO CF ) )
2+
2 3 2 2
−
. (Reproduced from Wishart, J.F. et al., Radiat. Phys. Chem., 72, 99, 2005. With
permission.)
NN
+
+
Rn
R
SAn: R = CH
2
CH
2
CH
2
CH
3
SEn: R = CH
2
CH
2
OCH
2
CH
3
SHn: R = CH
2
CH
2
CH
2
OH
Figure 11.6 Molecularstruc-
ture of alcohol-functionalized
ionic liquids. (Reproduced from
Wishart, J.F. et al., Radiat.
Phys. Chem., 72, 99, 2005. With
permission.)
272 Charged Particle and Photon Interactions with Matter
solvation response with an average solvation time of 260ps (Wishart etal., manuscript in prepara-
tion). In comparison, the average solvation time for the uorescent solvation probe counarin-153 is
350ps in the same ionic liquid (Funston etal., 2007). Electron solvation dynamics in ionic liquids
is often difcult to measure because of the need to exchange (ow) the sample to avoid the accu-
mulation of radiolytic damage. Solvatochromic dye solvation measurements, which are much more
extensively studied in ionic liquids (Arzhantsev etal., 2007; Funston etal., 2007; Jin etal., 2007),
are a useful proxy for estimating electron solvation times.
Since electron solvation processes in normal liquids occur on the time scale of picoseconds, excess
electrons in ionic liquids can persist hundreds to thousands of times longer in mobile, pre-solvated
states with reactivity proles that differ from fully solvated states. As already shown in Figure 11.3,
the solvated electron yield near time zero decreased with the concentration of added electron scav-
enger. In water and most other molecular solvents, such decreases in the “initial” yield cannot be
observed in nanosecond pulse radiolysis measurements. This decreased yield is due to scavenging
of the pre-solvated electron by the solute (Lam and Hunt, 1975; Jonah etal., 1977, 1989; Glezen et al.,
1992; Lewis and Jonah, 1986). For each solvent and solute, the efciency of pre-solvated electron
scavenging can be quantied by the constant C
37
dened as follows:
G
G
c
C
c
37
exp
0
=
−
(11.1)
where
G
c
is the yield of solvated electrons at a given scavenger concentration c
G
0
is the yield in the absence of scavenger
C
37
is the concentration where only 1/e (37%) of the electrons survive to be solvated
Examples of C
37
values and reaction rate constants of solvated electrons with scavengers
k( )
s
e
−
are
listed in Table 11.1. In
MeBu N N(SO CF )
3
+
2 3 2
−
, the values of C
37
for benzophenone, pyrene, and
phenanthrene are 0.062, 0.063, and 0.084M, respectively. The C
37
value for Bmim
+
is 0.057M in
TMPA N(SO CF )
+
2 3 2
−
, suggesting that the pre-solvated electron reacts with Bmim
+
at least as
efciently
as with the aromatics.
It
is reported that C2-alkylation of imidazolium cations is effective in extending the electro-
chemical redox windows of the imidazolium-based ionic liquids (Hayashi et al., 2005), suggest-
ing that C2-alkylation may reduce the reaction rate with solvated electrons. The solvated electron
table 11.1
rate
Constants and C
37
values for the reactions
of
s
olvated
and p
re-solvated
electrons
with
s
everal
s
cavengers
ionic liquid scavenger
k( )
s
e
−
(m
−1
s
−1
) C
37
(m)
MeBu
3
N
+
N(SO CF )
2 3 2
−
Benzophenone (1.6 ± 0.1) × 10
8
0.062
MeBu
3
N
+
N(SO CF
2 3
)
2
−
Pyrene (1.7 ± 0.1) × 10
8
0.063
MeBu
3
N
+
N(SO CF )
2 3 2
−
Phenanthrene (1.3 ± 0.1) × 10
8
0.084
MeBu
3
N
+
N(SO CF )
2 3 2
−
Indole (4.3 ± 0.3) × 10
7
0.22
TMPA
+
N(SO CF )
2 3 2
−
Bmim
+
5.3 × 10
8
0.057
TMPA
+
N(SO CF )
2 3 2
−
EDmim
+
3.8 × 10
8
0.081
TMPA
+
N(SO CF )
2 3 2
−
BDmim
+
3.0 × 10
8
0.13
TMPA
+
(N(SO CF )
2 3 2
−
HDmim
+
3.0 × 10
8
0.12
Radiation Chemistry and Photochemistry of Ionic Liquids 273
capture rate constants and C
37
values for N-ethyl-2,3-dimethylimidazolium (EDmim
+
), N-butyl-2,3-
dimethylimidazolium (BDmim
+
), and N-hexyl-2,3-dimethylimidazolium (HDmim
+
) are summarized
in Table 11.1 (Takahashi etal., 2008). For each of the C2-alkylated imidazolium cations, there is only
a slight reduction of the rate constant compared to Bmim
+
. This is quite reasonable because the rate
constants are considered to be diffusion limited. On the other hand, the C
37
values for BDmim
+
and
HDmim
+
are signicantly larger than that for non-alkylated imidazolium Bmim
+
. These results have
interesting implications for the reaction mechanisms of pre-solvated and solvated electron capture and
the nature of the electron–cation adduct, which may suggest a strategy for designing ionic liquids that
are more stable under irradiation.
11.5 photoexCitation oF solvated eleCtrons
There have been several studies for dielectrons in molten salts and solid salts, such as KCl (Lynch
and Robinson, 1968). A recent ab initio simulation for electrons in LiF (Zhang etal., 2008) also
suggested the presence of two types of dielectrons, namely, the singlet dielectron and the triplet
dielectron. The stabilization of two electrons in one trap site is considered to be due to not only
electrostatic interactions but also through hole–orbital coupling among solvent molecules (Zhang
et al., 2008). Because ionic liquids can be considered to have similar characteristics as molten
salts, we can postulate the presence of the dielectron in ionic liquids. To examine this possibility,
an experiment was performed in which the solvated electron was excited by light pulses at 532 and
1064 nm. If the dielectron exists, photoexcitation might produce two solvated electrons, which could
be detected as an increase in the absorption signal in the near-infrared wavelength region. The sol-
vated electrons were produced by electron photodetachment from iodide in an ionic liquid solution
by a 248nm KrF excimer laser pulse, as described in Section 11.3. After a variable delay, a 532nm
Nd-YAG
laser pulse irradiated the sample (Takahashi etal., 2009b).
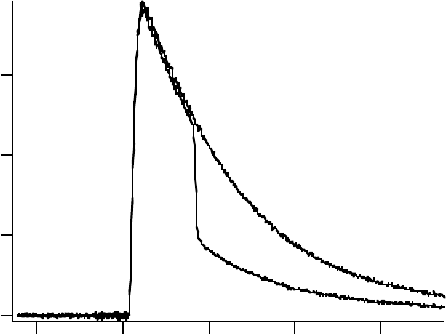
Figure
11.7 shows a transient absorption signal observed at 1000nm. When a 532nm laser pulse
was used to irradiate the sample during the lifetime of the solvated electron, the transient absorp-
tion signal displayed an instrument-limited decrease, and the decrease of absorption did not recover
within the lifetime of the solvated electron. Therefore, a fraction of the solvated electron population
was permanently removed by a 532 nm excitation. Transient absorption signals were monitored at
several different wavelengths, and a similar permanent bleaching was observed at each wavelength.
0.3
0.2
0.1
0.0
OD
6004002000–200
Time (ns)
1000 nm
Figure 11.7 Transient absorption signals observed in
TMPA N(SO CF )
+
2 3 2
−
at 1000nm with and without a
532nm pulse. (Reproduced from Takahashi, K. etal., Radiat. Phys. Chem., 78, 1129, 2009b. With permission.)
274 Charged Particle and Photon Interactions with Matter
The above results suggest that a portion of the solvated electrons react and disappear during the
532nm laser pulse. One possible explanation may be the reaction of the dry electron with its parent
iodine
atom via the following reaction scheme:
I I
nm
p
− −
→ +
hν( )
e
248
(11.2)
e e
p s
− −
→
(11.3)
e (e )*
( )
s
nm
s
− −
→
hν 532
(11.4)
(e )*
s
I I
− −
+ →
(11.5)
I I I
− −
+ →
2
(11.6)
I I I I
2 2 3
− − − −
+ → +
(11.7)
where
e
p
−
is the pre-solvated electron
e
s
−
is the solvated electron
(e
)*
s
−
indicates the electron after excitation with a 532nm light pulse
If permanent bleaching of the solvated electron takes place due to the above reaction scheme, it may
be possible to observe a decrease in
I
2
−
concentration, because the iodine atoms would disappear
on reacting with
( )e *
s
−
. Therefore, the transient absorption of
I
2
−
was measured with a wide time
window. The absorption spectrum of
I
2
−
in TMPA-NTf
2
is similar to that in water, with absorption
maxima located at 400 and 740 nm. The evolution of the absorption signal from
I
2
−
was monitored at
830nm. At this wavelength, both the
I
2
−
species and also the reactivity of solvated electrons can be
observed. The results are shown in Figure 11.8. In the early time regime (t < 500ns), the absorption
signal occurs mostly due to the solvated electron. After the decay of the solvated electron, a slow
buildup of
I
2
−
was seen. As shown in Figure 11.8, when the sample was irradiated by a 532nm laser
pulse approximately 200 ns after the production of the solvated electron, a permanent bleaching was
observed; however, in the microsecond regime, where the absorption is mainly due to
I
2
−
, there was
no signicant change in the absorption. It can be concluded that the iodine atom is not a reaction
partner of the excited electrons, since the yield of
I
2
−
at longer time periods remains unchanged.
Although at the present time we cannot identify the reaction mechanism(s) for the photo-induced
bleaching of solvated electrons with a 532nm light pulse, we can gain some insight from the previ-
ous work done on water and alcohols (Silva etal., 1998; Yokoyama etal., 1998; Kambhampati etal.,
2001). In these studies, pump-probe spectroscopy of the solvated electron in water, methanol, and
ethanol was studied with a 300fs time resolution. At low pump power, the observed dynamics were
assigned to s → p excitation and subsequent relaxation of the localized solvated electron. In contrast,
at high pump power, two-photon absorption produces mobile conduction band electrons, which are
subsequently trapped and relax at a resonant site far away from the initial equilibrated electron site.
Interestingly, in the two-photon excitation, they observed an ultrafast proton-transfer reaction from
the alcohol molecules, resulting in a permanent bleach. Although we cannot conclusively say that we
are observing the same process, we tentatively conclude that the excitation of the solvated electron
results in the generation of a reactive dry electron or pre-solvated electron in the ionic liquid.
Radiation Chemistry and Photochemistry of Ionic Liquids 275
11.6 radiCal reaCtions and kinetiC salt eFFeCts
11.6.1 reactionS between diiodide anion radicalS in ionic liQuidS
Ionic liquids are nding increasing uses in electrochemical devices, such as electric double-layer
capacitors, lithium batteries, fuel cells, and dye-sensitized solar cells (DSSCs). In DSSCs, a photo-
oxidized dye molecule must be reduced by a redox couple (Papageorgiou etal., 1996; Wang etal.,
2004). The
I /I
− −
3
redox couple has been used for this purpose and plays an important role in charge
(hole) transport in DSSCs. The diffusion of ions in ionic liquids is generally slow because of the high
viscosity of ionic liquids, and this fact is a disadvantage when an ionic liquid is used as an electro-
lyte. Recently, using an ultramicroelectrode technique, it has been proposed that in the
I /I
− −
3
redox
couple system, a Grotthuss-like mechanism involving the exchange of I
2
between
I
3
−
and I
−
is respon-
sible for efcient hole transport in ionic liquid electrolytes for DSSCs (Kawano and Watanabe, 2003,
2005). The exchange reaction would occur by the following process:
I I I I I I I
2
− − − − − −
+ → → +
3 3
(11.8)
To have this type of exchange take place, I
−
and
I
3
−
must be in close proximity. However, the close
approach of I
−
and
I
3
−
is hindered in molecular solvents because of Coulombic repulsion between the
two ions. On the other hand, the ions of an ionic liquid may weaken the electrostatic repulsion by
Coulombic shielding, allowing I
−
and
I
3
−
to approach each other without a large Coulombic energy
barrier. The effect of Coulombic shielding was examined through the reaction between two diiodide
(
I
2
−
) anion radicals. The
I
2
−
species can be formed by the 248nm laser photolysis of iodide through
the
following reactions:
I I
s
− −
+ → +hν e
(11.9)
0.16
0.14
0.12
0.10
0.08
0.06
Absorbance
0.04
0.02
0.00
10
–9
10
–8
10
–7
10
–6
Time (s)
10
–5
10
–4
10
–3
Figure 11.8 Time courses of the transient absorbance at 830nm after 248nm excitation of KI in
TMPA N(SO CF )
+
2 3 2
−
with (cross symbols) and without (dots) a subsequent 532nm pulse. The 532 nm pulse
was delayed by 200ns after the 248 nm pulse. (Reproduced from Takahashi, K. etal., Radiat. Phys. Chem.,
78,
1129, 2009b. With permission.)

276 Charged Particle and Photon Interactions with Matter
I I I+ →
− −
k
2
2
(11.10)
I I I I
2 2 3
3
− − − −
+ → +
k
(11.11)
In molecular solvents, the diffusion-controlled rate for an ionic reaction can be calculated by the
Debye–Smoluchowski
equation (Debye, 1942):
k R D f
diff ab ab
= 4π ε( ) (11.12)
f
r R
r R
( )
/
exp /
ε =
( )
−
c ab
c ab
1
(11.13)
r
Z Z e
k T
c
a b
B
=
2
0
4πε ε
(11.14)
where
R
ab
is the reaction distance of the closest approach
D
ab
is the relative diffusion coefcient of reactants
f(ε)
is the so-called Debye correction factor
e is the charge on the ions
k
B
is Boltzmann’s constant
ε
0
is the permittivity of free space
ε
is the dielectric constant
T is the absolute temperature
If
the diffusion coefcient can be expressed by a simple hydrodynamic model, that is, by the
Stokes–Einstein equation, D
a
= k
B
T/(6πηR
a
), the diffusion-limited rate constant (Equation 11.12)
may
be written as follows:
k
RTf
diff
=
8000
3
( )ε
η
(11.15)
where
η
is the viscosity (in Pa s)
R
is the gas constant (8.3144
J
K
−1
mol
−1
)
The Debye correction factors, f(ε), for H
2
O, MeOH, and EtOH were calculated from the dielectric
constants of the solvents, and are listed in Table 11.2. The Debye correction factor for an aqueous
solution was calculated to be 0.52, whereas the values for methanol and ethanol were calculated
to be 0.18 and 0.091, respectively, indicating that the Coulombic repulsion between the
I
2
−
radical
anions in the alcohol solvents is greater than that in water. These calculated Debye correction fac-
tors predict that the reaction between
I
2
−
anion radicals in the alcohol solvents is much slower than
that in water. As indicated in Table 11.2, the reaction rate constants in MeOH and EtOH are four to
ve
times lower than in water.
The
decay rate constants, k
3
, for reaction 11.11 in the molecular solvents and the ionic liquids
are plotted in Figure 11.9 as a function of the reciprocal of viscosity. The solid line in Figure 11.9
isthe plot for the diffusion-limited rate constants calculated from Equation 11.15 with f(ε) = 1,
that is, the calculations for reaction between neutral molecules (denoted as k
0
). For the molecular
solvents, the k
3
/k
0
ratios for H
2
O, MeOH, and EtOH are 0.37, 0.064, and 0.086, respectively, reect-
ing the importance of electrostatic repulsion between the diiodide anion radicals. In contrast, the
reaction rate constants for the ionic liquids are very close to the solid line. The k
3
/k
0
ratios for the
Radiation Chemistry and Photochemistry of Ionic Liquids 277
table 11.2
solvent
p
roperties
and r
ate
Constants for the b
imolecular
r
eactionbetween
I
2
−
anion radicals
no. solvent η
a
Ɛ
k
0
b
k
3
c
f(Ɛ)
d
k
3
/k
0
1 H
2
O 0.89 78.4 7.4 × 10
9
(2.8 ± 0.2) × 10
9
0.52 0.37
2 MeOH 0.55 32.7 1.2
× 10
10
(7.7 ± 0.5) × 10
8
0.18 0.064
3 EtOH 1.01 24.6 6.1
× 10
9
(5.3 ± 0.7) × 10
8
0.091 0.086
4 40%
Gly
e
3.1 68 2.1 × 10
9
(9.1 ± 0.8) × 10
8
0.47 0.43
5 60%
Gly
e
8.6 61 7.7 × 10
8
(3.0 ± 0.2) × 10
8
0.43 0.37
6 70%
Gly
e
17 56 3.8 × 10
8
(1.7 ± 0.3) × 10
8
0.39 0.44
7 80%
Gly
e
43 51 1.5 × 10
8
(6.8 ± 0.9) × 10
7
0.35 0.45
8 TMPA
+
TFSI
−f
69 9.6 × 10
7
(1.1 ± 0.2) × 10
8
1.15
9 P13
+
TFSI
−f
55 1.2 × 10
8
(1.3 ± 0.2) × 10
8
1.08
10 P14
+
TFSI
−f
69 9.6 × 10
7
(9.1 ± 0.5) × 10
7
0.95
11 PP13
+
TFSI
−f
135 4.9 × 10
7
(6.6 ± 0.4) × 10
7
1.35
12 DEME
+
TFSI
−f
63 1.0 × 10
7
(1.2 ± 0.5) × 10
8
1.14
13
DEME
+
BF
4
−
300 2.2 × 10
7
(4.0 ± 0.7) × 10
7
1.82
Source: Data
from
Takahashi,
K. etal., J. Phys. Chem. B, 111, 4807, 2007.
a
Viscosity, in mPa s.
b
Calculated diffusion-limited rate constant with f(ε) = 1, in M
−1
s
−1
.
c
Experimental rate constant for reaction 3, in M
−1
s
−1
.
d
Debye correction factor.
e
Glycerol–H
2
O mixture.
f
TFSI
−
=
N(SO CF )
2 3 2
−
.
10
10
10
9
10
8
10
7
0.001 0.01
13
11
8
12
9
10
7
6
5
4
3
2
1
1/viscosity (mPa
–1
s
–1
)
k (M
–1
s
–1
)
0.1 1
2
4
6
2
2
4
4
6
6
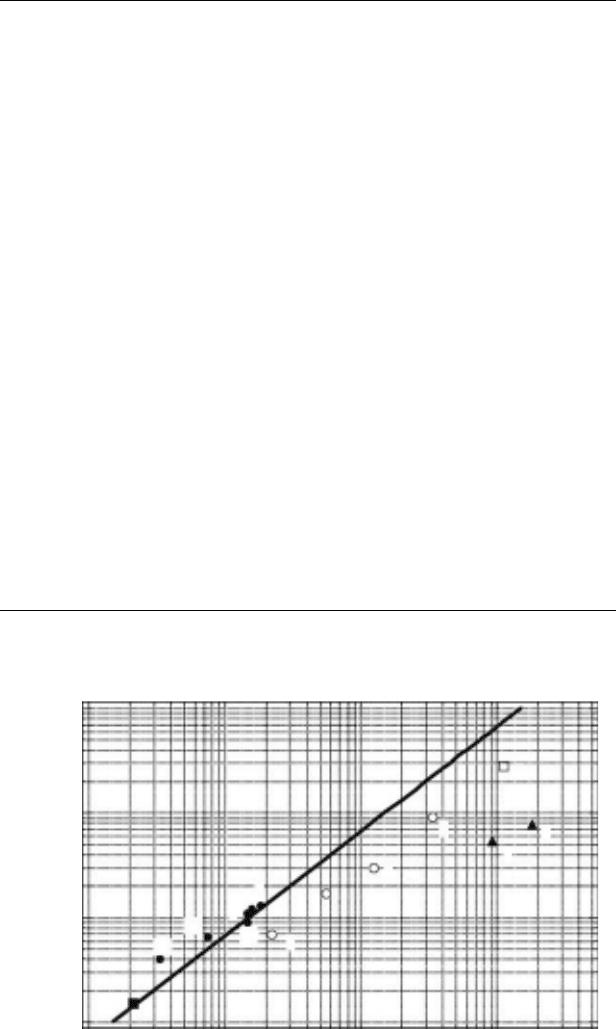
Figure 11.9 Plots of rate constants for reaction between
I
2
−
anion radicals in molecular solvents and ionic
liquids as a function of the inverse of viscosity: (◻) H
2
O, (▴) alcohols, (⚬) glycerol–H
2
O mixtures, (⦁) ionic
liquids. The numbers correspond to the numbers in Table 11.2. The lled square is data for reaction between
Br
2
−
anion radicals in an ionic liquid. The line plots the calculated diffusion-limited rate constants for reaction
between neutral molecules. (Reproduced from Takahashi, K. etal., J. Phys. Chem. B, 111, 4807, 2007. With
permission.)
278 Charged Particle and Photon Interactions with Matter
ionic liquids (except
PP13 N(SO CF )
+
2 3 2
−
and
DEME BF
+
4
−
) are almost unity (Table 11.2), indicating
that the Debye factors, f(ε), for the ionic liquids are close to unity and that the electrostatic repulsion
between
the diiodide anion radicals is unimportant.
The
coincidence of the experimental rate constants of
I
2
−
in the ionic liquids with those calcu-
lated based on the viscosity was, to a certain extent, unusual. In previous pulse radiolysis experi-
ments on the reaction of butylpyridinyl radical with duroquinone (Skrzypczak and Neta, 2003), the
experimental reaction rates deviated signicantly from calculated values based on viscosities with
the simple hydrodynamic assumption. In that work, the experimental rates were almost an order of
magnitude higher than the values estimated from the viscosities of the ionic liquids. Skrzypczak
and Neta (2003) suggested that this difference was due to the voids that exist in ionic liquids and the
possibility that reacting species diffuse through the movement of small groups of ions, whereas vis-
cosity is related to simultaneous movement of all ions. Furthermore, Maroncelli etal. have pointed
out the limitation of the continuum approximation of solvents and the importance of considering
the molecularity of solvents in solvation dynamics (Maroncelli and Fleming, 1987; Papazyan and
Maroncelli, 1995; Ito etal., 2004). The reason the simple hydrodynamic model can predict the pres-
ent results for the reaction between
I
2
−
anion radicals could be due to the relatively simple molecular
shape of the diiodide anion radicals. However, for the reaction of
I
2
−
in
PP13 N(SO CF )
+
2 3 2
−
and
DEME BF
+
4
−
, the agreement between the measured reaction rate and the calculated rate was not
good. These results could reect a limitation of the Stokes–Einstein approximation and indicate the
importance
of considering the molecularity of ionic liquids.
The
diffusion coefcients of
I
2
−
anion radicals in ionic liquids have been measured recently by
Kimura and Nishiyama by the transient grating method (Nishiyama etal., 2009). The diffusion
coefcients of
I
2
−
, calculated rate constants by the Smoluchowski and Debye–Smoluchowski treat-
ments, and the experimental rate constants for comparison are listed in Table 11.3. An important
point is that the calculated values by the Smoluchowski equation, k
(Smoluchowski)
, are not consistent
with the experimental rate constants in the molecular solvents, while the calculated values by the
Smoluchowski equation are nearly consistent with the experimental rate constants in ionic liquids.
These results suggest that the Coulombic screening effect efciently shields the electrostatic inter-
action
between the
I
2
−
anion radicals in ionic liquids.
Kimura and Nishiyama also compared the measured diffusion coefcients of
I
2
−
with the calcu-
lated values from the Stokes–Einstein relation (Nishiyama etal., 2009). The calculated diffusion
coefcients in
TMPA N(SO CF )
+
2 3 2
−
and
PP13 N(SO CF )
+
2 3 2
−
are 1.2 and 0.62 × 10
11
m
2
s
−1
, respec-
tively, whereas the measured values are 2.5 and 1.17 × 10
11
m
2
s
−1
, respectively. Therefore, the hydro-
dynamics approximation predicts a smaller value by a factor of 1/2 than the measured value in
ionic liquids. Hence, we can say again that the viscosity is not a measure for the diffusion rate of
molecules
in ionic liquids.
table 11.3
diffusion
Coefcients of
I
2
−
and Calculated rate Constants in
molecular
s
olvents
and i
onic
l
iquids
solvent
D
I
2
a
k
(smoluchowski)
b
k
(debye–smoluchowski)
b
k
(exp)
b
Methanol 120 550 100 77
Ethanol 79 380 35 53
TMPA
+
N(SO CF )
2 3 2
−
2.5 12 0.036 11
PP13
+
N(SO CF )
2 3 2
−
1.17 5.6 0.017 6.6
Source: Data
from Nishiyama,
Y.
etal., J. Phys. Chem. B, 113, 5188, 2009.
a
The unit is 10
–11
m
2
s
–1
.
b
The unit is 10
7
M
–1
s
–1
.
Radiation Chemistry and Photochemistry of Ionic Liquids 279
11.6.2 kinetic Salt effectS in ionic liQuid/Methanol MixtureS
It is well known that the addition of salt increases or decreases the rates of ionic reactions,
depending on whether the reactant charges are like or unlike each other. These effects have been
treated with Debye–Hückel theory, although the applicable ion concentration range is limited to
about 0.01mol L
−1
. In contrast, the concentrations of ions in ionic liquids may be in the range of
2–5molL
−1
. Therefore, ionic reactions under such high ionic strength conditions are of interest. In
Section 11.6.1, the disproportionation of diiodide anion radicals
I
2
−
in ionic liquids was discussed.
In this section, we examine the kinetic salt effect on the reaction between diiodide anion radicals in
methanol using a wide concentration of ionic liquid salts. To extract the specic salt effect of ionic
liquids in organic solvents, we also used the inorganic salt lithium bis(triuoromethylsulfonyl)
imide (
Li N(SO CF )
+
2 3 2
−
) for comparison. Using ionic liquids, it is possible to examine the salt effect
under
very high mole fraction conditions (Takahashi etal., 2009a).
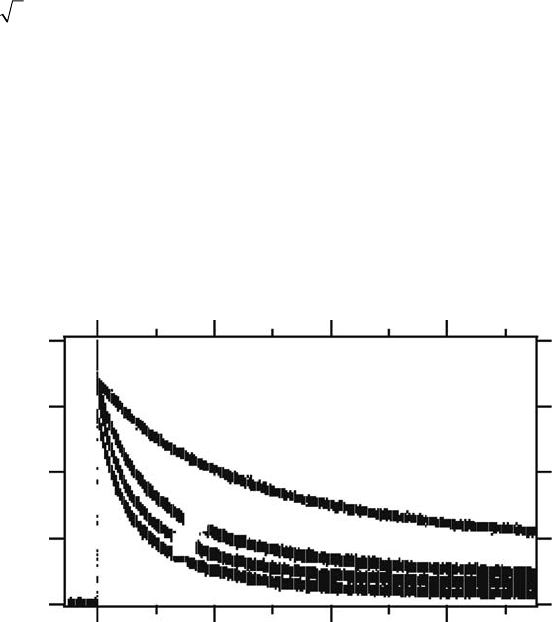
Figure
11.10 shows examples of transient absorption signals at 700nm with different concen-
trations of
TMPA N(SO CF )
+
2 3 2
−
in methanol. These decay signals correspond to the dispropor-
tion reaction of
I
2
−
. As the concentration of TMPA N(SO CF )
+
2 3 2
−
increases, the decay rate becomes
faster, indicating that the addition of the
TMPA N(SO CF )
+
2 3 2
−
salt accelerates the reaction rate
between
I
2
−
anion radicals. Because the
I
2
−
anions are expected to be surrounded by TMPA
+
cations,
the Coulombic repulsion between the
I
2
−
anion radicals is screened. In Figure 11.11, log(k/k
0
) was
plotted against the square root of the ionic strength, I, for three different salts, where k and k
0
are
rate constants with and without salt, respectively. At low ionic strength, the values of log(k/k
0
) are
proportional to
I
, indicating that the Debye–Hückel limiting law could be applicable. However,
as the ionic strength increases, the plots deviate from a straight line. As can be seen in Figure 11.11,
the effect of ionic strength on the rate constants depends on the kind of salts used. Because these
salts are composed of the common anion
NTf
2
−
, the difference in the effect of ionic strength can
be attributed to specic effects of the cations. At low ionic strength, the TMPA
+
cation increases
the reaction rate most effectively, while the Li cation is less effective. There are a few possibilities
to explain this result, such as screening effects, reduction of
I
2
−
anion mobility, and electrolyte
ion association. At high salt concentrations, it can be expected that the degree of dissociation of
Li N(SO CF )
+
2 3 2
−
is lower than for ionic liquids; hence, the kinetic salt effect may be less effec-
tive in
Li N(SO CF )
+
2 3 2
−
than in ionic liquids. The screening effect on the electrostatic repulsion
between
I
2
−
anions may depend on the size of the cations. A larger cation could effectively screen
30
40×10
–3
20
10
0
0 40
(a)
(b)
(c)
(d)
80
Time (μs)
Absorbance
120
Figure 11.10 Transient absorption signals at 700nm with different concentrations of
TMPA N(SO CF )
+
2 3 2
−
in
methanol. (Reproduced from Takahashi, K. etal., Chem. Lett., 38, 236, 2009a. With permission.)
