Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

310 Charged Particle and Photon Interactions with Matter
with the published experimental and theoretical studies discussed above. The changes observed
in the 0–50 ps timescale are supported according to the authors by ultrafast photolysis experi-
ments that also show an initial decay assigned to a “dispersive dynamics of ultrafast radicals
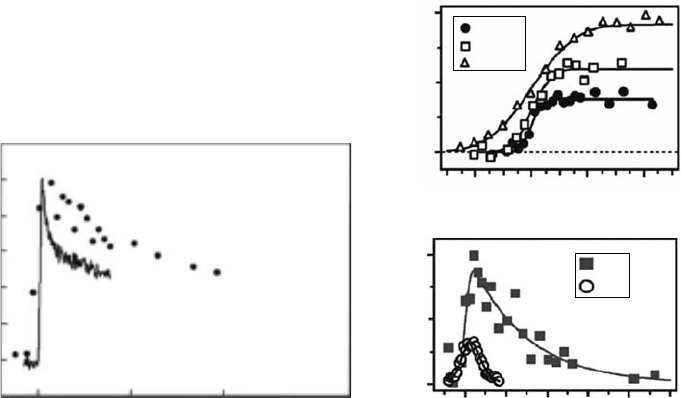
events.” The results obtained by Oulianov etal., (2007) are also reported in Figure 12.17 (right):
in contrast, as expected for the solvated electron, a quasi-at absorption signal is observed during
the rst tens of picoseconds. To obtain these results, the authors conducted the experiments with
10 Hz LWA systems, and combined their pulse-probe setup with the measurement of hydrated
electron decay at the nanosecond timescale as a normalization source to correct the dose uc-
tuations shot to shot. Theauthors also outline the difculty to keep this normalization constant
during the series of experiments because of laser pointing instabilities.
12.4.2.2 solvent
e
ffect
on the t
ime-dependent
G-
value
of s
olvated
electron
Tetrahydrofuran
(THF) is an aprotic solvent that is largely used in organic chemistry. Considering
the spurs reactivity, the interest of THF comes from its dielectric constant that is 10 times lower
than that of water. In THF, the Coulombic eld is very strong and favors long-range interactions
between charged reactive centers. It is also known that ionized THF formed by irradiation is
rapidly deprotonated and that solvated electrons can recombine with both the proton adduct and
the residual C-centered radical (Salmon etal., 1974). Based on a systematic study of solvated
electron scavenging by methyl bromide and ethyl bromide with different concentrations in THF,
it was concluded (Kadhum and Salmon, 1984) that the initial formation yield of solvated electron
in THF is around (4.2 ± 0.4) ×10
−7
mol J
−1
and that, after spur reactions, the value is decreased to
(0.36 ± 0.02) × 10
−7
mol J
−1
at microsecond timescale (or (0.4 ± 0.02) × 10
−7
mol J
−1
depending on
data treatment). The transient absorption signals of solvated electron in water and THF recorded
at 790 nm after radiolysis by a picosecond electron pulse are reported in Figure 12.18 (De Waele
et al., 2006, 2007). The ratio between the initial absorbance (at 30 ps) and the absorbance at
0
0
1
Normalized signal
25 50
Time (ps) Time (ps)
Time (ps)
ΔOD ΔOD
Low energy radiation
(UV photons: 2 × 4 eV)
Pure liquid water
294 K
Femtolysis
high energy radiation
(relativistic electrons)
10×10
–3
0
15×10
–3
–20(a)
(b)
0 20
2000 400
1 M
5 M
10 mm
5 mm
2 mm
40
0
Probe:820 nm
Figure 12.17 Pulse-radiolysis experiments performed using relativist electron bunches generated by laser-
wakeeld acceleration as irradiation beam and a laser pulse around 800 nm as a probe beam. Left: comparison
of the decays of solvated electrons formed by two-photon ionization, and pulse radiolysis of water. (Reprinted
from Brozek-Pluska, B. etal., Radiat. Phys. Chem., 72, 149, 2005.) Right: decay of solvated electrons formed
by pulse radiolysis of water measured for different cell thicknesses (a) and in the presence of 1 and 5 M acid
(b). (Adapted from Oulianov, D.A. etal., J. Appl. Phys., 101, 053102, 2007.)
Time-Resolved Study on Nonhomogeneous Chemistry Induced in Polar Solvents 311
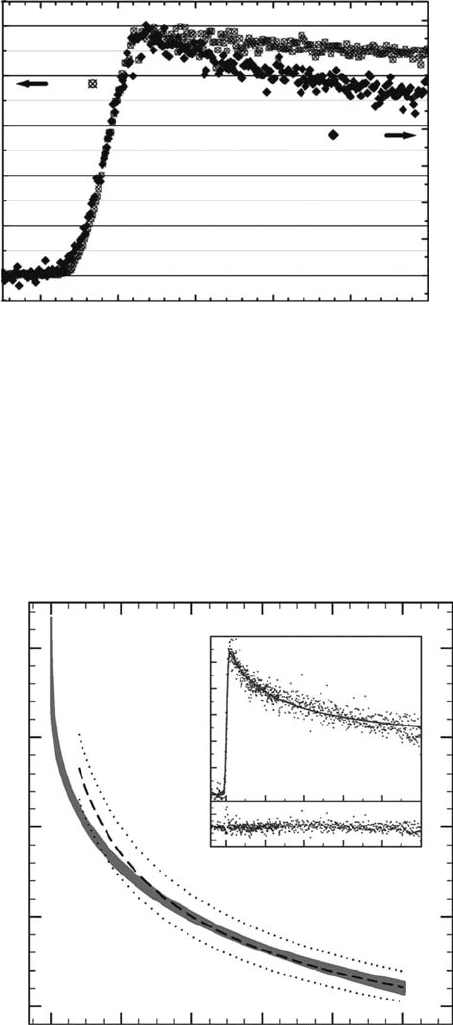
200 ps is 0.95 and 0.79 in water and in THF, respectively. Figure 12.19 (inset) presents the decay
of solvated electrons in THF at longer times, up to 2.5 ns. The G-value of solvated electrons in
THF is much more time dependent than those in polar solvents like water and alcohols. From
the t of solvated electron decay, it was found that the initial separation distance between sol-
vated electrons and the cation is around 4 nm (De Waele etal., 2007). This distance is shorter
than the Onsager radius for this solvent (7 nm). The low yield of solvated electron in THF can be
100
80
60
H
2
O
THF
40
20
0
–50 0 50
Pulse-probe delay (ps)
Absorbance (mOD)
100 150 200
0
2
4
Absorbance (mOD)
6
8
10
12
14
Figure 12.18 Decay of solvated electrons measured in water (right scale) and in THF (left scale), see
(De Waele etal., 2006) for details. (Reprinted from De Waele, V. etal., Chem. Phys. Lett., 423, 30, 2006.)
0.01
0.008
0.006
AbsorbanceResidues
0.004
0.002
0
0.002
–0.002
0 500 1000
Time (ps)
1500 2000 2500
0
0
1.0
1.5
2.0
G (10
–7
mol J
–1
)
2.5
3.0
500 1000 1500
Time (ps)
2000 2500
Figure 12.19 Comparison of the time-dependent G-value of solvated electrons in THF, determined by
scavenging method and direct measurement of the time-dependent decay. The dashed line corresponds to the
time-dependent G-value recalculated from the scavenging data from Salmon etal. (1974), the dotted-line gives
the data uncertainty on these values. The grey area is the direct time-dependent G-value obtained from the
tted experimental decay given in inset and represents the 99% condence interval, given data uncertainty.
Details are given in De Waele etal. (2006).
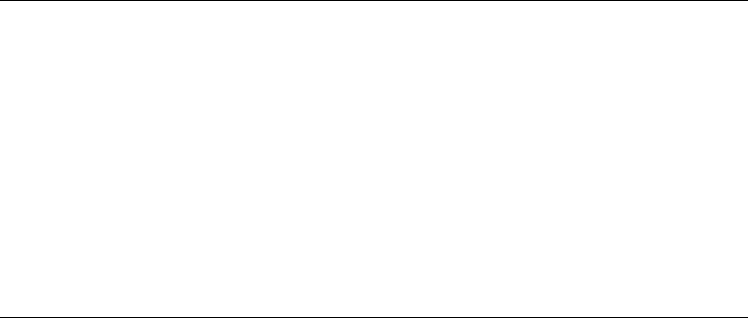
312 Charged Particle and Photon Interactions with Matter
understood in terms of the short thermalization distance of the electron compared to the Onsager
radius in this solvent.
The time-dependent G-value obtained from the scavenging data of (Kadhum and Salmon, 1984)
is compared with the t of the time-resolved measurement in Figure 12.19. A very good agree-
ment between the kinetics decay and the value obtained by the scavenging method is obtained
between 500ps and 2.5ns. At short times (less than 500ps), there is a discrepancy, which is under-
standable because the scavenging method is not appropriate at a very short time. According to the
time-resolved measurements in THF, the G-value at 30ps is around 2.7 × 10
−7
mol J
−1
and from
the deconvolved signal at zero time it is around 3 × 10
−7
mol J
−1
that is noticeably smaller than the
value reported by scavenging method (4 × 10
−7
mol J
−1
). This suggests that either the initial yield in
THF (at zero time) is lower than that in water or that a very fast decay occurs within the electron
pulse. Results reported by Scwartz etal. (Martini etal., 2000) and obtained by femtosecond laser
photolysis show that the decay in the rst hundred picoseconds is indeed very fast. Recently, the
reactivity between Biphenyl molecules and the precursors of the solvated electron in THF was stud-
ied by picosecond pulse-radiolysis (Saeki etal., 2007). The results also support an initial G-value of
solvated
electrons equal to 2.7 × 10
−7
mol J
−1
at 30ps in THF.
The yield of solvated electron was also measured in different alcohols by picosecond pulse-
radiolysis (Lin etal., 2006; Muroya etal., 2008). The data are summarized in Table 12.2, together
with the values for water and THF. Compared to the situation in water, the initial G-values in
alcohols are also found close to the value of 4.0 × 10
−7
mol J
−1
. However, the decays are more
pronounced than that in water, in good agreement with the dielectric constant lower in alcohols
than in water.
12.4.2.3 time-dependent
G-
value
of oh
•
radical
The G-value of OH
•
after 100 ns is well known. Nevertheless, its initial G-value is still contro-
versial. Direct measurements of the G-values of the OH
•
radical were performed (Jonah and
Miller, 1977). The authors reported the time dependence of G(OH
•
) by following the decay of
its weak absorption band in the UV. A value of 6.1 × 10
−7
mol J
−1
was estimated at 200 ps. Other
evaluations were obtained from scavenging studies using formate or hexacyanoferrate ions.
These data are limited to a scavenging power of 0.7 × 10
10
s
−1
, and different stochastic and deter-
ministic modelings of water radiolysis were performed (Figure 12.20). The often-cited value of
G(OH
•
) = 6.1 × 10
−7
mol J
−1
for OH
•
radical at 200ps was based on an estimated G
200ps
(
e
s
−
) = 4.7 ×
10
−7
mol J
−1
for solvated electrons. The OH
•
yield was reduced to G
200 ps
(OH
•
) = 5.3 × 10
−7
mol J
−1
on
the basis of the recent G-value of hydrated electron. Jay-Gerin and Ferradini reported that the
G-value for OH
•
at 100 ps is 4.6 × 10
−7
mol J
−1
(Jay-Gerin and Ferradini, 2000). This controversy
table 12.2
G-values
m
easured
for the s
olvated
electron in a
lcohols,
w
ater
and thF
η
25°C
(mpa · s)
dielectric
Constant λ
max
of
e
s
-
(nm)
- 2008 — 2025 «СтудМед»
