Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

740 18. Discrete PEG Reagents
used with a reactive group on one end and a blocked hydroxyl group on the other end (e.g., as
the methyl ether). In addition, large, branched PEG molecules have been created to add more
bulk or exclusion volume at a modifi cation site, thus increasing the protective effect of the PEG
molecule toward the biological molecule.
Discrete PEG compounds also have been developed in various reactive forms with methyl
ether blocking groups on the terminal end (Thermo Fisher, Quanta BioDesign). Unlike the
original PEG polymer reagents that display polydispersity, these PEG compounds are pure and
consist of only one chain length per reagent type. The chain lengths in discrete PEG modifi -
ers include, for example, polyethylene oxide repeat units of 3, 4, 8, 12, and 24. NHS–mPEG
n
modifi cation reagents can be used directly to couple with amine-containing molecules or pro-
teins through amide bond formation. This reaction occurs in aqueous buffers at physiological
pH or slightly alkaline pH conditions. Conversely, maleimide–mPEG
n
reagents are designed to
conjugate with thiol-containing molecules, and these may be used to target reduced disulfi de
bonds in proteins or thiols created on molecules using a thiolation reagent.
In addition, branched chain compounds have been developed consisting of a functional
group or a reactive group followed by a PEG
4
chain, which then leads to three branches each
having an mPEG
12
arm on them. Such compounds are expected to provide large exclusion vol-
umes in aqueous solution to surround, protect, and solubilize modifi ed molecules.
Another type of PEG modifi cation reagent that has been developed contains a functional
group on each end that can be used for conjugation purposes and which can be used to build
structures on solid supports, surfaces, or on other molecules. Some of these reagents have been
developed to contain an amine group on one end and a carboxylate on the other end. Such PEG-
based amino acids can be used as spacer arms to mask surfaces or provide highly hydrophilic
tethers for the attachment of affi nity ligands. A thiol-PEG
n
-carboxylate, for instance, can be used
to modify metal particles or surfaces through dative binding of the thiol to the metal and then
create PEG–carboxylic acids for further conjugation.
Figures 18.24 through 18.26 illustrate these PEG-based modifi cation reagents. The meth-
ods for their use follow the same general protocol guidelines as discussed in previous sections
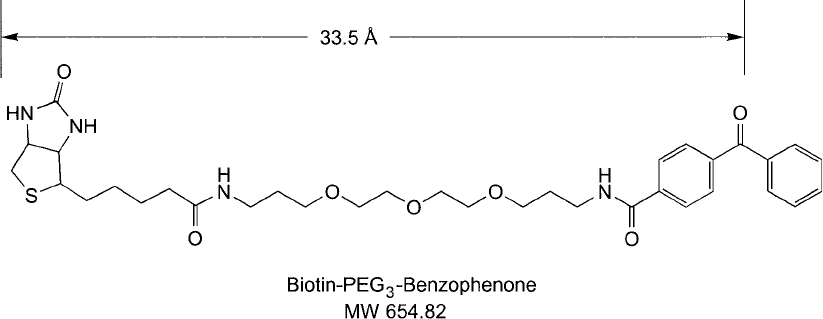
Figure 18.23 Biotin–PEG
3
–benzophenone is a water-soluble photoreactive biotinylation reagent that can be
used to add a biotin group to surfaces or molecules containing no easily derivatized functional groups.

Figure 18.24 Discrete PEGylation reagents are available to provide a range of different chain lengths for adding
mPEG modifi cation arms to biomolecules. They also can be used to add water-soluble mPEG groups to organic
molecules that are normally not very soluble in aqueous solution. The NHS ester end of the mPEG compounds
reacts with amine-containing molecules to form amide bonds, leaving the mPEG chain to interact with the
aqueous environment.
Figure 18.25 Amino-PEG
n
-carboxylate compounds contain a primary amine on one end and a carboxylate
group on the other end. They can be used to add water-soluble spacer arms to molecules or surfaces. Using an
amine-reactive group, the amino-PEG
n
-carboxylate compound can be coupled via an amide bond, thus leaving
the carboxylate end free for further conjugation reactions. Avoid the use of single-step EDC conjugation reac-
tions, as this will polymerize the amino-PEG
n
-carboxylate by reacting with both ends.
4. Discrete PEG Modifi cation Reagents 741
742 18. Discrete PEG Reagents
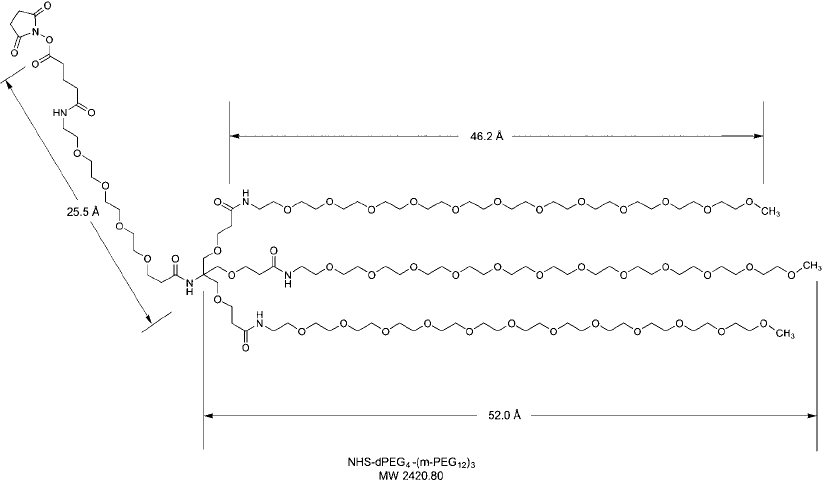
Figure 18.26
The branched PEGylation compound NHS-dPEG
4
-(mPEG
12
)
3
contains three mPEG arms, which
provide an increased sphere of hydration around modifi ed molecules compared to straight-chain PEGylation
compounds.
of this chapter for the corresponding reactive group or functional group. When modifying
biomolecules with mPEG-based reagents, a series of modifi cation levels should be investigated
to determine the optimal performance in an intended application. For surface or particle modi-
fi cation, reference should be made to Chapter 14, especially the section on covalent coupling to
particles.

The technology of bioconjugation has affected every conceivable
discipline in the life sciences. The application of a myriad of available
chemical reactions and reagent systems for creating novel complexes
with unique activities has made possible the assay of minute quantities
of substances, the in vivo targeting of molecules, and the modulation
of specifi c biological processes. Modifi ed or conjugated molecules also
have been used for purifi cation, detection, or location of specifi c sub-
stances, and in the treatment of disease.
Crosslinking and modifying agents can be applied to alter the native
state and function of peptides and proteins, sugars and polysaccha-
rides, nucleic acids and oligonucleotides, lipids, and almost any other
molecule imaginable that can be chemically derivatized. Through care-
ful modifi cation or conjugation strategies, the structure and function of
proteins can be investigated, active site conformation discovered, or
receptor–ligand interactions revealed. Some of these techniques are
so well characterized and standardized that general protocols can be
used with broad application and with excellent prospects for success.
The following sections describe how to prepare modifi ed or conjugated
biological macromolecules for use in specifi c applications. The chosen
applications represent some of the most popular uses of these reagent
systems, but are by no means exhaustive.
PART III
Bioconjugate
Applications
This page intentionally left blank

745
19
This chapter describes the design, preparation, and use of hapten–carrier conjugates used to
elicit an immune response toward a coupled hapten. The chemical reactions discussed for these
conjugations are useful for coupling peptides, proteins, carbohydrates, oligonucleotides, and
other small organic molecules to various carrier macromolecules. The resultant conjugates are
important in antibody production, immune response research, and in the creation of vaccines.
1. The Basis of Immunity
The essence of adaptive immunity is the ability of an organism to react to the presence of for-
eign substances and produce components (antibodies and cells) capable of specifi cally inter-
acting with and protecting the host from their invasion. An “ antigen ” or “ immunogen ” is the
name given for a substance which is both able to elicit this type of immune response and also is
capable of interacting with the sensitized cells and antibodies which are manufactured against it.
The immune system has two basic components which respond to a challenge of a foreign
substance: a cellular response mediated by T lymphocytes and a humoral response mediated
by secreted proteins called antibodies produced by B-lymphocytes, also called plasma cells. The
B-lymphocytes recognize antigens through cell-surface immunoglobulins that bind to discrete
chemical and structural epitopes on the antigen molecule. Each B cell possesses surface immu-
noglobulin of a single type (i.e., is monoclonal) and has a binding capability that is directed
against a discrete epitopic target.
Antigen binding by a complementary immunoglobulin molecule on the surface of B cells
starts a process of cellular internalization of the foreign substance by pinocytosis. Once inter-
nalized by endosomes, systematic processing of the antigen takes place which breaks it down
into smaller components.
At this point, the endosome may fuse with vesicles containing newly synthesized or recycling
major histocompatibility complex (MHC) antigens. Some of the partially degraded antigenic
fragments may form a complex with the MHC and be transported back to the cell surface.
There they are “ presented ” to the circulating T helper (T
h
) cells which contain receptors
able to bind specifi cally to particular structural and chemical characteristics of the degraded
antigen–MHC complex. If a T
h
cell recognizes and binds to the presented antigen on the
Preparation of Hapten–Carrier
Immunogen Conjugates
746 19. Preparation of Hapten–Carrier Immunogen Conjugates
surface of the antigen presenting cells (APC), the T
h
cell proliferates and begins to produce
various lymphokines. Finally, the recognition and binding of the presented antigen by the T
h
cells, coupled with the release of lymphokines, stimulates the associated B cells to proliferate
and produce antibodies which recognizes the intact antigen (Germain, 1986; Pier et al ., 2004).
Antigens usually are macromolecules that contain distinct antigenic sites or “epitopes”,
which can be recognized and interact with the various components of the immune system. They
can exist as individual molecules composed of synthetic organic chemicals, proteins, lipopro-
teins, glycoproteins, RNA, DNA, polysaccharides—or they may be parts of cellular structures
(bacteria or fungi) or viruses (Male et al ., 1987; Harlow and Lane, 1988).
Small molecules like short peptides, although normally able to interact with the products of
an immune response, often cannot cause a response on their own. These “haptens”, as they are
called, actually are incomplete antigens, and while not able by themselves to cause immunogenic-
ity or to elicit antibody production, they can be made immunogenic by coupling them to a suit-
able carrier molecule ( Figure 19.1 ). Carriers typically are antigens of higher-molecular weight
that are able to cause an immunological response when administered in vivo .
Antibodies typically are able to recognize peptide sequences as small as 5–6 amino acids in
length. For instance, IgE auto-antibodies were found to have clinical signifi cance in multiple
sclerosis by binding specifi cally to short 5- and 6-amino acid epitopes on the surface of myelin
proteins (Mikol et al ., 2006).
In an immune response, antibodies are produced and secreted by the B-lymphocytes in con-
junction with the T
h
cells. In the majority of hapten–carrier systems, the B cells end up producing
antibodies that are specifi c for both the hapten and the carrier. In these cases, the T lymphocytes
will have specifi c-binding domains on the carrier, but will not recognize the hapten alone. In a
kind of synergism, the B- and T-cells cooperate to induce a hapten-specifi c antibody response.
After such an immune response has taken place, if the host is subsequently challenged with only
the hapten, usually it will respond by producing hapten-specifi c antibodies from memory cells
formed after the initial immunization. For a review of immunobiology (see Janeway, 2004).
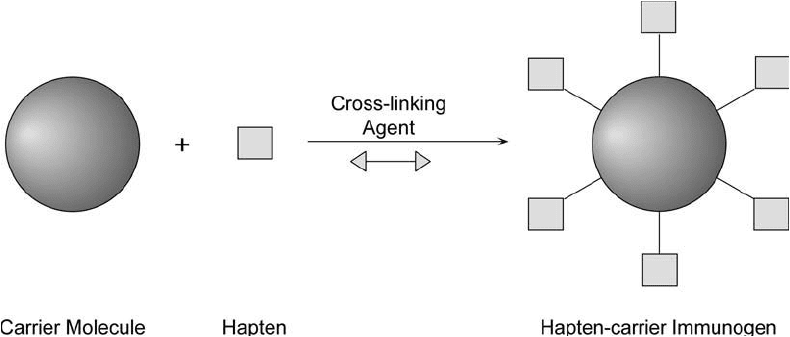
Figure 19.1 Immunogens are made by the crosslinking of a hapten molecule with a carrier using a conjugation
reagent.
Synthetic haptens mimicking some critical epitopic structures on larger macromolecules are
often conjugated to carriers to create an immune response to the larger ‘ parent ’ molecule. For
instance, short peptide segments can be synthesized from the known sequence of a viral coat
protein and coupled to a carrier to induce immunogenicity toward the native virus. This type
of synthetic approach to immunogen production has become the basis of much of the current
research into the creation of vaccines.
The complete picture of the immune system is much more complex than this brief discus-
sion can justly describe. In many instances, merely creating a B cell response by using synthetic
peptide-carrier conjugates, however well designed, will not always guarantee complete protec-
tive immunity toward an intact antigen. The immune response generated by a short peptide
epitope from, say, a larger viral particle or bacterial cell may only be suffi cient to generate
memory at the B cell level. In these cases, it is generally now accepted that a cytotoxic T-cell
response is a more important indicator of protective immunity. Designing peptide immunogens
with the proper epitopic binding sites for both B-cell and T-cell recognition is one of the most
challenging research areas in immunology today.
Hapten–carrier conjugates also are being used to produce highly specifi c monoclonal anti-
bodies that can recognize discrete chemical epitopes on the coupled hapten. The resulting mono-
clonals often are used to investigate the epitopic structure and interactions between native
proteins. In many cases, the haptens used to generate these monoclonals are again small pep-
tide segments representing crucial antigenic sites on the surface of larger proteins. Monoclonals
developed from known peptide sequences will interact in highly defi ned ways with the protein
from which the sequence originated. These antibodies then can be used, for example, as com-
petitors to the natural interactions between a receptor and its ligand. Thus, using antibodies
generated from hapten–carrier conjugates, information can be obtained as to the precise sites
of binding between macromolecules.
The preparation of hapten–carrier conjugates using peptide sequences can be control-
led to produce immunogens that generate high-affi nity antibodies when administered in vivo .
Pedersen et al. (2006) determined that antibody titers increased in response to increasing the
peptide-to-carrier ratio of conjugation. However, just the opposite effect was found for gen-
erating high affi nity antibodies. The lower the peptide-to-carrier conjugation ratio, the higher
the relative affi nity of the antibodies produced. In addition, it also was found that coupling
peptides to the carrier through a central amino acid residue caused higher antibody titers than
using a terminal amino acid residue for conjugation. For this reason, for the preparation of
particular immunogen conjugates, several ratios and methods of conjugation may have to be
investigated to result in the optimal level and affi nity of antibodies produced.
2. Types of Immunogen Carriers
The most commonly used carriers are all highly immunogenic, large molecules that are capa-
ble of imparting immunogenicity to covalently coupled haptens. Some of the more useful ones
are proteins, but other carriers may be composed of lipid bilayers (liposomes), synthetic or
natural polymers (dextran, agarose, poly-L-lysine), or synthetically designed organic molecules
(i.e., dendrimers, see Chapter 7). The criteria for a successful carrier molecule are the potential
for immunogenicity, the presence of suitable functional groups for conjugation with a hapten,
2. Types of Immunogen Carriers 747
748 19. Preparation of Hapten–Carrier Immunogen Conjugates
reasonable solubility properties even after derivatization—although this is not an absolute require-
ment, since precipitated molecules can be highly immunogenic—and lack of toxicity in vivo .
Some synthetic carriers actually are designed to have low immunogenicity on their own to
minimize the potential for antibody production against them. When a hapten is coupled to
these molecules, the immune response is directed principally toward the modifi cation, not at the
carrier. This design approach guides most of the immune response toward the desired target and
minimizes the production of carrier-specifi c antibodies.
2.1. Protein Carriers
The fi rst carrier molecules used for immunogen conjugation were proteins. A foreign protein
administered in vivo by any one of a number of potential routes nearly assured the elicitation
of an immune response. In addition, protein carriers could be chosen to be highly soluble and
possessed of abundant functional groups that could facilitate easy conjugation with a hapten
molecule. When proteins are used as carriers in immunogen complex, the conjugates can be
injected in any animal except the animal of origin for the carrier protein itself. In other words,
the use of bovine serum albumin (BSA) would not be suitable for administration into cows,
since self-proteins would not be expected to elicit good immune responses, even when attached
with hapten molecules.
The most common carrier proteins in use today are keyhole limpet hemocyanin (KLH;
MW 4.5 10
5
to 1.3 10
7
), BSA (MW 67,000), aminoethylated (or cationized) BSA (cBSA),
thyroglobulin (MW 660,000), ovalbumin (OVA; MW 43,000), and various toxoid proteins,
including tetanus toxoid and diphtheria toxoid. Other proteins occasionally used include
myoglobin, rabbit serum albumin, immunoglobulin molecules (particularly IgG) from bovine
or mouse sera, tuberculin purifi ed protein derivative, and synthetic polypeptides such as poly-
L-lysine and poly-L-glutamic acid.
KLH
Perhaps the most popular carrier protein is KLH. The hemocyanin from keyhole limpets (the
mollusk Megathura crenulata) is the oxygen-carrying protein of these primitive sea creatures.
KLH is an extremely large, multi-subunit protein that contains chelated copper of non-heme
origin. In concentrated solutions above pH 7.0, it displays a characteristic opalescent blue
color that betrays its near insolubility and copper prosthetic groups. In acidic solutions, the
blue color changes to green. At physiological pH, the protein exists in various subunit aggre-
gate states of large molecular weight. For instance, in Tris buffer at pH 7.4 it is known to
associate in fi ve different aggregate forms (Senozan et al., 1981). In highly alkaline or acidic
environments, KLH disassociates into subunits (Hersckovits, 1988). The protein exhibits
increased immunogenicity when it is disassociated into subunits, probably due to exposure of
additional epitopic sites to the immune system (Bartel and Campbell, 1959). The intact pro-
tein usually creates considerable light-scattering or iridescent effects due to its size and almost
colloidal nature in aqueous solutions. Subunits of KLH that are highly soluble in aqueous solu-
tion are available commercially (Thermo Fisher, Biosyn). KLH is a frequent choice for devel-
oping immunogen conjugates, especially for the treatment of cancer (Curigliano et al., 2006;
Sabbatini and Odunsi, 2007).
Since keyhole limpets are marine creatures existing in a high-salt environment, native KLH
maintains its best stability and solubility in buffers containing at least 0.9 M NaCl (not 0.9 per-
cent). As the concentration of NaCl is decreased below about 0.6 M, the protein begins to pre-
cipitate and denature. Conjugation reactions using multi-subunit KLH, therefore, should be done
under high-salt conditions to preserve the solubility of the hapten–carrier complex. KLH used in
the form of discrete subunits does not have this requirement of high salt to maintain solubility.
Native, multi-subunit KLH also should not be frozen. Freeze-thaw effects cause extensive
denaturation and result in considerable amounts of insoluble material. Commercial prepara-
tions of native KLH are typically freeze-dried solids that no longer fully dissolve in aqueous
buffers and do not display the protein ’s typical blue color due to loss of chelated copper. The
partial denatured state of these products often makes conjugation reactions diffi cult.
KLH contains an abundance of functional groups available for conjugation with hapten
molecules. On a per-mole basis (using an average multi-subunit MW of 5,000,000 D), KLH
has over 2,000 amines from lysine residues, over 700 sulfhydryls from cysteine groups, and
over 1,900 tyrosines. Activation of the protein with succinimidyl-4-( N -maleimidomethyl)cyclo
hexane-1-carboxylate (SMCC) (Section 5, this chapter) typically results in 300–600 maleimide
groups per molecule for coupling to sulfhydryl-containing haptens.
The preparation of immunogen conjugates often requires the coupling of a sparingly soluble
hapten to a carrier molecule. Pre-dissolving the hapten in an organic solvent and adding an
aliquot of this solution to an aqueous reaction mixture typically is done to maintain at least
some solubility of the hapten in the conjugation solution. Dimethyl sulfoxide (DMSO) may be
used for this purpose with KLH while maintaining very good solubility characteristics of the
protein as well as the hapten. KLH is completely soluble in 50 percent (v/v) DMSO, becomes
cloudy at a level of 60 percent, and defi nitely precipitates at 67 percent. Therefore, conjugation
reactions may be done by adding a volume of aqueous KLH to an equal volume of hapten dis-
solved in DMSO. Care should be taken, however, to avoid buffer salt precipitation upon addi-
tion of organic solvent.
BSA and cBSA
BSA (MW 67,000) and cationized BSA (cBSA) are highly soluble proteins containing numer-
ous functional groups suitable for conjugation. Even after extensive modifi cation with hap-
ten molecules these carriers usually retain their solubility. The exception to this statement is
when hydrophobic peptides or other sparingly soluble molecules are conjugated to the pro-
teins. Modifi cation of any carrier with hydrophobic haptens may cause enough masking of
the hydrophilic surface to result in precipitation. Depending on the degree of precipitation,
such conjugates often are useful in generating an immune response. To limit the production of
insoluble complexes, however, the conjugation reaction can be scaled back to reduce the level
of carrier modifi cation.
BSA possesses a total of 59 lysine -amine groups (with only 30–35 of these typically avail-
able for derivatization), 1 free cysteine sulfhydryl (with an additional 17 disulfi des buried within
its three-dimensional structure), 19 tyrosine phenolate residues, and 17 histidine imidazole
groups. The presence of numerous carboxylate groups gives BSA its net negative charge (pl 5.1).
cBSA is prepared by modifi cation of its carboxylate groups with ethylene diamine (Chapter
1, Section 4.3) ( Figure 19.2 ). Controlled aminoethylation using the water-soluble carbodiimide
EDC results in blocking many of BSA ’s aspartic and glutamic acid side chains (and possibly the
2. Types of Immunogen Carriers 749
