Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

720 18. Discrete PEG Reagents
In use, NHS–PEG–maleimides fi rst are reacted in at least a 10-fold molar excess with an amine-
containing molecule, such as a protein, to form an intermediate derivative with terminal male-
imide groups. Depending on the number of maleimide modifi cations needed, the molar excess of
crosslinker may be adjusted to provide the desired level of activation. The maleimide groups are
more stable in aqueous solution than the NHS esters, so the modifi ed protein can be purifi ed from
excess crosslinker before reacting it with a sulfhydryl-containing molecule or protein. After puri-
fi cation, the maleimide–PEG–protein derivative is reacted with a second protein containing sulf-
hydryls to form the fi nal conjugate via a thioether bond ( Figure 18.9 ). The higher the number
of maleimide–PEG modifi cations on the fi rst protein, the greater the number of potential sulfhy-
dryl-containing proteins may be conjugated to it. The ratio of the reactants is often chosen to
give at least several thiol–proteins conjugated to each of the maleimide–PEG–protein derivatives.
Optimization of this ratio should be done to determine the best conjugate for a given application.
The intermediate maleimide–PEG–protein derivative maintains excellent solubility due to the
presence of the PEG chains. Unlike a hydrophobic NHS–maleimide-type crosslinker made from
an aliphatic cross-bridge, the PEG chains have a tendency to increase the solubility of modi-
fi ed proteins or other molecules. This effect is amplifi ed when using PEG compounds of longer
dimensions. The result is that modifi ed proteins remain soluble, and even if not every maleim-
ide group gets conjugated to a thiol-containing molecule, the presence of unreacted crosslinkers
on the protein surface doesn ’t contribute to nonspecifi c interactions of the conjugates.
Most of these PEG crosslinkers come as thick, sticky, viscous liquids or low melting sol-
ids (PEG
2
). For this reason, weighing out a small sample of a compound can be diffi cult or
impossible. It usually is best to dissolve an entire vial or a larger amount in organic solvent
at a known concentration to permit accurate dispensing of a smaller amount into a reaction.
Suitable solvents to prepare a stock solution include dry (molecular sieved) DMSO, DMF,
DMAC, acetonitrile, or methylene chloride (for non-water-miscible reactions).
The following protocol is a general guide for using NHS–PEG
n
–maleimide crosslinkers. This
method may be used as a starting point for developing an optimized procedure for creating a
unique conjugate.
Protocol
1. In a fume hood, dissolve the NHS–PEG
n
–maleimide compound of choice in a dry, water-
miscible organic solvent to make a concentrated stock solution. For instance, to pre-
pare a 100 mM solution of the NHS–PEG
6
–maleimide reagent (MW 601.6), dissolve an
entire 100 mg vial of the crosslinker in 1.66 ml of DMAC (dry DMSO or DMF work
well, too).
2. Dissolve the amine-containing protein to be activated in 0.1 M sodium phosphate, pH
7.2 (coupling buffer). A protein concentration of 1–10 mg/ml in buffer will work well in
this protocol. For more dilute protein solutions, greater quantities of the NHS–PEG
n
–
maleimide compound may have to be added to obtain equivalent levels of modifi cation.
3. Add a quantity of the crosslinker solution to the protein solution to provide at least a
10-fold molar excess of reagent over the amount of protein present. Higher levels of
reagent-to-protein may be used, even up to a 50-fold molar excess, to obtain a large
number of active groups for subsequent coupling to a thiol–protein. Conversely, the use
of lower molar ratios will limit the number of maleimide–PEG modifi cations on each
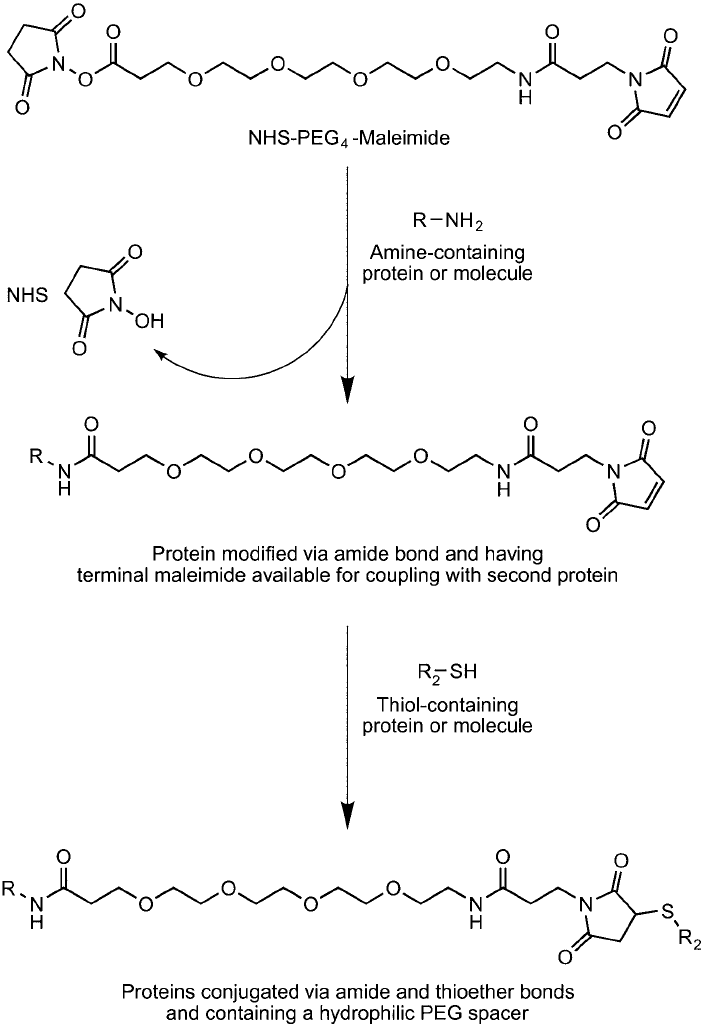
2. Heterobifunctional PEG Reagents 721
Figure 18.9 NHS–PEG
4
–maleimide conjugation reactions are carried out in two steps involving modifi cation
of an amine-containing molecule with the NHS ester end with subsequent coupling of the maleimide end with a
thiol-containing molecule.
722 18. Discrete PEG Reagents
protein to just a few. A series of reactions using different concentrations of crosslinker
may have to be done to determine the optimal level to use for a particular application.
4. React with gentle mixing for 30 minutes at room temperature or 60 minutes at 4°C.
5. Remove excess crosslinker by centrifugal dialysis or gel fi ltration using a molecu-
lar weight exclusion of 5,000. This procedure should be done quickly due to the labile
nature of the maleimide group in aqueous solution, which will hydrolyze to a ring-open
maleamic acid that is ineffective at coupling to thiols.
6. Dissolve a sulfhydryl-containing protein in coupling buffer containing 10 mM EDTA at
a concentration of at least 1–10 mg/ml. Thiols may be generated in proteins by disulfi de
reduction or through use of a thiolation reagent (Chapter 1, Section 4.1). The amount of
the thiol–protein needed is determined by the optimal molar ratio desired in the fi nal con-
jugate of the thiol-protein to the maleimide-activated protein purifi ed in Step 5. Often,
this means doing a reaction of the thiol–protein to the maleimide–PEG–protein in at least
a 4:1 molar ratio, but higher ratios (e.g., 15:1) also can be used, depending on the appli-
cation. Note that the number of thiol–protein molecules that can be conjugated with the
maleimide–PEG–protein is limited by the number of reactive maleimide groups present
and also by the molecular size of the two molecules being conjugated. Steric crowding
will limit how many thiol–proteins can be attached to the maleimide-activated protein.
7. Add the thiol–protein to the maleimide–PEG–protein in the desired molar ratio to initiate
the conjugation reaction.
8. React with gentle mixing for at least 2 hours at room temperature or overnight at 4°C.
9. The conjugate may be purifi ed to remove unconjugated protein using gel fi ltration on
a column of resin having a molecular weight cut-off able to accommodate the proteins
being separated.
2.2. NHS-PEG
n
-Azide/Alkyne Compounds for Chemoselective Ligation
Another family of heterobifunctional compounds containing an internal PEG spacer is reagents
with an NHS ester on one end and either an azide or alkyne group on the other end ( Figure
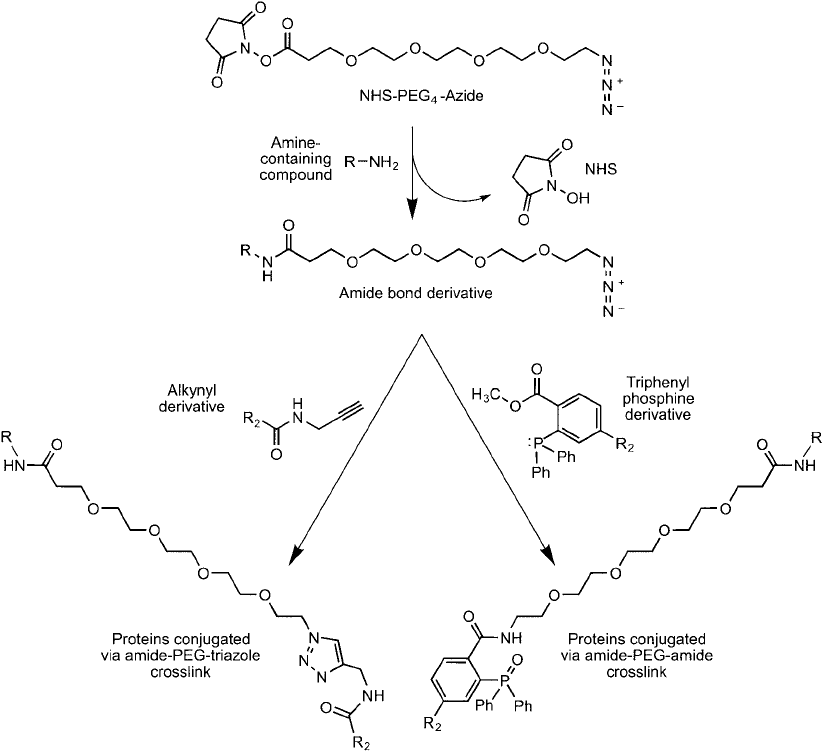
18.10 ). These reagents react with amines on proteins or other molecules to create an amide
bond derivative having a terminal functionality suitable for use in chemoselective ligation reac-
tions, particularly click chemistry cycloaddition and Staudinger ligation (Chapter 17, Sections
4 and 5). Click chemistry is the reaction between an azide and an acetylene group in the pres-
ence of a catalytic amount of Cu(I) yielding a triazole ring linkage, which is a very effi cient pro-
cess for bioconjugation purposes. NHS ester heterobifunctional compounds containing these
groups may be used to functionalize biomolecules, organic compounds, particles, or surfaces
with azides or alkynes. Since both the azido and acetylene groups are highly stable in aqueous
solution or in the presence of biomolecules, the modifi ed substances or devices maintain nearly
indefi nitely the ability to conjugate molecules containing the opposite functionality.
The azide-containing PEG compounds also can be used in the Staudinger ligation process
with a phosphine-containing group having an electrophilic trap. This reaction, like the click
chemistry process, is chemoselective and highly bioorthogonal in that the functional groups
won’t react with typical functionalities present in biological solutions. The Staudinger reac-
tion with PEG-azide compounds proceeds to give an amide bond with either retention of the
phosphine component or, in the traceless version of the reaction, with loss of the phosphine
and formation of a zero-length crosslink between the two reacting molecules ( Figure 18.11 ).
Click chemistry or Staudinger ligation equipped heterobifunctional crosslinkers containing a
hydrophilic PEG spacer provide a stable, yet highly effi cient means to conjugate or immobilize
biomolecules. The PEG spacer provides water solubility, low nonspecifi c binding character, and
low immunogenicity.
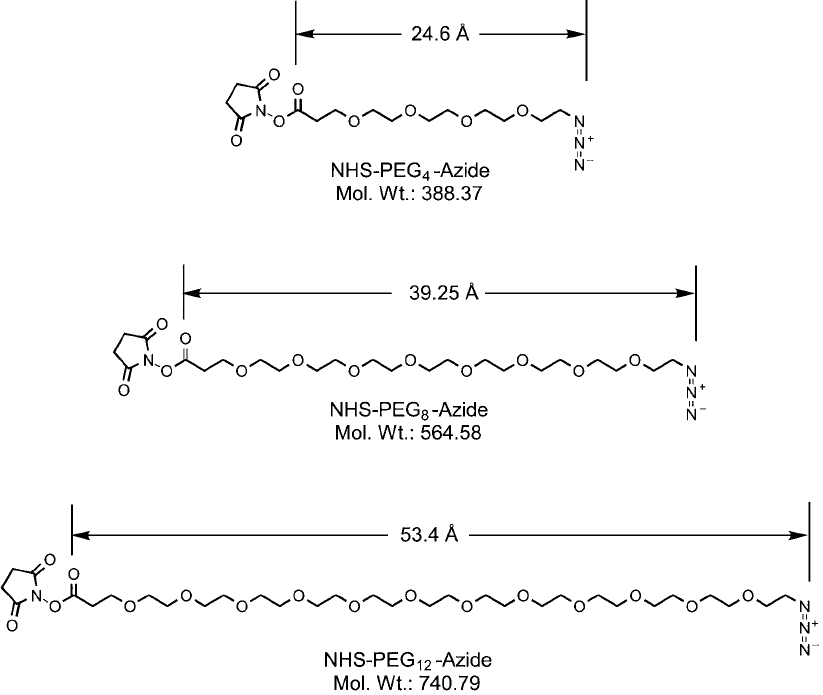
Three PEG lengths are available in NHS-PEG
n
-azide compounds, including n 4, 8, or 12
ethylene oxide repeat units ( Figure 18.10 ) (Quanta BioDesign). The NHS-PEG
4
-azide com-
pound provides a 16-atom spacer, which is 17.7 Å long after reaction with an amine-containing
molecule. The NHS-PEG
8
-azide and the NHS-PEG
12
-azide reagents provide longer spacer arms
having molecular distances of 32.2 Å and 46.4 Å, respectively. All of these measurements are
done using three-dimensional molecular models that are linearized structures with distances
taken from the NHS ester carbonyl group to the fi rst nitrogen atom of the azide. The actual
2. Heterobifuncational PEG Reagents 723
Figure 18.10 NHS-PEG-azide compounds can be used to modify amine-containing proteins or other molecules
for subsequent conjugation using either the click chemistry reaction or Staudinger ligation.
724 18. Discrete PEG Reagents
molecular dimensions between crosslinked molecules will differ from these values due to the
different conformations that the PEG chains can take in aqueous solution and due to the for-
mation of the triazole ring after the click reaction has taken place.
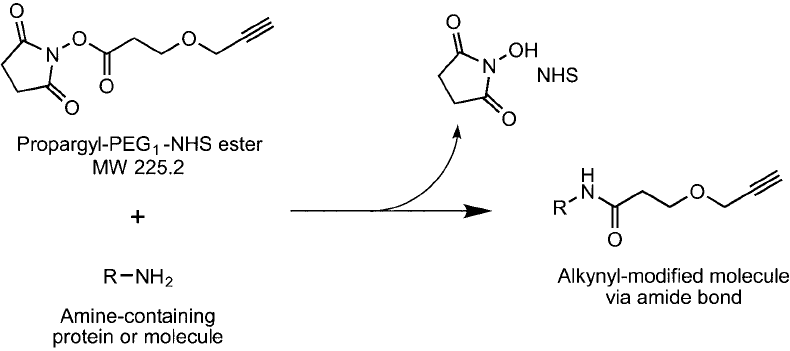
Only one compound is currently available for modifying amine-containing molecules with
an acetylene group, which is the propargyl–PEG
1
–NHS ester containing only a single ethyl-
ene oxide unit ( Figure 18.12 ) (Quanta BioDesign). Other simple acetylene-based raw materials
also are commercially available with functional groups, such as propargylamine and various
carboxylic acid derivatives (4-pentynoic acid, Aldrich), which can be used in a carbodiimide
reaction to modify molecules or surfaces. However, at the time of this writing, no longer chain
Figure 18.11 NHS-PEG
4
-azide can be used to modify an amine-containing molecule to create an amide deriva-
tive terminating in azido groups. The azide modifi cations then can be used in a click chemistry reaction that
forms a triazole linkage with an alkyne-containing molecule. Alternatively, the azide derivative can be used in a
Staudinger ligation reaction with a phosphine derivative, which results in an amide bond linkage.
PEG-based reagents are available with the terminal alkyne needed for a click chemistry biocon-
jugation reaction.
The following protocol may be used to modify a protein for coupling to a surface using the
NHS-PEG
n
-azide and propargyl–PEG
1
–NHS ester compounds. Similar procedures may be used
to conjugate two molecules together, such as forming an antibody–enzyme conjugate.
Protocol
Modifi cation of protein 1 with NHS-PEG
n
-azide groups
1. Dissolve a fi rst protein to be modifi ed with an azide group in 100 mM sodium phosphate,
pH 7.2 (PBS), at a concentration of at least 1–10 mg/ml.
2. In a fume hood, prepare a stock solution of an NHS-PEG
n
-azide by dissolving it at a
concentration of 20 mM in DMAC, DMSO, or DMF (using highly pure and dry solvent).
3. Add a quantity of the crosslinker solution to the protein solution with mixing to provide
at least a 10-fold molar excess of crosslinker over the amount of protein present.
4. React with mixing for 30–60 minutes at room temperature.
5. Purify the azido-PEG–protein by dialysis or gel fi ltration using PBS to remove excess
reactants and solvent. The azide modifi cations are stable to aqueous conditions, so the
protein derivative may be stored in this form until needed, provided the protein is stable.
Modifi cation of protein 2 with propargyl–PEG
1
–NHS ester
1. Dissolve a second protein to be functionalized with alkyne groups at a concentration of
1–10 mg/ml in PBS.
2. In a fume hood, prepare a stock solution of propargyl–PEG
1
–NHS ester in DMAC,
DMSO, or DMF (highly pure and dry) at a concentration of 20 mM (4.5 mg/ml).
3. Add a quantity of the propargyl–PEG
1
–NHS ester solution with stirring to the protein
solution to obtain a 10-fold molar excess. Optimization of reactant ratios may be done
to determine the best modifi cation for a particular conjugation.
2. Heterobifunctional PEG Reagents 725
Figure 18.12 Propargyl–PEG
1
–NHS ester can be used to react with amine-containing molecules to add short
alkyne modifi cation sites for subsequent click chemistry reactions with azide-containing molecules.
726 18. Discrete PEG Reagents
4. Gently mix for 30–60 minutes at room temperature.
5. Purify the alkyne-modifi ed protein from excess linker by dialysis or gel fi ltration. The
acetylene modifi cation is stable in aqueous solution at this point.
Coupling the alkyne-protein to the azide-protein
1. With mixing, add a quantity of the azide-modifi ed protein to the alkyne-modifi ed protein
to provide the desired molar excess over the quantity of protein and amount of alkyne
groups present. Maintain the overall protein concentration at 1–10 mg/ml or greater to
obtain the best reaction kinetics. Often, a one protein is reacted in 4- to 15-fold molar
excess over a second protein to create a conjugate having several molecules of the fi rst
protein attached to the second protein. However, the appropriate molar excess may have
to be determined by doing a series of reactions at different ratios and determining the
best ratio for use in a particular application.
2. Add 2.5 l of a solution containing 10 mM CuSO
4
5H
2
O and 50 mM ascorbic acid dis-
solved in water per ml of the protein mixture. Mix to dissolve.
3. React with mixing at room temperature for at least 4 hours (or at 4°C overnight, if the
protein is not stable at ambient temperature). The optimal time of reaction is depen dent
on the proteins being coupled together and the number of azide and alkyne reactive
groups available on them. An alkynyl-protein added to the azide-protein at a high molar
ratio probably would reach maximal coupling yield in a matter of hours.
4. Purify the protein conjugate by dialysis or gel fi ltration using a molecular weight cut-off
appropriate for the sizes of the proteins being separated.
A similar protocol may be used to couple proteins or molecules modifi ed with an azide or
alkyne to a particle or surface modifi ed with the other functionality.
3. Biotinylation Reagents Containing Discrete PEG Linkers
Biotin modifi cation reagents are widely used to attach a biotin group to proteins or other
molecules for subsequent use in avidin, streptavidin, or NeutrAvidin separations or assays.
Traditional biotin compounds containing aliphatic or other hydrophobic linker arms are dis-
cussed in detail in Chapter 11. In this section, the biotin–PEG compounds exclusively are
discussed due to their unique hydrophilic properties, which include low nonspecifi c binding
character and low immunogenicity.
Traditional biotinylation compounds include the very popular (sulfo)NHS-LC-biotin, which
contains either an uncharged NHS ester or a negatively charged sulfo-NHS ester in addition to
a C
6
alkyl chain leading to the biotin group. Although the sulfo-NHS ester provides a degree
of water solubility for the entire compound prior to using it to modify a protein, the resultant
LC-biotin modifi cation left on the protein is extremely hydrophobic. The result of adding such
hydrophobic groups to a protein surface often is a balance between maintaining water solubil-
ity or tending toward protein aggregation and precipitation. Many antibodies that are bioti-
nylated with NHS-LC-biotin undergo slow aggregation and loss of activity despite limiting the
degree of biotinylation during production.
The use of discrete PEG spacers in the construction of biotinylation compounds not only
increases the water solubility of the modifi cation reagent itself, but signifi cantly increases the
hydrophilicity and stability of proteins modifi ed with them. Even when high modifi cation levels
are used with PEG–biotin compounds, the resultant biotinylated protein typically is very water
soluble and does not aggregate in solution.
The following compounds represent some of those that are commercially available for add-
ing a PEG–biotin group to proteins and other molecules. Reactive group options include NHS
esters for coupling to amines, maleimide groups for coupling to thiols, hydrazides for conju-
gation with carbonyl compounds, and a photoreactive benzophenone for nonselective inser-
tion into molecular structures (Thermo Fisher, Quanta BioDesign, Solulink, and Molecular
Biosciences). In addition, a chromogenic biotin compound containing a PEG spacer is available
from Thermo Fisher and Solulink. This unique compound allows exact measurement of the
amount of biotinylation by measuring the absorbance of the fi nal purifi ed conjugate.
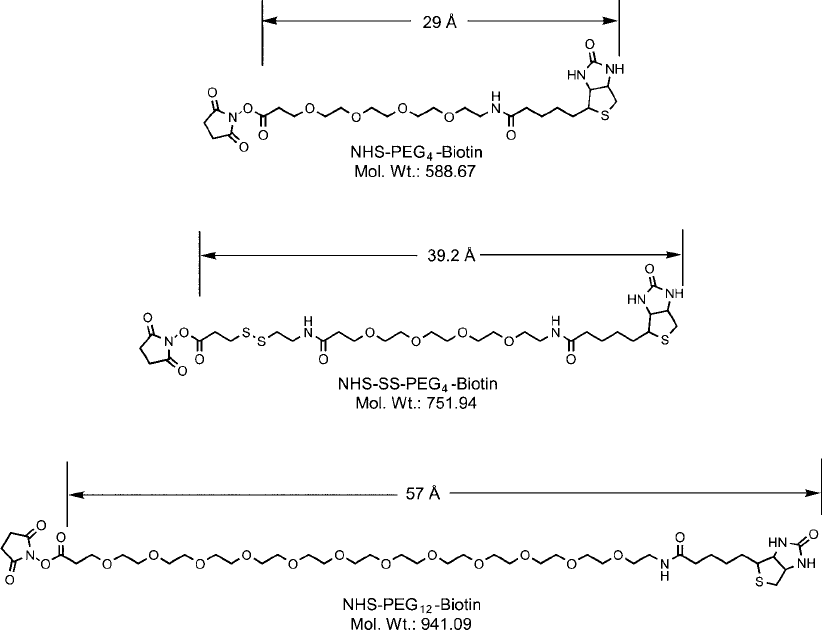
3.1. NHS–PEG
n
–Biotin Compounds
NHS ester biotinylation reagents are the most popular choice for adding a biotin group to
another molecule that contains an available amine. The ester reacts with an amine at neutral or
slightly alkaline pH values to form a stable amide bond with the amine-containing protein or
molecule. Discrete PEG biotinylation compounds containing an NHS ester are available in chain
lengths of 4 or 12 repeating polyethylene oxide units and in one form that contains a cleavable
disulfi de in the cross-bridge.
The NHS ester compounds are sensitive to hydrolysis in aqueous solution, and they likely
will hydrolyze faster than more hydrophobic biotinylation compounds due to their hydrophilic-
ity. If a stock solution is made at a higher concentration to facilitate the addition of a small
amount to a reaction solution, the initial solution should be made in a water-miscible organic
solvent that is dried with a molecular sieve. Suitable solvents include DMAC, DMSO, or DMF.
If using DMF, use only highly pure solvent, as it may contain amines that can react with the
NHS ester groups ( Figure 18.13 ).
Reactions done with NHS–PEG
n
–biotin compounds typically are done with the reagent
in molar excess over the amount of protein being modifi ed. The effi ciency of the reaction is
dependent on the concentrations of reactants and the solvent exposed area of the amine groups
on the protein. Reactions done with a 10-fold molar excess of NHS–PEG
n
–biotin usually will
result in at least 2–3 biotin labels per protein, while doubling the molar excess should provide
4–6 biotinylations. The optimal number of biotin groups added to a particular protein should
be determined experimentally to provide the best performance in the intended application.
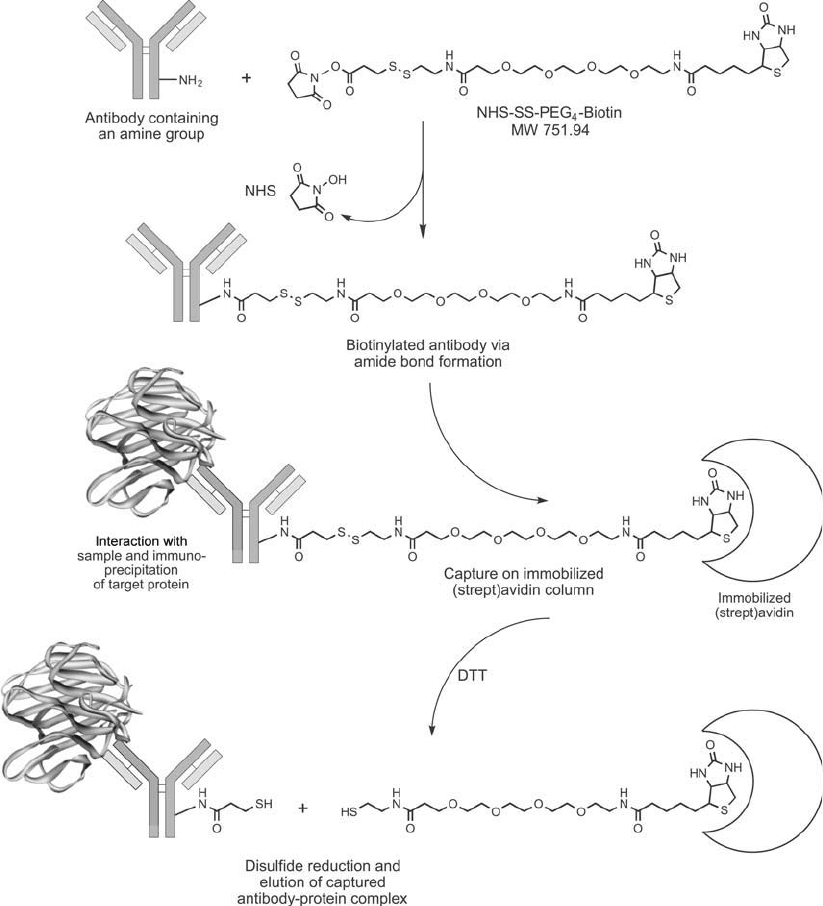
NHS-SS-PEG
4
-biotin contains a cleavable disulfi de bridge next to the PEG chain. This fea-
ture allows the biotin group to be released from the biotinylated molecule using a disulfi de
reducing agent, such as DTT or TCEP ( Figure 18.14 ). This is useful for doing immunopre-
cipitation (IP) or co-immunoprecipitation (co-IP) assays, because a biotinylated antibody can
be used to capture and affi nity isolate a target protein on immobilized (strept)avidin and then
elute the bound proteins by reduction (using 50 mM DTT for 2 hours at room temperature or
for 30 minutes at 50°C). This eliminates the severe denaturing conditions usually required to
break the (strept)avidin–biotin bond, thus better preserving the native structure and activity
of the captured proteins. Compounds of this type also may be used successfully in cell-surface
biotinylation, as described in Chapter 11, Section 1, under sulfo-NHS-SS-biotin.
The following protocol is based on the applications done at Thermo Fisher (formerly Pierce).
The use of higher pH values for the NHS ester reaction than those recommended may result
3. Biotinylation Reagents Containing Discrete PEG Linkers 727
728 18. Discrete PEG Reagents
in lower biotinylation yields due to increased hydrolysis, especially when using an extremely
hydrophilic PEG compound.
Protocol
1. Dissolve an antibody or protein to be modifi ed at a concentration of 1–10 mg/ml in 0.1 M
sodium phosphate, 0.15 M NaCl, pH 7.2–7.5. Lower concentrations of protein may result
in decreased reaction yields and require increased quantities of reagent to obtain acceptable
levels of biotinylation. Avoid amine-containing buffers or components, such as Tris or imi-
dazole, which will react with the NHS ester and interfere with the biotinylation process.
2. Dissolve the NHS–PEG
n
–biotin compound in DMAC, DMSO, or DMF (pure and dry) at
a concentration of 10–20 mM. Prepare fresh in a fume hood.
3. With mixing, add an aliquot of the biotinylation stock solution to the protein solution to
provide at least a 10-fold molar excess over the concentration of protein present. Doing
a series of reactions with different molar amounts of the NHS–PEG
n
–biotin compound
may be done to optimize the modifi cation level.
4. React with gentle mixing for 30–60 minutes at room temperature or 2 hours at 4°C.
Figure 18.13 NHS–PEG
n
–biotin compounds.
3. Biotinylation Reagents Containing Discrete PEG Linkers 729
Figure 18.14 NHS-SS-PEG
4
-biotin can be used to label a primary antibody molecule that has specifi city for
a protein or interest. Incubation of the biotinylated antibody with a sample, such as a cell lysate, allows the
antibody to bind to its target. Capture of the antibody–antigen complex on an immobilized streptavidin reagent
effectively isolates the targeted protein from the other proteins in the sample. The disulfi de linkage in the spacer
arm of the biotin tag permits elution of the immune complex from the streptavidin support using DTT and
without using the strong denaturing condition typically required to break the streptavidin–biotin interaction.
