Iwamoto M., Kwon Y.-S., Lee T. (Eds.) Nanoscale Interface for Organic Electronics
Подождите немного. Документ загружается.

Structure Optimization for High Efficiency WOLEDs 99
An exciplex-based WOLED was reported by Kim et al. which
demonstrated exciplex between a charge transport layer and an
EML.
33
The authors developed a WOLED using exciplex formation
between active layers and a charge transport layer consisting of near
ultraviolet and yellow emission. The white device showed a current
efficiency of 12 cd/A at 1000 cd/m
2
and Commission Internationale
de I’Eclairage (CIE
x,y
) coordinates of (0.34, 0.34) at 8.6 mA/cm
2
,
respectively.
2.4. PIN- and Tandem-WOLEDs
The highly conductive p-doped material in the hole transporting layer
and n-doped material in the electron transporting layer could improve the
charge injection from the metals (anode and cathode) reduce the drive
voltage in OLEDs.
34-36
The operating voltages of OLEDs are close to the
thermodynamic limit. The device concept from LEDs has generalized to
OLEDs. Ho et al. demonstrated PIN WOLEDs with high power
efficiency and stable color which were comprised of 50% v/v
tungsten oxide (WO
3
)-doped N,N'-bis(naphthalen-1-yl)-N,N'-bis(phenyl)-
benzidine (NPB) as the p-doped transport layer and 2% cesium carbonate
(Cs2CO
3
)-doped 4,7-diphenyl-1,10-phenanthroline (BPhen) as the n-doped
transport layer.
37
Tandem WOLEDs were first introduced in 1996 with stacked
multiple emitting units in a single OLED.
38
The charge generation layers
(CGL) are formed by contact of a n-doped ETL and a p-doped HTL or
transparent inorganic conductors. The current efficiency of the tandem
device with N units is N times as high as that of the single-unit device,
normally. Kanno et al. developed tandem WOLEDs based on
a combination of fluorescent and phosphorescent emitters.
39
CGL
consisted of MoO
3
as p-type material and Li-doped BPhen as n-type
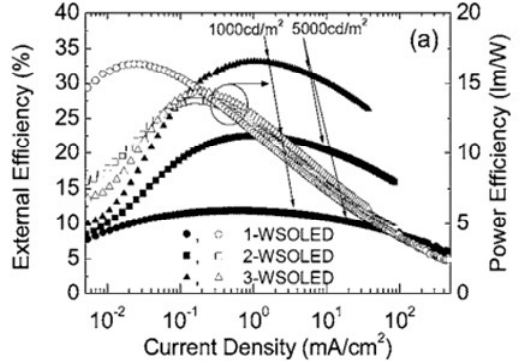
material, respectively. Figure 4 shows EQE and power efficiencies of
one- (circles), two- (squares), and three- (triangles) element tandem
WOLEDs as functions of current density. 1-, 2-, and 3-element white
devices showed a peak EQE of 12 ± 1, 23 ± 2, and 33 ± 3% at 0.78, 1.0,
and 1.1 mA/cm
2
, respectively.
J. H. Seo, J. H. Seo and Y. K. Kim 100
Fig. 4. EQE and power efficiencies of one- (circles), two- (squares), and three- (triangles)
element tandem WOLEDs as functions of current density.
2.5. Sensitizer- and Microcavity-WOLEDs
WOLEDs can be obtained from phosphorescent emitters as sensitizer
and fluorescent emitter.
40,41
OLEDs that use a phosphorescent sensitizer
were first introduced in 2000 reported by Baldo et al.
42
The device
with a phosphorescent sensitizer showed an EQE three times higher
than the control device without a phosphorescent sensitizer. Lei et al.
reported blue phosphorescent dye as an effective sensitizer and emitter
for WOLEDs, consisting of iridium(III) bis[(4,6-di-fluoropheny)-
pyridinato-N,C2] picolinate as the blue emitter and sensitizer and
4-(dicyanomethylene)-2-t-butyl-6-(1,1,7,7-tetramethyljulolidyl-9-enyl) as
the fluorescent emitter.
40
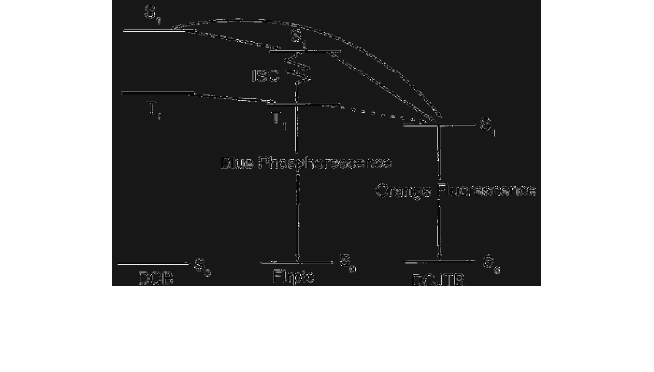
The mechanism showed that a blue
phosphorescent sensitizer can harvest both singlet and triplet excitons,
then transfer energy to the fluorescent emitter and emit blue
phosphorescence for white emissions; Fig. 5 illustrates energy transfer
mechanism in the white light device. The white device showed a
maximum luminance of 18200 cd/m
2
and a peak current efficiency of
9.2 cd/A.
Structure Optimization for High Efficiency WOLEDs 101
A microcavity is one of the most effective methods to improve the
luminance and enable brightness. A microcavity resonator for OLEDs
consists of a pair of mirrors facing each other across an EML. Typically,
one of the mirrors is a metal electrode and the other is a half mirror.
WOLEDs can also be obtained from a microcavity mechanism.
Multiwavelength resonant cavities for WOLEDs were reported by
Shiga et al. in 2003.
43
The authors designed WOLEDs with a
multiwavelength resonant cavity (MWRC) by using optical simulation.
The white device with MWRC was 1.3 times as bright as that of a
control device.
3. WOLEDs Based on Wet-Process
Thermal evaporation process has been used as a deposition method for
the organic materials, because it can process relatively uniform layers as
compared with wet-process.
30,44-47
However, its high fabrication cost (due
to wasting material) and its limited substrate size are weak points. On the
other hand, the fabrication of OLEDs by wet-process is well-known to
be low cost, as it does not require vacuum processing.
48-53
It has been
reported that solution processes such as spin-coating,
54,55
doctor blade,
56
ink-jet printing,
57
gravure printing,
58,59
and screen printing
60
remain cost
efficient because of large-area OLED fabrication.
Fig. 5. Energy transfer mechanism in the sensitizer WOLEDs.
J. H. Seo, J. H. Seo and Y. K. Kim 102
In the wet-process, WOLEDs typically consist of a white emissive
single-emission layer. WOLEDs are not manufactured with separated
red, green, and blue emission layer since it is difficult to form multilayer
structures because of solvent erosion of previously deposited layers
during spin-coating. This session is devoted to exploring methods to
improve WOLEDs performance by optimizing the device structure and
materials used in the device.
3.1. White Emission from Single Polymer
Poly(p-phenylene vinylene) (PPV) was used first for the operation of
polymer organic light-emitting diodes (PLEDs); increasing attention has
been paid to using poly(fluorine) (PF) because it is possible to develop
the blue emission and easily control the emission spectrum by bringing
the comonomer in a PF molecule. Various white emissive polymers can
be also synthesized based on the copolymerization of the PF backbone.
Some examples are shown in Fig. 1, where the PF with the blue
emission was copolymerized with red and green emissive structure
for the white emission.
61-65
Phenylenevinylene oligomer (Fig. 6(a)),
naphthalimide (Figs. 6(b)-6(d)), and fluorine-benzothiadiazole oligomer
(Figs. 6(e) and 6(f)) with the triphenylamine are introduced the for
the green emission, while the 4-(dicyanomethylene)-2-methyl-6-(p-
dimethylaminostyryl)-4H-pyran (DCM) derivative (Fig. 6(a)) and
thiophene-benzothiadiazole oligomer (Figs. 6(b)-6(f)) are induced
for the red emission in polymer. In contrast, Fig. 7 shows the polymers
with two-color white emission using the complementary color
relation.
66-71
The PF with blue emission is copolymerized with reddish-
yellow emitting comonomers, naphthalimide (Fig. 7(a)), triphenylamine-
benzothiadiazole oligomer (Figs. 7(b) and 7(c)), DCM derivative
(Fig. 7(d)), quinacridone derivative (Fig. 7(e)), and cyanovinylene
complex (Fig. 7(f)) based on phenothiazine. The triphenylamine and
oxadiazole are introduced in almost all chemical structures for the
efficient transport of hole and electrons, respectively, as well as color
tuning of PF. Even if naphthalimide moiety is the same, the emission
color can be changed as its connected location, as shown in Figs. 6(b)-6(d)
and Fig. 7(a). Each naphthalimide moiety connected to the conjugated
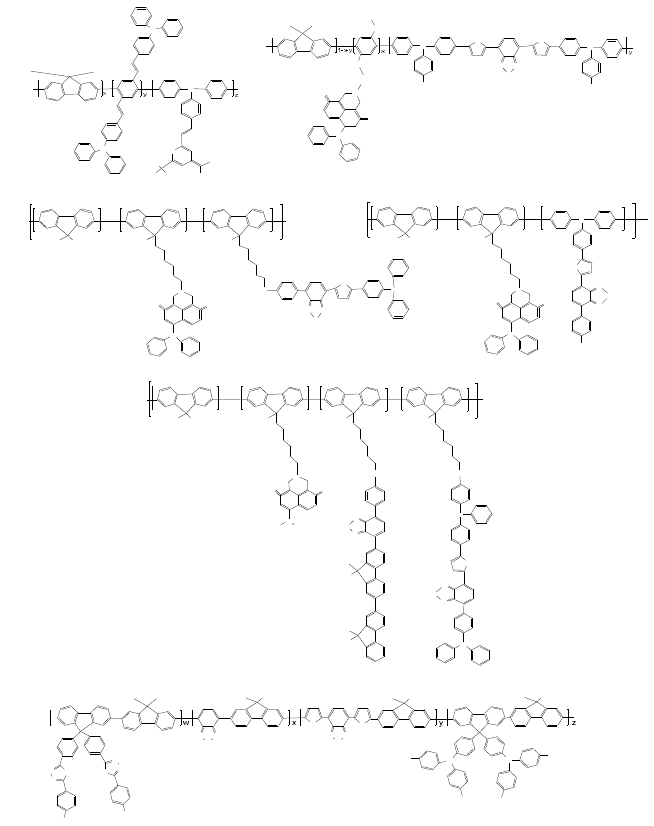
Structure Optimization for High Efficiency WOLEDs 103
Fig. 6. Single-component white emissive polymers with red, green, and blue emission. (a)
CIE
x,y
(0.33, 0.35), 0.10 cd/A, x = 0.95, y = 0.03, z = 0.02, (b) CIE
x,y
(0.31, 0.34), 1.59 cd/A,
0.83 lm/W, x = 0.0002, y = 0.0003, (c) CIE
x,y
(0.31, 0.32), 7.30 cd/A, 4.17 lm/W,
x = 0.0002, y = 0.0002, (d) CIE
x,y
(0.30, 0.31), 3.80 cd/A, 1.99 lm/W, x = 0.0002,
y = 0.0002, (e) CIE
x,y
(0.33, 0.36), 8.6 cd/A, 5.4 lm/W, x = 0.0005, y = 0.0005, z = 0.0002,
(f) CIE
x,y
(0.37, 0.36), 4.87 cd/A, w = z = 0.4938 n, x = 0.0080 n, y = 0.0044 n.
N
XHe HeX
N
N
O
CH
3
CH
3
CH
3
CN
CN
Oct
Oct
N
S
S
N
N N
S
CH
3
CH
3
O
C
2
H
4
N
O
O
N
O
Oct
Oct
Oct
*
1-x-y
Hex
x
Hex
y
*
n
N
O
O
N
O
S
N
N N
S
Oct
Oct
*
1-x-y
Hex
x
y
n
N
O
O
N
N
S
N
N
S
OMe
Oct
Oct
*
1-x-y-z
Hex
x
y
n
N
O
O
N
CH
3
CH
3
Hex
z
Hex
O
O
N
N
S
Hex
Hex
Hex
Hex
S
N
N
N
N
S
Oct
Oct
N
N
O
t
-
B
u
O
N
N
t-Bu
N
S
N
Oct
Oct
N
S
N
Oct
Oct
Oct
Oct
S
S
N N
n-Bu
n-Bu
n-Bu
n-Bu
(
a
)
(
b
)
(c)
(d)
(e)
(f)
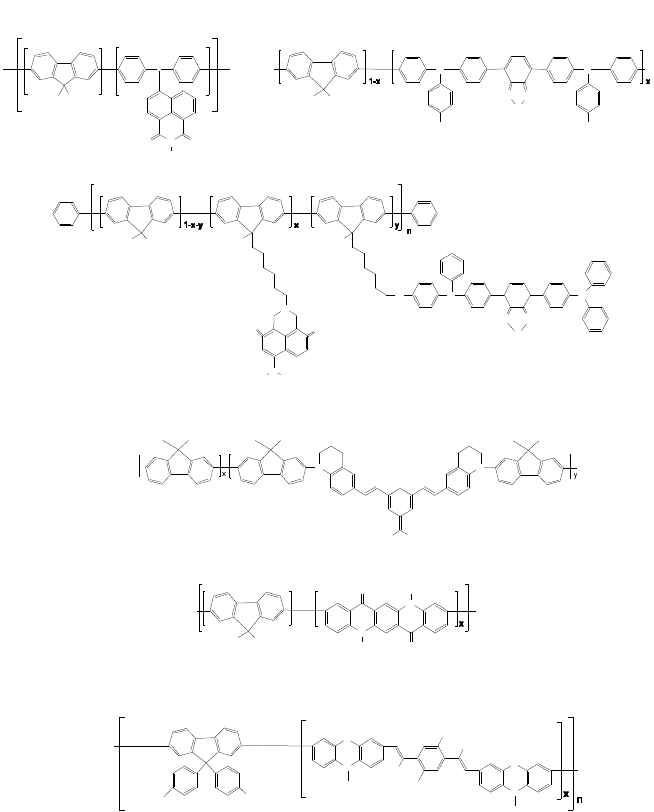
J. H. Seo, J. H. Seo and Y. K. Kim 104
Fig. 7. Single-component white emissive polymers with reddish-yellow and blue emission.
(a) CIE
x,y
(0.25, 0.35), 5.3 cd/A, 2.8 lm/W, x = 0.0005, (b) CIE
x,y
(0.35, 0.34), 8.99 cd/A,
5.75 lm/W, x = 0.0003, (c) CIE
x,y
(0.31, 0.36), 12.8 cd/A, 8.51 lm/W, x = 0.0005,
y = 0.0003, (d) CIE
x,y
(0.33, 0.31), 0.60 cd/A, x = 0.995, y = 0.005, (e) CIE
x,y
(0.27, 0.35),
3.47 cd/A, 2.18 lm/W, x = 0.0003, (f) CIE
x,y
(0.33, 0.39), 1.95 cd/A, 0.97 lm/W,
x = 0.005.
Oct Oct
N
NO O
C
10
H
21
1-x
x
n
Oct
Oct
*
N
CH
3
N
CH
3
N N
S
Oct
Oct Hex Hex
N
O
O
O
N
H
3
C CH
3
N N
N N
S
Hex
Hex
Hex
Hex
N
NC CN
N
Hex
Hex
OC
8
H
17
OC
8
H
17
N
S
CN
OMe
CN
N
S
OEH
Hex
H
e
x
(
a
)
(c)
(d)
(e)
(f)
(
b
)
Oct
Oct
*
1-x
N
N
n
C
10
H
21
O
O C
10
H
21
Structure Optimization for High Efficiency WOLEDs 105
Fig. 8. Single-component white emissive polymers with phosphorescent emitter. (a)
CIE
x,y
(0.31, 0.36), 4.6 cd/A, x = 0.0003, y = 0.003, (b) CIE
x,y
(0.35, 0.38), 8.2 cd/A,
7.2 lm/W, x = y = 0.2496 n, z = 0.004 n, w = 0.0004 n.
main-chain and non-conjugated side-chain of PF emits the reddish-
yellow and green color, respectively. Moreover, as shown in Figs. 6(e)
and 7(c), the dimethylamine-substituted naphthalimide, when used
instead of diphenylamine, is functionalized like a blue dopant and
improving the WOLED efficiency because its blue emission is more
efficient than fluorescent emission efficiency from PF.
Fluorescent material limits the realization of efficient devices due to
its low quantum yield; thus, the efficiency of WOLEDs can be enhanced
by using the phosphorescent emitters as comonomers. All iridium
complexes shown in Fig. 8 are used as red phosphorescence emitters.
72-75
A remarkable thing is that the complexes of Figs. 6(f) and 8(b) were
reported by same researchers and their chemical structures are the same
except for the red emitting site. The device efficiency with fluorescent
emitter shows a maximum efficiency of 4.87 cd/A, whereas the device
Oct
Oct
Oct
Oct
N
N N
S
O
O
CF
3
Ir
N
Oct
Oct
N
N
O
t-Bu
O
N
N
t-Bu
Oct
Oct
N
S
N
Oct
Oct
Oct
Oct
N
N N
n-Bu
n-Bu
n-Bu
n-Bu
O
O
Ir
N
(
a
)
(b)
J. H. Seo, J. H. Seo and Y. K. Kim 106
with phosphorescent emitter has a much higher efficiency of 8.20 cd/A.
Therefore, it can be concluded that the introducing the phosphorescent
emitter is an effective method to improve the efficiency of WOLEDs based
on the polymer. The CIE coordinates, efficiency, and copolymerization
ratio of all complexes shown in Figs. 6-8 are presented in each figure
caption.
3.2. White Emission from Blended Red, Green,
and Blue Emitters
3.2.1. Polymer/Polymer Blending
There are many reports that WOLEDs can be also fabricated by blending
the red, green, and blue emitting polymers (or reddish-yellow and blue
emitting polymers) in the emission layer.
76-79
For example, when the
reddish-yellow emitting polymer (PFD) copolymerized with DCM
derivative and the blue emitting polymer (PF) are blended, they emit
the white color of CIE (0.33, 0.35) and demonstrate an efficiency of
0.26 cd/A.
79
However, these blended polymers lead to relatively low
efficiency and high driving voltage, due to their difficult purification and
wide energy band gap. Therefore, most contemporary researchers prefer
to use phosphorescent small molecular red, green, and blue dopants
rather than a polymer emitter.
3.2.2. Polymer Host/Phosphorescent Small Molecular
Dopants Blending
In order to improve the device efficiency, it is advisable to apply
the host-dopant system with phosphorescent small molecular dopants.
Recently, poly(N-vinyl-carbazole) (PVK) and poly(9,9-dioctylfluorene)
(PFO) shown in Fig. 9 have been used as polymer hosts for the
fabrication of OLEDs by wet-process.
80-84
Wu et al. fabricated a highly
efficient WOLED based on a PVK host.
80
The device consisted of
bis(2-(4,6-difluorophenyl)-pyridinato-N,C2') picolinate (Firpic), iridium
tris(2-(4-tolyl)pyridinato-N,C2') (Ir(mppy)
3
), and iridium bis(1-
phenylisoquinoline) (acetylacetonate) (Ir(piq)
2
(acac)) as blue, green, and
red phosphorescent dopants, respectively, in a PVK host. This device had
Structure Optimization for High Efficiency WOLEDs 107
current efficiency, EQE, and power efficiency of 24.3 cd/A, 14.4%, and
9.5 lm/W, respectively, with CIE
x,y
coordinates of (0.34, 0.46). Although
this device emitted a reddish-white color, these efficiency values are the
best among wet-processed WOLEDs reported so far. PVK is a very
suitable polymer host, due to its excellent film-forming properties,
high glass transition temperature (T
g
) (~160°C) and hole transport
characteristics.
85
However, PVK has a relatively low-lying triplet state
(T
1
) (ca. 2.5 eV). When using dyes to tune the emission color of a device,
the host material should not act as a quencher of the dye emission
via energy transfer from the dye to the host. When the dye is a
phosphorescent emitter, its emission can be quenched by energy transfer
to the triplet excited-state of the host.
86,87
To prevent this, the triplet
energy of the host must be higher than that of the dopant. In general, red,
green, and blue phosphorescent emitters have T
1
of approximately 2.0 eV,
2.5 eV, 2.7 eV, respectively. Thus, even if PVK is an excellent host
material for the red and green emitters, it is not suitable for the blue emitter
in terms of energy transfer, because it leads to endorthermic energy
transfer and exchange energy loss.
85
To obtain highly efficient WOLEDs,
it is necessary to develop a host material with high T
1
.
3.2.3. Small Molecular Host/Phosphorescent Small Molecular
Dopants/Polymer Binder Blending
In recent years, it has been shown that the small molecular hosts of
carbazole-type shown in Fig. 9 are suitable for the phosphorescent
N
n
N N
N N
n
PVK
PFO
C
B
P
m
C
P
Fig. 9. Polymers and small molecules generally used as host in wet-process OLEDs.
J. H. Seo, J. H. Seo and Y. K. Kim 108
dopant in wet-process OLEDs as well as thermal-evaporated OLEDs.
88-90
This is because they have relatively high T
1
, narrow energy band gap,
and excellent charge transport ability in comparison with polymer hosts.
The CBP (T
1
; 2.6 eV) and mCP (T
1
; 2.9 eV) are the most generally used
host materials in phosphorescent OLEDs. However, although high
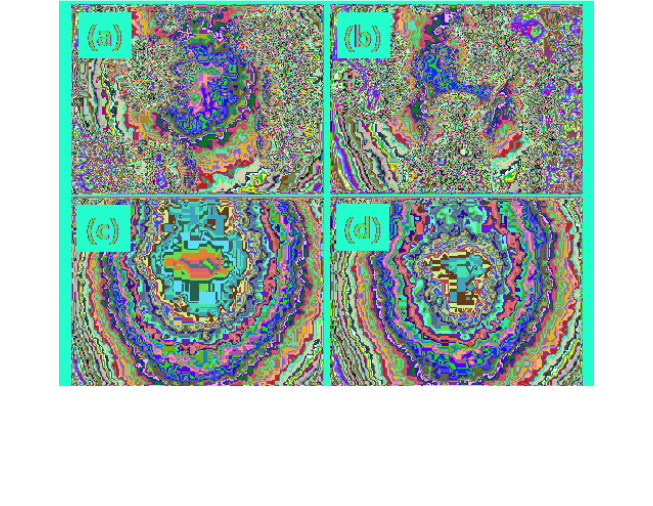
efficiency can be obtained by using mCP or CBP, crystallization often
occurs during the annealing process (Figs. 10(a) and 10(b)) required by
wet-process for the exclusion of solvents remaining in the film.
88
As such,
small molecular hosts with high T
1
and T
g
need to be developed for
higher efficiency and device stability. However, Lee et al. reported that
crystallization of small molecules can be suppressed by adding a polymer
binder into emission layer (Figs. 10(c) and 10(d)).
88
They added a PVK
or polystyrene (PS) binder into the emission layer, consisting of CBP,
Firpic, and iridium bis(2-phenylbenzothiozolato-N,C2')(acetylacetonate)
(Bt
2
Ir(acac)) as host, blue emitter, and red emitter, respectively. The
Fig. 10. Optical microscopy images of thin films stored at room temperature for 24 h
after spin-coating with toluene solution of (a) CBP, (b) CBP and Firpic, (c) PS, CBP, and
Firpic, and (d) PVK, CBP, and Firpic.
