Jung Han. Innovations in Food Packaging
Подождите немного. Документ загружается.

Lipid-based edible
films
and coatings
365
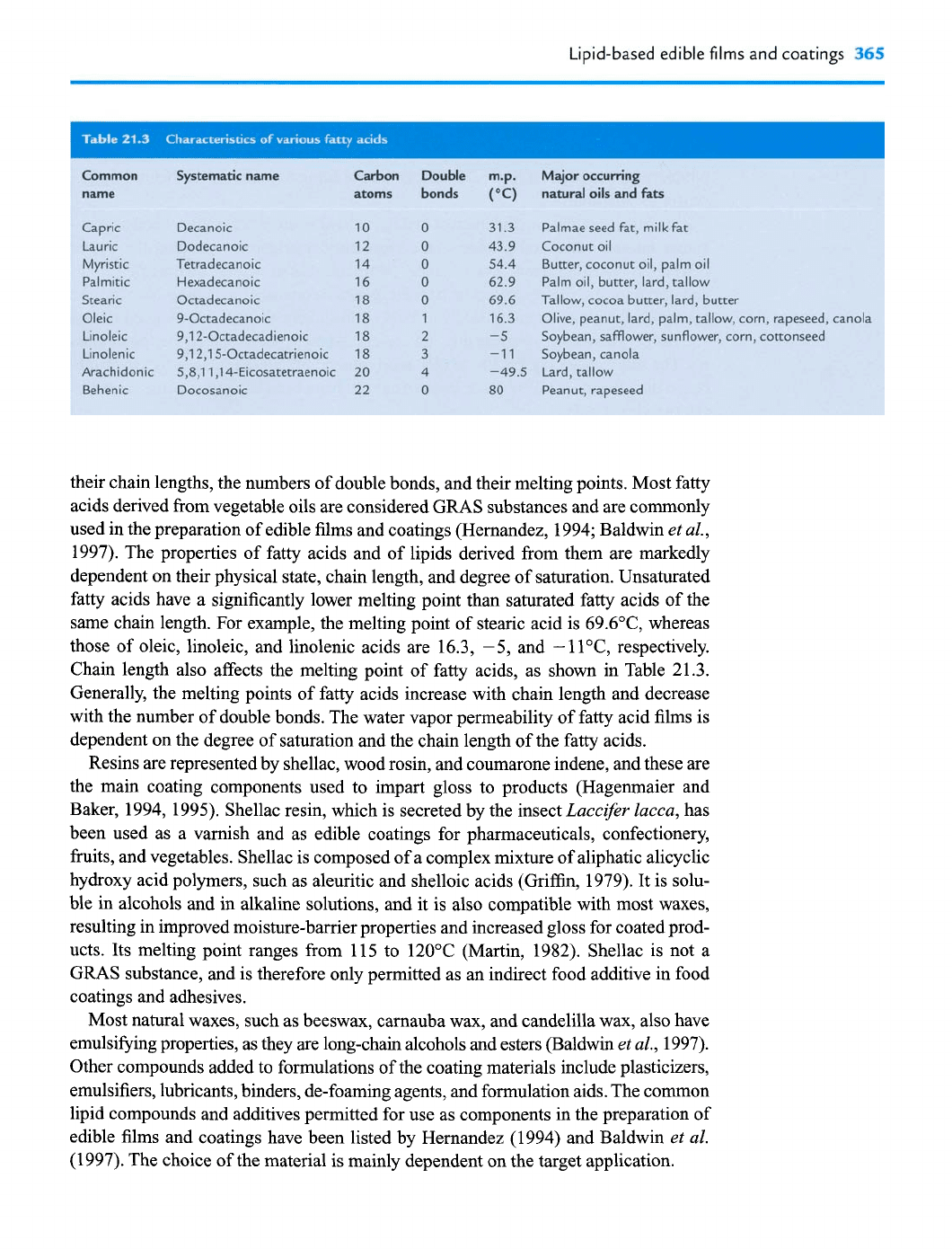
Common
name
Capric
Lauric
Myristic
Palmitic
Stearic
Oleic
Linoleic
Linolenic
Arachidonic
Behenic
Systematic name
Decanoic
Dodecanoic
Tetradecanoic
Hexadecanoic
Octadecanoic
9-Octadecanoic
9,12-Octadecadienoic
9,12,15-Octadecatrienoic
5,8,11,14-Eicosatetraenoic
Docosanoic
Carbon
atoms
Double
bonds
m.p.
Major
occuning
(OC)
natural oils
and
fats
31.3
Palmae seed
fit,
milk fat
43.9 Coconut oil
54.4 Butter, coconut oil, palm oil
62.9 Palm oil, butter, lard, tallow
69.6 Tallow, cocoa butter, lard, butter
16.3
Olive, peanut, lard, palm, tallow, corn, rapeseed, canola
-5
Soybean, safflower, sunflower, corn, cottonseed
-1 1 Soybean, canola
-49.5 Lard, tallow
80
Peanut, rapeseed
their chain lengths, the numbers of double bonds, and their melting points. Most fatty
acids derived from vegetable oils are considered GRAS substances and are commonly
used in the preparation of edible films and coatings (Hernandez, 1994; Baldwin
et
al.,
1997). The properties of fatty acids and of lipids derived from them are markedly
dependent on their physical state, chain length, and degree of saturation. Unsaturated
fatty acids have a significantly lower melting point than saturated fatty acids of the
same chain length. For example, the melting point of stearic acid is 69.6"C, whereas
those of oleic, linoleic, and linolenic acids are 16.3, -5, and
-1
1°C, respectively.
Chain length also affects the melting point of fatty acids, as shown in Table 21.3.
Generally, the melting points of fatty acids increase with chain length and decrease
with the number of double bonds. The water vapor permeability of fatty acid films is
dependent on the degree of saturation and the chain length of the fatty acids.
Resins are represented by shellac, wood rosin, and coumarone indene, and these are
the main coating components used to impart gloss to products (Hagenmaier and
Baker, 1994, 1995). Shellac resin, which is secreted by the insect Laccifer lacca, has
been used as a varnish and as edible coatings for pharmaceuticals, confectionery,
fruits, and vegetables. Shellac is composed of a complex mixture of aliphatic alicyclic
hydroxy acid polymers, such as aleuritic and shelloic acids (Griffin, 1979). It is solu-
ble in alcohols and in alkaline solutions, and it is also compatible with most waxes,
resulting in improved moisture-barrier properties and increased gloss for coated prod-
ucts. Its melting point ranges from 115 to 120°C (Martin, 1982). Shellac is not a
GRAS substance, and is therefore only permitted as an indirect food additive in food
coatings and adhesives.
Most natural waxes, such as beeswax, carnauba wax, and candelilla wax, also have
emulsifying properties, as they are long-chain alcohols and esters (E3aldwin
et
al., 1997).
Other compounds added to formulations of the coating materials include plasticizers,
emulsifiers, lubricants, binders, de-foaming agents, and formulation
aids.
The common
lipid compounds and additives permitted for use as components in the preparation of
edible films and coatings have been listed by Hernandez (1994) and Baldwin
et
al.
(1997). The choice of the material is mainly dependent on the target application.

366
Innovations in Food Packaging
When lipid-based edible films and coatings are a part of the food product and are
consumed with its contents, it is not only important that the films or coatings be com-
patible with the product, but also that the edible films or coatings are benign from a
sensory standpoint.
Two forces are driving the interest in natural lipid sources for coating foods; a con-
sumer interest in natural products, and regulatory restrictions regarding the use of
petroleum-based products. For instance, petroleum-based waxes, such as polyethyl-
ene and parailk, are restricted or banned in some countries, including Norway, the
United Kingdom, and Japan (Baldwin, 1994). Most likely, the naturally derived waxes,
such as beeswax, carnauba wax etc., are considered more acceptable in the food indus-
try. The same reasoning applies to oils, with mineral oil being replaced by vegetable-
based oils. Resins are also under inspection, and have been banned in some countries
(Hernandez, 1994).
Preparation
Generally, edible films and coatings are made from a solution or a dispersion of the
film-forming agents, followed by a film-forming application method such as casting,
spraying, dipping, extrusion, falling film enrobing, etc. Edible films contain at least one
component with a high molecular weight polymer such as protein or polysaccharide,
particularly if a self-supporting film is desired. Long-chain polymeric structures are
required to yield film matrices with appropriate cohesive and tensile strength when
deposited from a suitable solvent (Banker, 1966). The solvent system used for film-
forming is also important in determining the properties of the finished films, and for
edible films and coatings it is limited primarily to water, ethanol, or a combination of
the two.
Environmental conditions during film formation can markedly influence the final
film properties. Application of
warm
film-forming solutions to a
warm
receiving surface
yields the most cohesive films. However, excessive temperatures resulting in an exces-
sive rate of solvent evaporation during
film
drylng may prematurely immobilize polymer
molecules, before they have an opportunity to coalesce into a continuous, coherent film,
possibly resulting in a brittle film (Banker, 1966). This may also result in film defects
such as pinholes or non-uniform film thickness, both of which increase water vapor or
gas permeability. Since solvents evaporate rapidly from atomized coating suspensions,
the potential for premature immobilization of polymer chains is greater in spray-
formed films than in films formed by casting or dipping (Kester and Fennema, 1986).
Another important factor is the relative humidity. Low relative humidity during film
drying may increase the rate of solvent removal, probably resulting in brittle films,
whereas high relative humidity may delay the drying time.
In contrast to the self-supporting films, edible coatings have been generally used as
protective coatings for fresh fruits and vegetables to prevent the decrease in turgor and
in
weight and to maintain high quality during commercialization (Avena-Bustillos
et
al.,
1994). The most well known and oldest coating method was the application of natural
waxes and lipid coatings on specific fruits and vegetables to reduce dehydration and

Lipid-based edible films and coatings
367
abrasion during processing and handling, and to improve appearance by adding gloss
(Baldwin,
1994;
Hagenmaier and Baker,
1994;
Baldwin
et
al.,
1997).
Limitations to
their use include their poor mechanical properties and oily appearance in some
products. Composite films and coatings have been developed to combine the advan-
tages of both lipid and hydrocolloid components (Baldwin
et
al.,
1997;
Krochta
and De Mulder-Johnston,
1997).
The lipid component in the formulation can serve as
a good barrier to water vapor, while the hydrocolloid component can provide a selec-
tive barrier to oxygen and carbon dioxide and the necessary supporting matrix
(Guilbert,
1986;
Kester and Fennema,
1986;
Baldwin,
1994;
Wong
et
al.,
1994;
Baldwin
et
al.,
1997).
Generally, two kinds of composite films are known, according to their preparation
method
-
lamination or emulsion. One is a bi-layer film in which a hydrophobic lipid
layer is laminated over a preformed hydrophilic
film,
resulting in the lipid being a distinct
layer within or atop the hydrophilic
film
(Park
et
al.,
1994;
Gontard
et
al.,
1995;
Weller
et
al.,
1998).
The other is an emulsion film in which the lipid material is uniformly dis-
persed throughout the hydrophilic film (McHugh and Krochta,
1994c;
Shellhammer and
Krochta,
1997;
Rhim
et
al.,
1999).
Figures
21.1
and
21.2
show the procedures for the
preparation of typical bi-layer and emulsion composite films, respectively.
Both bi-layer and emulsion films offer advantages. The laminate films are easier to
apply with regard to the temperature, due to the distinct nature of the support matrix
and lipid (Koelsch,
1994).
During the casting of the lipid onto protein or polysaccha-
ride film, the temperature of the film and lipid can easily be controlled separately.
Film forming solution (1 0% whey protein)
J.
Protein denaturation
by
heating at 90°C
for
30
min
C
Add lipid materials (camauba, candelilla or beeswax)
C
Coarse homogenization (high-shear probe mixer)
1st
:
1
minat 15000rpm
2nd
:
2
min at
22
000 rpm
(while heating at 80 or 90°C)
C
Fine homogenization (microfluidizer)
7
passed at
41
MPa
(while heating at 80 or 90°C)
C
Cooling in an ice bath
C
Casting
C
Drying over
18
h at
4050°/.
RH,
22-25°C
C
Whey protein emulsion film
Fipm
21.1
Procedure for the preparation ofwhey protein emulsion films (Shellhammer and Krochta,
1997).
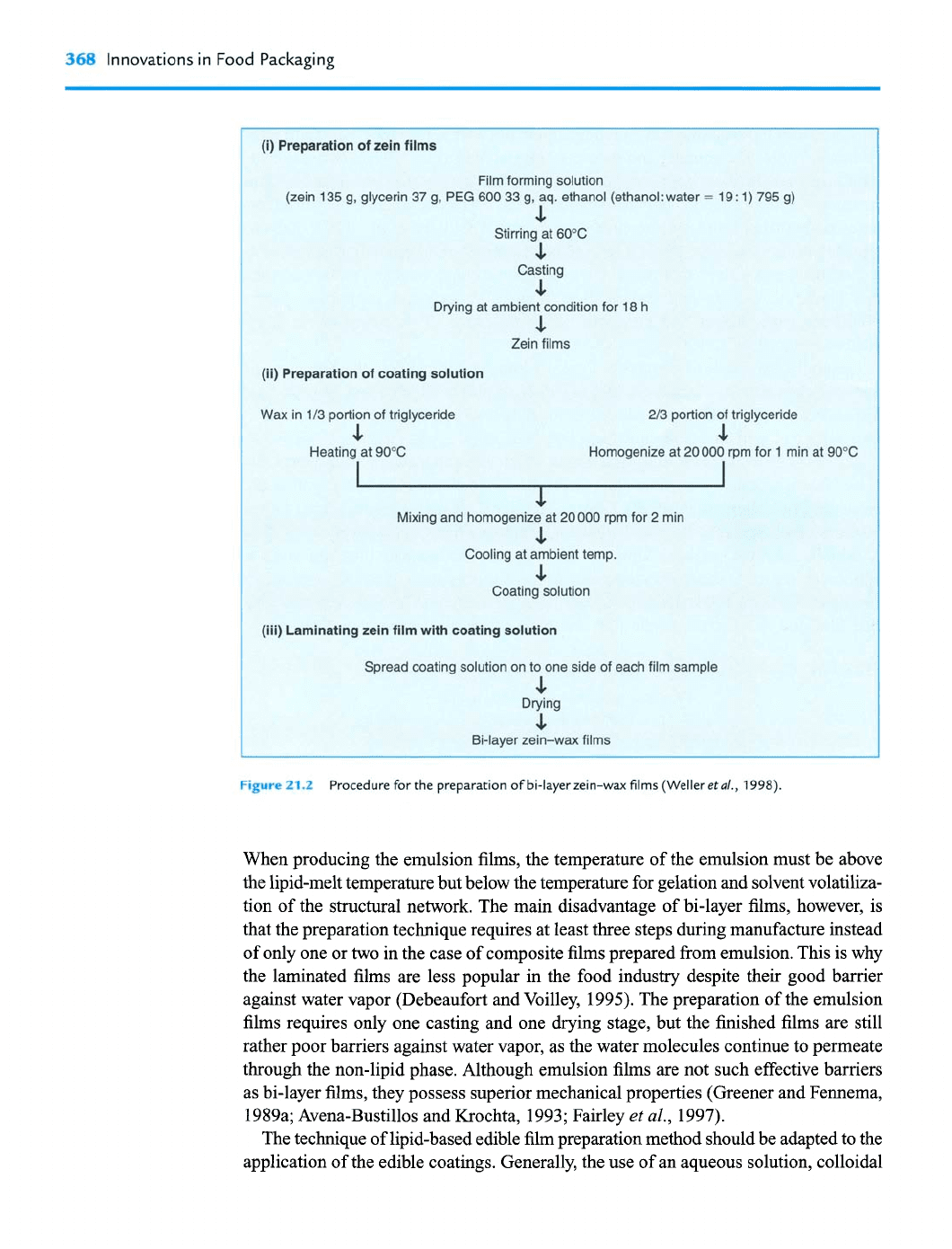
368
Innovations in Food Packaging
21@
Procedure for the preparation of bi-layer zein-wax films (Weller
et
a/.,
1998).
When producing the emulsion films, the temperature of the emulsion must be above
the lipid-melt temperature but below the temperature for gelation and solvent volatiliza-
tion of the structural network. The main disadvantage of bi-layer films, however, is
that the preparation technique requires at least three steps during manufacture instead
of only one or two in the case of composite films prepared from emulsion. This is why
the laminated films are less popular in the food industry despite their good barrier
against water vapor (Debeaufort and Voilley, 1995). The preparation of the emulsion
films requires only one casting and one drying stage, but the finished films are still
rather poor barriers against water vapor, as the water molecules continue to permeate
through the non-lipid phase. Although emulsion films are not such effective barriers
as bi-layer films, they possess superior mechanical properties (Greener and Fennema,
1989a; Avena-Bustillos and Krochta, 1993; Fairley
et
al.,
1997).
The technique of lipid-based edible
film
preparation method should be adapted to the
application of the edible coatings. Generally, the use of an aqueous solution, colloidal

Lipid-based edible films and coatings
369
dispersion or emulsion of the film-coating materials with a relatively high concentra-
tion is preferred. The application and distribution of the film-coating material in a liq-
uid form can be achieved by (Guilbert, 1986):
Hand spreading with a paint brush
Spraying
Falling
film
enrobing
Dipping and subsequent dripping
Distribution in a revolving pan (pan coating)
Bed fluidizing or air brushing.
When considering possible edible coating applications, attention must be paid to the
requirement that edible coating formulations have to be wet when spread on the food
surface and upon drying form a film coating that has adequate adhesion, cohesion,
and durability to function properly. These properties are influenced by both edible film
formulation, and methods of coating and drying. Lack of attention to these facts has
resulted in inconsistent and unsatisfactory results in many studies. In addition, edible
coatings must provide a satisfactory appearance, aroma, flavor, and mouthfeel.
Selecting the best application for the appropriate foods and good control of environ-
mental conditions is necessary to ensure microbial stability.
Physical
properties
The intended use of edible films and coatings requires a clear understanding of their
moisture barrier and mechanical properties. These properties depend strongly on the
film composition, its formation, and the methods of application onto the food prod-
ucts (Cuq
et
al., 1995; Debeaufort
et
al., 1998).
Many researchers have examined the water vapor permeability
(WVP)
and the
mechanical properties of composite films made from proteins or polysaccharides with
added lipids. For example, composite protein-lipid films had lower WVP values than
control protein films from caseinates (Avena-Bustillos and Krochta, 1993), whey pro-
tein (McHugh and Krochta, 1994a, 1994b; Bannerjee and Chen, 1995; Pkrez-Gago and
Krochta, 1999), zein (Weller
et
al., 1998), and wheat gluten (Gennadios
et
al., 1993;
Gontard
et
al., 1994). Accordingly, the barrier against water vapor was improved with
composite polysaccharide-lipid films made from methylcellulose (MC) (Greener and
Fennema, 1989a, 1989b; Kester and Fennema, 1989a, 1989b; Koelsch and Labuza,
1992; Debeaufort and Voilley, 1999, and hydroxypropyl methylcellulose (HPMC)
(Kamper and Fennema, 1984a, 1984b; Hagenmaier and Shaw, 1990).
Morillon
et
al.
(2002) listed the WVP of lipid-based edible films as a function of
their composition and structure, and part of their list is shown in Table 2 1.4. While
some of the lipid and food-grade hydrophobic substances (principally waxes) have
WVP values close to those of synthetic plastic films, such as low-density polyethyl-
ene (LDPE) or polyvinyl chloride (PVC), they are nonetheless more permeable to
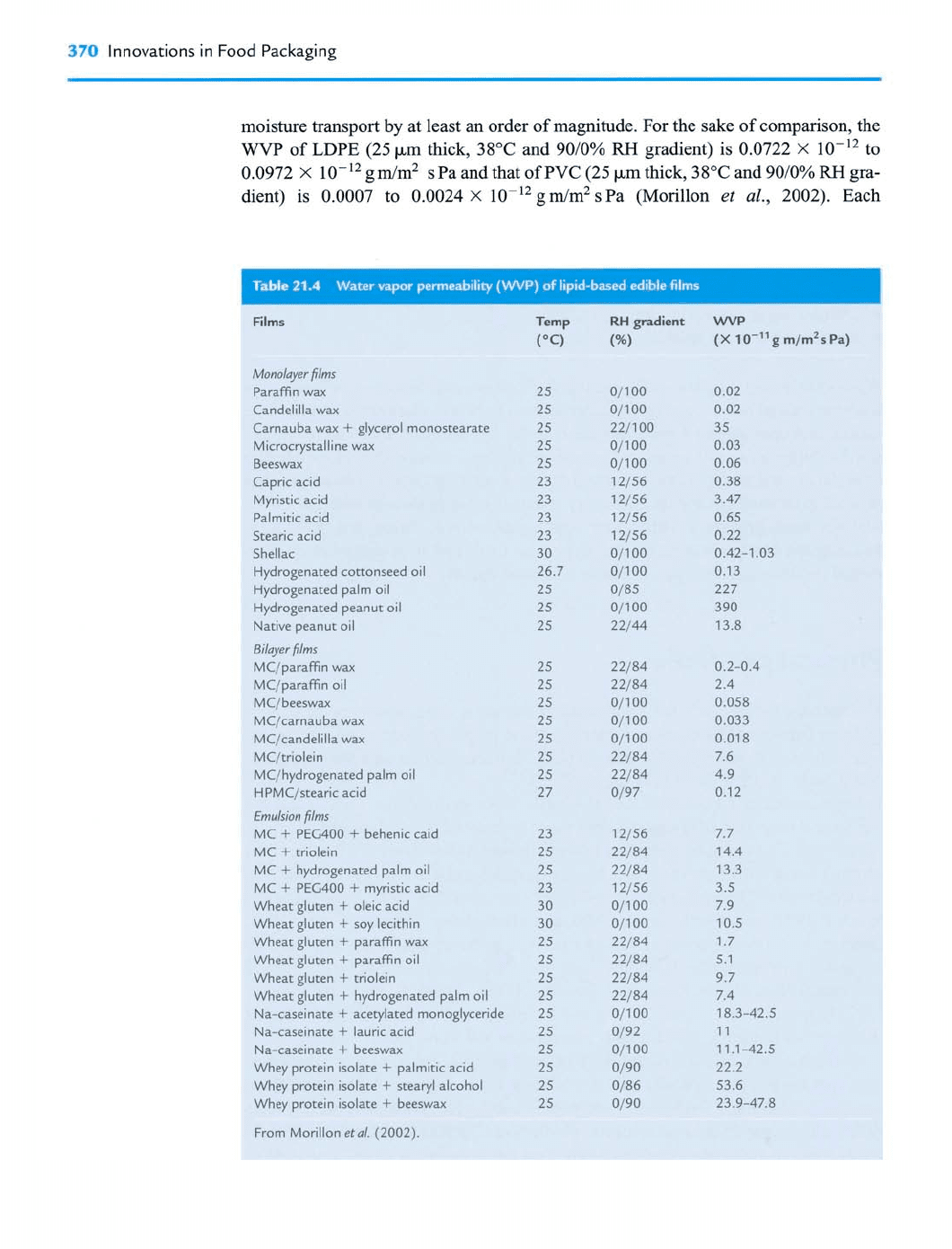
370
Innovations in Food Packaging
moisture transport
by
at least an order of magnitude. For the sake of comparison, the
WVP
of LDPE (25 pm thick, 38°C and 9010%
RH
gradient) is 0.0722
X
10-l2 to
0.0972
X
10-l2 gdm2 s Pa and that of PVC (25 pm thick, 38°C and 9010%
RH
gra-
dient) is 0.0007 to 0.0024
X
1
0-l2 g rn/m2 s Pa (Morillon
et
al.,
2002). Each
Films
I
T.bk
21.4
Water
vapor
pennclbility
(wvp)
of
lid-bad
sdi
fib
I
Monolayer
films
Paraffin
wax
Candelilla wax
Camauba wax
+
glycerol monostearate
Microcrystalline wax
Beeswax
Capric acid
Myriaic acid
Palmitic acid
Stearic acid
Shellac
Hydrogenated cottonseed oil
Hydrogenated palm oil
Hydrogenated peanut oil
Native peanut oil
Bilayar
films
MCIparaffin
wax
MC/paraffin oil
MC/ beeswax
MC/camauba wax
MC/candelilla wax
MC/triolein
MCIhydrogenated palm oil
HPMC/stearic acid
Emulsion
films
MC
+
PEG400
+
behenic caid
MC
+
triolein
MC
+
hydrogenated palm oil
MC
+
PEG400
+
myristic acid
Wheat gluten
+
oleic acid
Wheat gluten
+
soy lecithin
Wheat gluten
+
paraffin
wax
Wheat gluten
+
paraffin oil
Wheat gluten
+
triolein
Wheat gluten
+
hydrogenated palm oil
Na-caseinate
+
acetylated monoglyceride
Na-caseinate
+
lauric acid
Na-caseinate
+
beeswax
Whey protein isolate
+
palmitic acid
Whey protein isolate
+
steaiyl alcohol
Whey protein isolate
+
beeswax
From Morillon
eta/.
(2002).
. .
,
.
.
m
RH
gradient
(%I

Lipid-based edible films and coatings
371
hydrophobic substance has its own physicochemical properties, and thus edible films
based on lipids have variable behaviors against moisture transfer. Generally, the
WVP
decreases with increasing hydrophobicity of the added lipid materials. Waxes are the
most efficient substances to decrease the
WVP
because of their high hydrophobicity
due to their high content of long-chain fatty alcohols and alkanes. The hydrophilic
groups of lipid molecules normally promote water vapor sorption, which may bring
about water vapor migration through the film. In addition, many other factors inside
or outside the lipid-based edible films affect its barrier properties.
lnfluence of
film
preparation methods on
WP
The structure and barrier properties of lipid-based films strongly depend on the film
preparation method (Kamper and Fennema, 1984a; Martin-Polo
et
al., 1992;
Debeaufort
et
al., 1993). The barrier efficiency of bi-layer films is in the same order
of magnitude as that of pure lipid or synthetic plastic films, and much lower than that
of emulsion films (Table 21.4). The structure and the state of the surface of the film
mainly depends on the film preparation technique. Debeaufort
et
al. (1993) used scan-
ning electron microscopy (SEM) to characterize the surfaces of both emulsified and
bi-layer films composed of MC and parafi wax. The emulsified films had an irregu-
lar surface and a heterogeneous structure. In this type of film, paraffin wax was dis-
persed in the form of globules in the MC matrix. In contrast, the lipid component of
the bi-layer or laminated film had a smooth surface and homogeneous structure. They
determined the
WVP
of both types of films, and found that the more homogeneously
the paraffin wax was distributed, the more the barrier efficiency increased. Nevertheless,
Kamper and Fennema (1984a) reported opposite results with both bi-layer and emul-
sion films composed of HPMC and stearic and palmitic acids. Their emulsion films
had 40 times lower permeability than the bi-layer films. These contradictory results
could be explained by considering film preparation conditions. Although the initial
emulsion prepared by Kamper and Fennema was homogeneous, a phase separation
was observed during drying that led to an apparent bi-layer structure in the emulsified
films. Such reorientation of lipid molecules during drylng and storage of the films
greatly influences the
WVP
of lipid-based composite films.
In
some cases, the
WVP
of
lipid-based edible films varies greatly with the orientation of the lipid molecules within a
film (Avena-Bustillos and Krochta, 1993; Kamper and Fennema, 1984a).
In
emulsion
films, water migrates preferentially through the continuous hydrophilic matrix and the
dispersed lipid phase only modifies the apparent tortuosity. McHugh and Krochta
(1994b) reported that lipid particle size and distribution significantly affected the
WVP
of whey proteinheeswax emulsion films. They found that as mean emulsion
particle diameters decreased, the
WVP
of the film decreased linearly.
lnfluence of fatty acids
Most fatty acids derived from vegetable oils are considered to be GRAS substances,
and they are commonly used
in
the preparation of lipid-based edible films and coatings.
372
Innovations in Food Packaging
The properties of fatty acids are markedly dependent on their chain length and on
their degree of saturation. As stated earlier, unsaturated fatty acids have a lower melt-
ing point than do saturated fatty acids of the same length (Table 21.3).
Similarly, the water vapor permeability of fatty acid films is dependent on the
degree of saturation and the chain length of the fatty acids. As chain length increases,
chain mobility decreases, making fatty acids with long chains good barriers to water
vapor transmission (McHugh and Krochta, 1994a;
Rhim
et
al.,
1999). On the contrary,
fatty acids with shorter chain lengths tend to have greater chain mobility within
the
structure of composite films, resulting
in
higher water vapor permeabilities (McHugh
and Krochta, 1994~). Although fatty acids such as stearic and palmitic acid lack the
structural integrity to form continuous coatings, they have been used successfully in
formulations for composite films. Furthermore, composite films containing unsatu-
rated fatty acids have been shown to be more permeable to water vapor than those
containing saturated fatty acids (Kamper and Fennema, 1984a). The poor barrier
properties of unsaturated fatty acids are a result of the expansion
in
the molecular vol-
ume that occurs with the introduction of a double bond, and of the lower melting point
with greater chain mobility (Greener and Fennema, 1992).
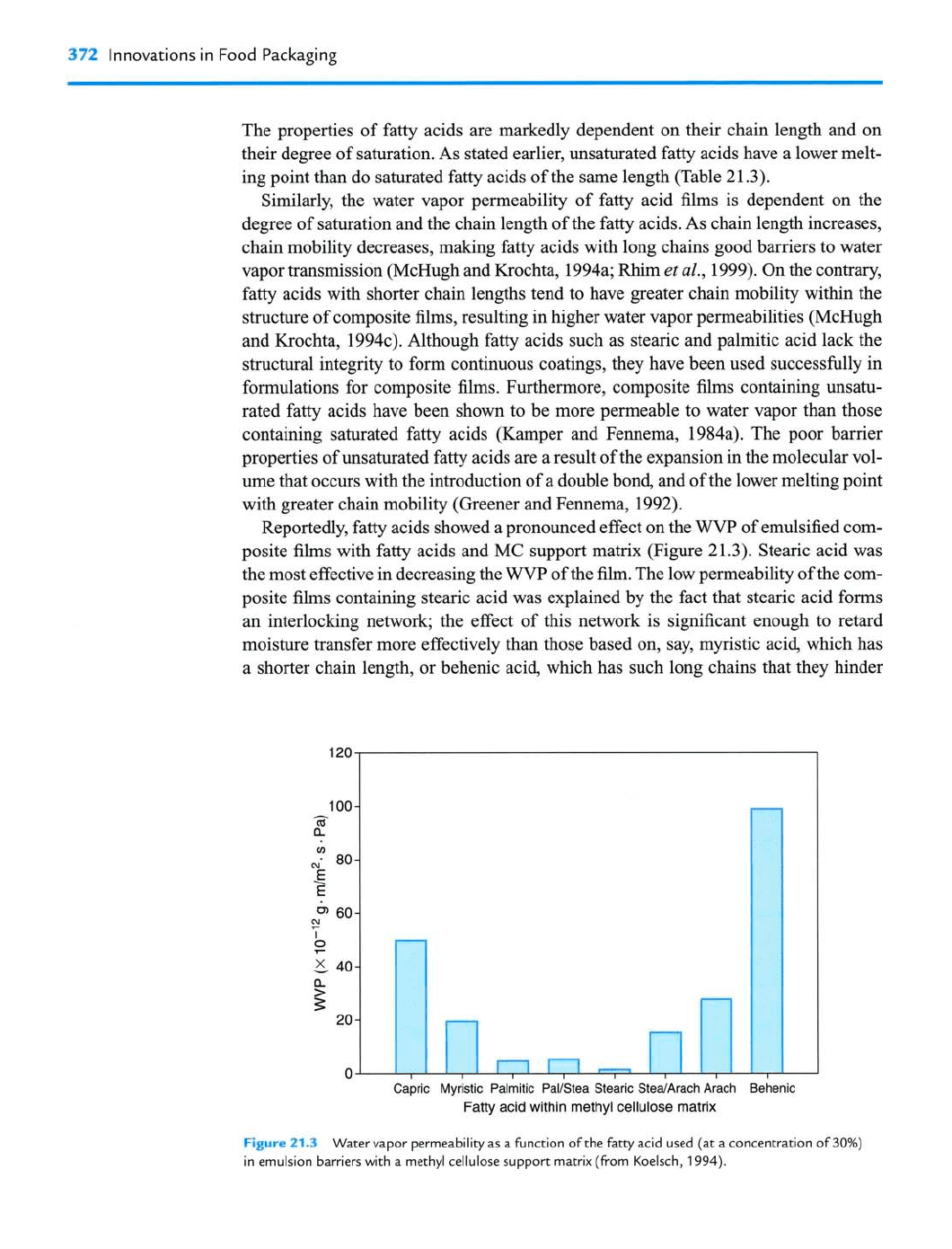
Reportedly, fatty acids showed a pronounced effect on the
WVP
of emulsified com-
posite films with fatty acids and
MC
support matrix (Figure 21.3). Stearic acid was
the most effective in decreasing the
WVP
of the film. The low permeability of the com-
posite films containing stearic acid was explained by the fact that stearic acid forms
an interlocking network; the effect of this network is significant enough to retard
moisture transfer more effectively than those based on, say, myristic acid, which has
a shorter chain length, or behenic acid, which has such long chains that they hinder
I.
Capric Myristic Palmitic PalIStea Stearic SteaIArach Arach Behenic
Fatty acid within methyl cellulose matrix
Figure
213
Water vapor permeability as a function of the fatty acid used (at a concentration of
30%)
in emulsion barriers with a methyl cellulose support matrix (from Koelsch,
1994).
Lipid-based edible
films
and coatings
373
the formation of a tightly interlocking network. In essence, stearic acid provides the
optimum chain length without hindering the formation of an interlocking network.
However, this is not always the case. Tanaka
et
al.
(2000) reported that among the lipid
materials tested for preparation of composite films with fish water-soluble proteins,
the incorporation of oleic acid was the most effective from the standpoint of the over-
all properties (tensile strength, elongation at break, and WVP) of the films.
Rhim
et
al.
(1999) showed that palrnitic acid was more effective in decreasing the WVP of emulsi-
fied soy protein isolate films with fatty acids. The effect of fatty acids on the perme-
ability properties of fatty acid-based composite films depended on the fatty acid
characteristics, and on the interactions between the fatty acid and the hydrocolloid
structural matrix.
Influence of the concentration of lipids
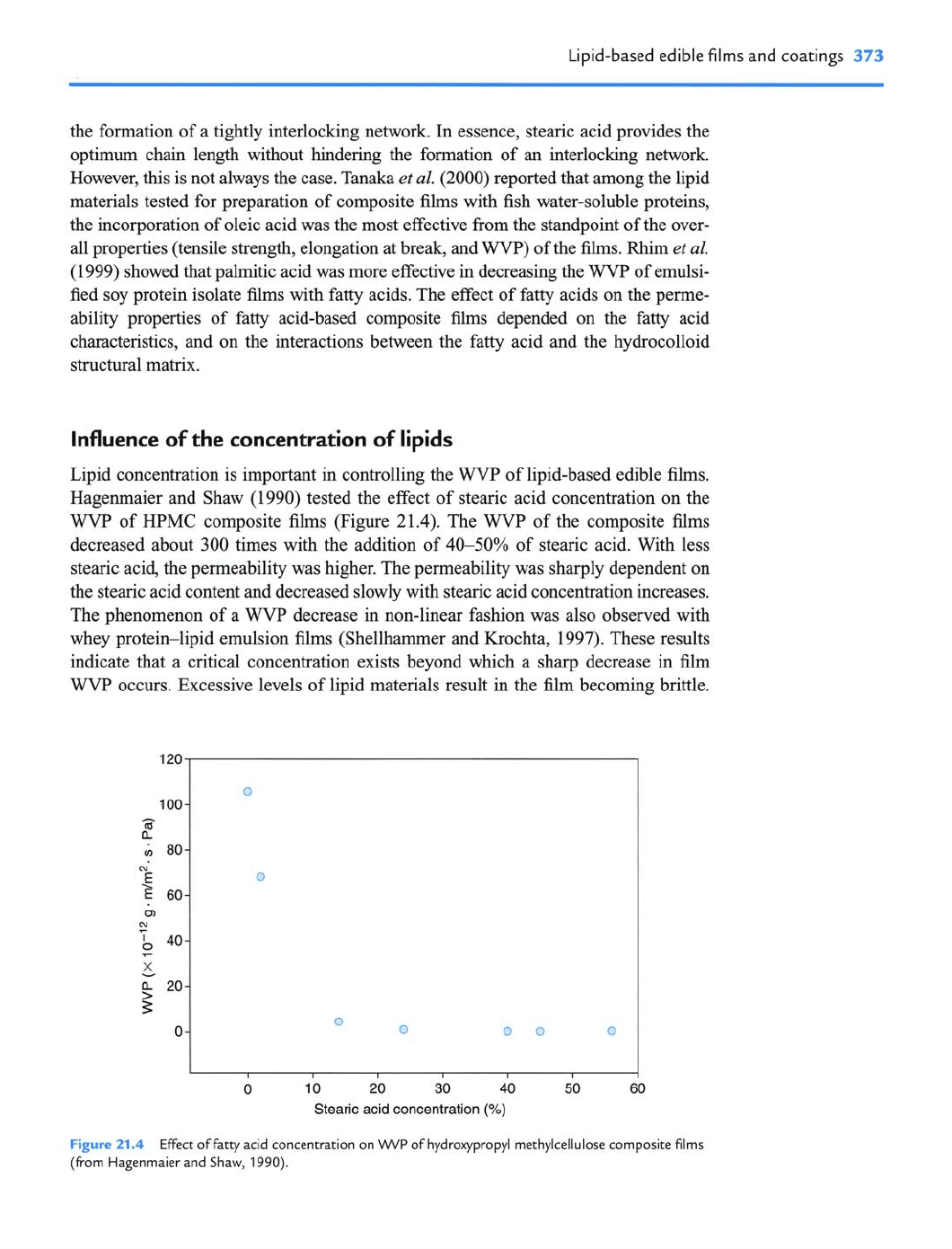
Lipid concentration is important in controlling the WVP of lipid-based edible films.
Hagenmaier and Shaw (1990) tested the effect of stearic acid concentration on the
WVP of HPMC composite films (Figure 21.4). The WVP of the composite films
decreased about 300 times with the addition of 40-50% of stearic acid. With less
stearic acid, the permeability was higher. The permeability was sharply dependent on
the stearic acid content and decreased slowly with stearic acid concentration increases.
The phenomenon of a
WVP
decrease in non-linear fashion was also observed with
whey protein-lipid emulsion films (Shellhammer and Krochta, 1997). These results
indicate that a critical concentration exists beyond which a sharp decrease in film
WVP
occurs. Excessive levels of lipid materials result in the film becoming brittle.
Stearic acid concentration
(%)
Figure
21.4
Effect of fatty acid concentration on
WVP
of hydroxypropyl methylcellulose composite films
(from Hagenmaier and Shaw,
1990).
374
Innovations in Food Packaging
Therefore, the optimum concentration of lipid material in the preparation of lipid-
based edible films should be determined by considering both the effect of decreasing
the
WVP
and the physical strength of the films. As shown in Figure 21.4, stearic acid
concentration of about 20% seems to be the optimum for modifying the water vapor
barrier properties.
Rhim
et
al. (1999) also found that the incorporation of 20% fatty acids
was most effective from the standpoint of the overall properties, including the
WVl?
Influence
of
film
thickness
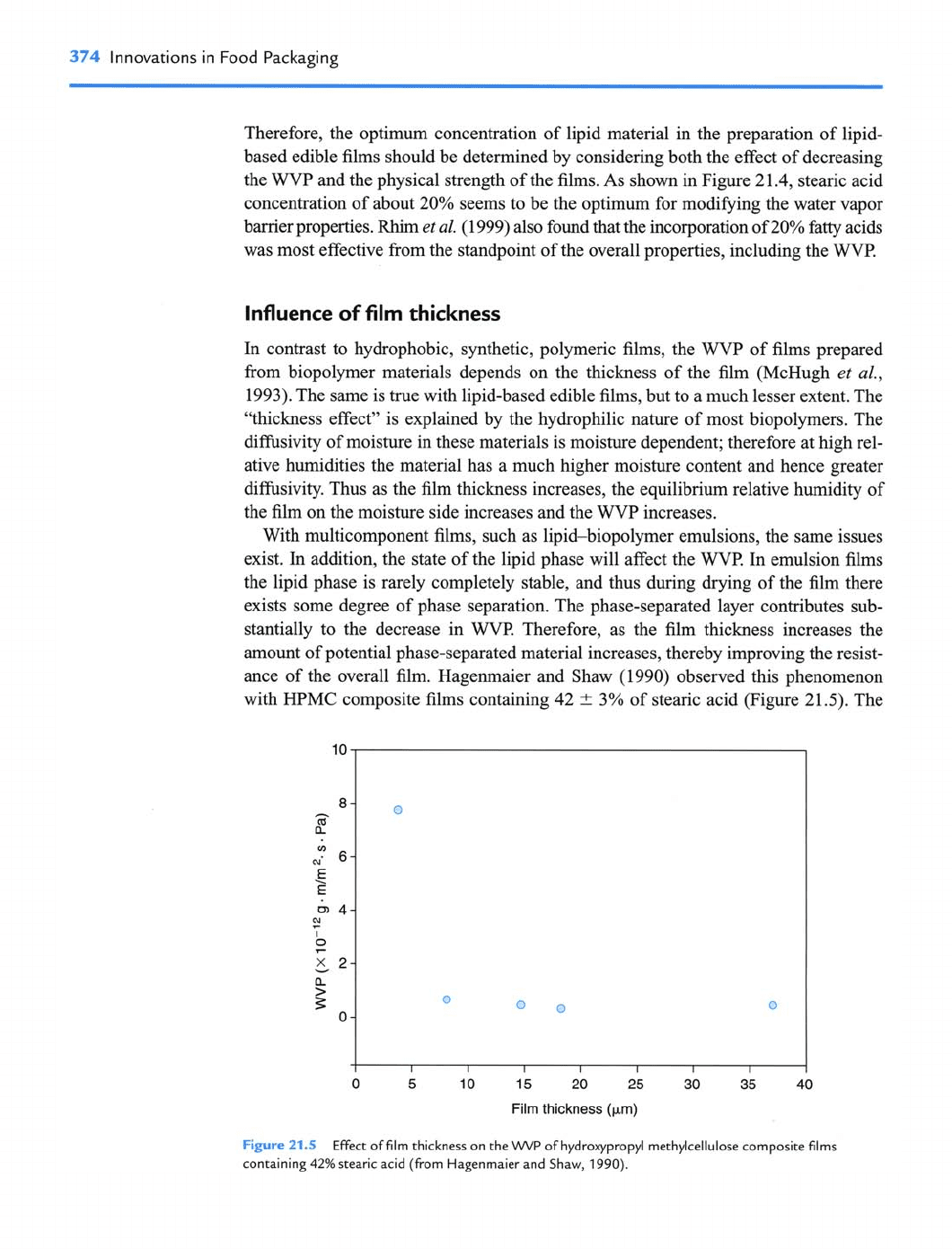
In contrast to hydrophobic, synthetic, polymeric films, the
WVP
of films prepared
from biopolymer materials depends on the thickness of the film (McHugh
et
al.,
1993). The same is true with lipid-based edible films, but to a much lesser extent. The
"thickness effect" is explained by the hydrophilic nature of most biopolymers. The
difisivity of moisture in these materials is moisture dependent; therefore at high rel-
ative humidities the material has a much higher moisture content and hence greater
difisivity. Thus as the film thickness increases, the equilibrium relative humidity of
the film on the moisture side increases and the
WVP
increases.
With multicomponent films, such as lipid-biopolymer emulsions, the same issues
exist. In addition, the state of the lipid phase will affect the
WVl?
In emulsion films
the lipid phase is rarely completely stable, and thus during drying of the film there
exists some degree of phase separation. The phase-separated layer contributes sub-
stantially to the decrease in
WVP.
Therefore, as the film thickness increases the
amount of potential phase-separated material increases, thereby improving the resist-
ance of the overall film. Hagenmaier and Shaw (1990) observed this phenomenon
with
HPMC
composite films containing 42
t
3% of stearic acid (Figure 21.5). The
0 5 10 15 20 25 30 35 40
Film
thickness
(pm)
Figure
21.5
Effect of film thickness on the
WVP
of hydroxypropyl methylcellulose composite films
containing
42%
stearic acid (from Hagenmaier and Shaw,
1990).
