Lal R., Shukla M.K. Principles of Soil Physics
Подождите немного. Документ загружается.


FIGURE 20.6 (a) Flood irrigation
without adequate drainage, (b)
Irrigation used for cotton-wheat
rotation in central Asia may lead to
secondary salinization if subsoil has
high salts, water is of poor quality, and
drainage is inadequate.
Soil salinity is used to designate a condition in which soluble salt concentration of soil
reaches a level that is harmful to crops. Moderate salinity can often go undetected
Principles of soil physics 594

because it causes no apparent injuries other than restricted growth. Leaves of plants
growing in salt infested areas may be smaller and darker blue to green in color than the
normal leaves. Salinity causes increased succulence, especially for a high concentration
of chloride ions in the soil solution. The appearance of plants in salt-affected soils and
moisture stress (drought) conditions is almost similar. The wilting of plants
FIGURE 20.7 (a) A saline soil with a
high water table, (b) Salt accumulation
in a poorly drained depressional land
in Haryana, India.
Freezing and thawing effects 595

is far less prevalent because the osmotic potential of the soil solution usually changes
gradually and plants adjust their internal salt content sufficiently to maintain turgor and
avoid wilting.
Symptoms of specific element toxicities, such as marginal or tip burn of leaves, occur
as a rule only in woody plants. Chloride and sodium ions and boron are the elements
most usually associated with toxic symptoms. Nonwoody species may often accumulate
as much or more of these elements in their leaves without showing apparent damage, as
do the woody species.
20.4.1 Osmotic Pressure
The water molecules are dipole and other ions in the solution are attracted to them by the
electric field to form clusters. The presence of solutes affects the thermodynamic
properties of water and lowers the potential energy. Consider two compartments, one
containing pure water and the other a solution, that are separated by a membrane
permeable to pure water and impermeable to solute. The pure water will continue to cross
over into the solution side, unless stopped by an opposing force. If the compartment on
the solution side is a flexible diaphragm type, then the pure water entry will expand it.
This will result in a rise in hydrostatic pressure that will eventually stop the flow of pure
water into the solution compartment. The hydrostatic pressure at equilibrium is known as
osmotic pressure (Π, erg cm
−3
) of a solution and for a dilute solution it is expressed as
follows
Π=C
s
RT
(20.23)
where C
s
is concentration of solution (moles cm
−3
), R is universal gas constant (8.32×10
7
erg moldeg
−1
), and T is absolute temperature (K). The solute potential differences tend to
become uniform through the system owing to the process of diffusion and do not affect
the value of any other soil water potential components at equilibrium. However, when
membrane or diffusion barriers are present within the soil-water system and solute
system, the solute potentials need to be included in the analysis of potentials. In order to
further explain the osmotic pressure potentials and other components of soil water
potentials, let us consider a vessel filled (Fig. 20.8) with a solution of osmotic pressure
(or π) and a capillary tube filled with pure water. One end of the capillary has a perfect
semiinfinite membrane, that restricts flow of solute into a capillary but allows flow of
water into a capillary. The capillary risestops at equilibrium at height h.
Principles of soil physics 596
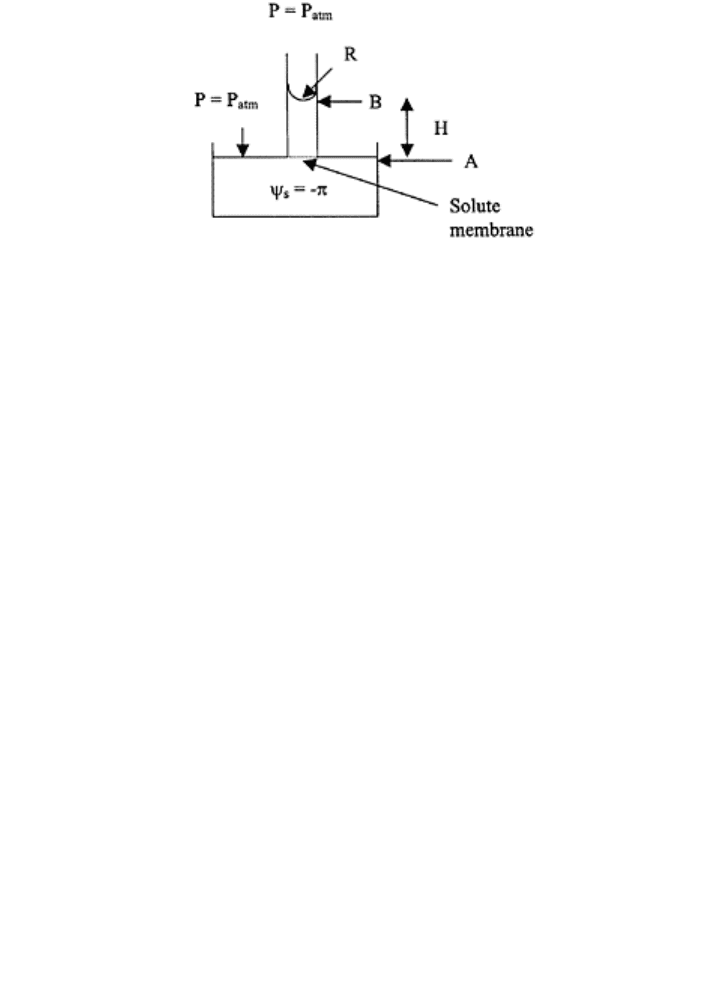
FIGURE 20.8 Schematic of a
capillary tube at equilibrium with a
solution of osmotic pressure (π).
At point A
The datum z=0 and P
0
=P
atm
Evaluating the components of total soil water potential (Φ
t
)
Gravitational pressure potential: Φ
z
=0 because z=z
0
=0
Air pressure potential: Φ
a
=0 because P=P
0
Matric potential: Φ
m
=0 because no soil is present
Hydrostatic pressure potential: Φ
p
=0 because no hydrostatic
pressure
Solute potential: Φπ=−π (by definition)
Therefore, Φ
τ
=Φ
z
+Φ
a
+Φ
m
+Φ
p
−Φ
π
Or, Φ
t
=−π at point A
(20.24)
At point B
Φ
a
=0 because P=P
0
=P
atm
Φ
s
=0 because pure water
Φ
z
=ρ
w
gh
Φ
m
=P
1
−P
a
=−2σ/R because the contact angle is zero, σ is
surface tension
Therefore, Φ
t
,=ρ
w
gh−2σ/R
(20.25)
Since the soil water system in the vessel and capillary is in equilibrium, Eqs. (20.24) and
(20.25) are equal
ρ
w
gh−2σ/R=−π
(20.26)
or
h=2σ/ρ
w
gR−π/ρ
w
g
(20.27)
Freezing and thawing effects 597
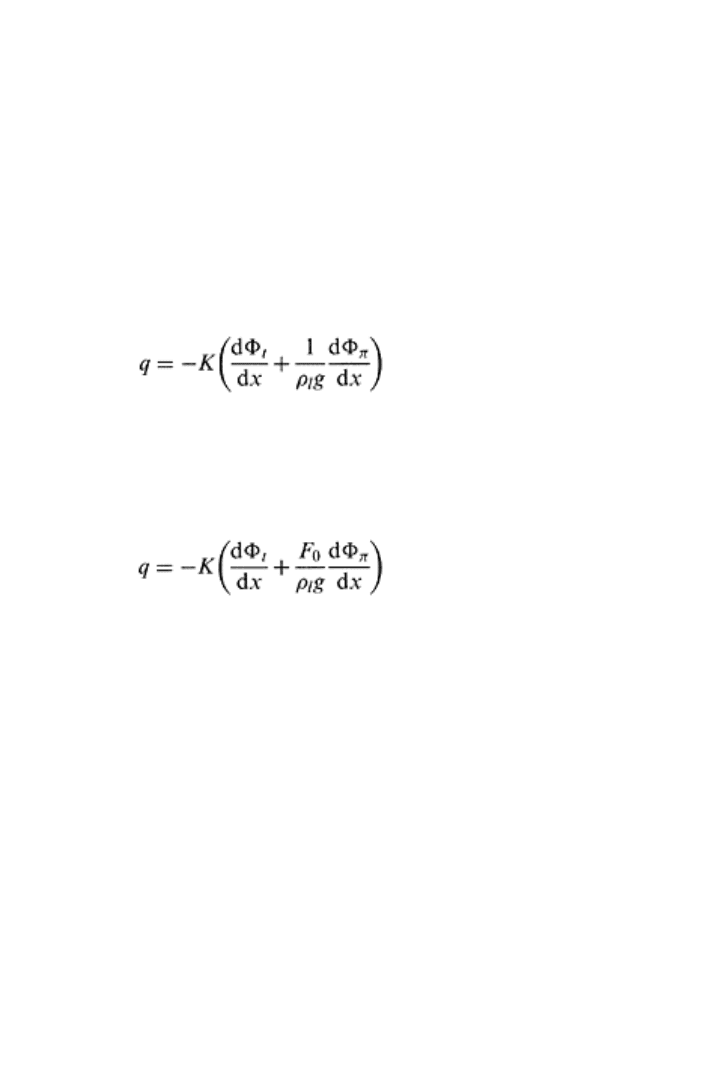
The equation shows that due to solute potential the rise of water in the capillary is smaller
than without it. If solute potential becomes larger than 2σ/R, there will be no capillary
rise.
20.4.2 Effects of Salinity on Water Movement
Darcy’s law states that the soil water flux is the product of hydraulic conductivity and the
driving force. The driving force consists of gravitational and pressure potentials for solute
free soils. For soils containing salts, the osmotic potential gradient is the additional
driving force for water movement through a semipermeable membrane by restricting the
flow of solutes and at the same time allowing the flow of water. For the situations where
solute flow is totally restricted the total hydraulic head is sum of all the three driving
forces (i.e., gravitational, pressure, and osmotic) and flux of water (q) for a soil of
hydraulic conductivity K can be given as follows
(20.28)
where Φ
t
,=Φ
m
+Φ
z
, ρ
l
is the density of solution, g is acceleration due to gravity, and x is
the distance along the direction of flow. When solutes are restricted to movement relative
to the water solvent, such a phenomenon is known as salt sieving. For field situations, a
total restriction of solute particles from flow seems unrealistic; therefore, an osmotic
efficiency factor (F
0
) is introduced in Eq. (20.28), which changes to
(20.29)
Experimental studies have demonstrated that F
0
is close to zero under saturated
conditions. However, for unsaturated conditions at high suction values, F
0
becomes
significant and is reported as 0.03 for suction of 0.25 to 1 bar (Letey, 1968). The solutes
have a profound influence on the saturated soil hydraulic conductivity (K
s
) because
aggregates tend to collapse by the dispersion of clay, which also blocks the interaggregate
pores, and high exchangeable sodium percentage and low salt concentrations cause
swelling and dispersion of clay—both of which ultimately reduce the K
s
of soil. The
negatively charged clay particles form a diffuse double layer by attracting cations. When
the solution concentration is less than 200–400 meql
−1
, this process of imbibition causes
swelling in soils, which reduces the osmotic pressure difference between the soil solution
(or more appropriately ambient solution, which is the soil solution away from soil
particles) and clay particle, and weakens interparticle bond (McNeal, 1974). This results
in dispersion of clay and reduction in K
s
of soil.
20.4.3 Leaching Requirement
In arid regions where irrigation with water containing salts is applied to crops, the twin
processes of evaporation and transpiration results in rise in salt concentration in the root
zone. On the other hand, if a shallow groundwater table exists in the area, then salt is
Principles of soil physics 598

brought in the root zone by the process of capillary rise. The excess salt present in the soil
is removed by leaching, which is a process in which the optimal quantity of water equal
to the leaching requirement is applied to the field and allowed to flow through and past
the root zone so that excess salts are removed (Richards, 1954). Leaching may result in a
slight increase in soil pH by lowering of salt concentration, but saline soils rarely become
strongly sodic upon leaching. Unless the water table is very deep and lateral movement of
water fast, the process of leaching can cause water table buildup. Therefore, an adequate
drainage system is a necessity for leaching. Leaching requirement (LR) is defined as the
fraction of irrigation water that must be leached out from the bottom of root zone to keep
soil salinity level within a specific limit (usually 4dSm
−1
). LR depends on the
evapotraspiration, salt tolerance of crops, and salt content of soil profile and irrigation
water. The LR can be obtained by first making a salt balance, which is the total salt input
and output for a given volume or depth of soil as follows:
ρ
w
(V
r
c
r
+V
i
c
i
+V
g
c
g
)+M
s
+M
a
−(M
p
+M
c
+ρ
w
V
s
c
s
)=∆M
(20.30)
where V and c are the volume of water entering or leaving the soil root zone (per unit
surface area or equivalent depth) and concentration (EC), respectively, subscript r, i, and
g are for rainfall, irrigation, and groundwater, respectively. M
s
and M
a
are the mass of
salts from soil and soil amendment or fertilizers, M
p
and M
c
are mass of salt precipitated
and removed by crop, V
s
and c
s
is the volume of water drained from soil and
concentration, respectively, and ∆M is the total change in mass of salt. Disregarding the
changes in salt balance in soil profile by precipitation, agricultural inputs,
evapotraspiration, drainage, and groundwater or capillary rise, Eq. (20.22) is simplified as
follows:
V
i
c
i
=V
s
c
s
(20.31)
Equation (20.31) is for the steady state conditions where water content and salinity of soil
profile is constant, and no precipitation or dissolution of salt is taking place.
V
s
=V
i
−V
ET
(20.32)
where V
ET
is volume of evapotranspiration. Transferring Eq. (20.24) into (20.23)
V
i
c
i
=(V
i
−V
ET
)c
s
(20.33)
or
(20.34)
or in terms of depth of irrigation water (d
i
), equivalent depth of evapotranspiration by
crop (d
ET
), EC of drainage (s) and irrigation water (i), the equation can be written as
follows (Richards, 1954)
Freezing and thawing effects 599

(20.35)
Equation (20.35) suggests that by varying the amount of water for leaching the
concentration of salts in root zone can be reduced to the desired level.
20.5 SOIL WATER REPELLENCY
Water repellency is defined as a phenomenon of repulsion of water by soil particles. Soil
hydrophobicity, also called “water repellency or non-wetting,” reduces the affinity of soil
for water. Hydrophobicity can reduce the infiltration capacity of a soil to the extent that
the soil does not wet up even after weeks of being in contact with water. This can lead to
inhibited plant growth, increased overland flow and accelerated soil erosion, uneven
wetting patterns, and preferential flow generation. Hydrophobicity is known to vary
temporally, being generally most extreme after long dry periods and reduced or absent
after long wet spells (DeBano, 2003).
Water repellency is mostly associated with organic matter and its decomposition,
particularly where fungi growth is involved. Exudates and biomass produced by plant
roots and soil microbes can alter the surface characteristics of soil particles and lead to
the development of hydrophobic particle surfaces that may reduce water transport and
retention. This type of organic coating does not necessarily require covering the entire
soil particle; just a partial covering can render it water-repellent. The degree of soil
hydrophobicity is most severe at the soil surface and within the top 5 cm of soil profile,
but can be as deep as 15 cm or have patches of a hydrophobic layer within the soil profile
(DeBano, 2003).
Water repellency has been a concern for both land managers and researchers since the
early part of the twentieth century. It is a soil property with important repercussions for
plant growth, surface and subsurface hydrology, and soil erosion. It is generaly confined
to coarse-textured soils in regions with specific vegetation types and seasonally dries
climate and/or areas affected by fire. However, research conducted during the 1980s and
early 1990s showed that its occurrence is far more widespread. Water repellency can
occur at much lower levels or a localized scale in soil profile and can contribute to
preferential flow of water and nutrients. At low levels, repellency may not have a
deleterious impact on water retention and may even enhance microbial diversity through
the preferential alteration of soil pores by organisms. The hydrophobic substances
causing water repellency are also beneficial for conserving water by reducing the
capillary rise of water and the attendant evaporation, and leaching of nutrients. The water
repellency is often characterized in terms of wetting coefficients (C
w
=cos θ; where C
w
is
wetting coefficient and θ is contact angle) (Bahrani et al., 1970), surface roughness (Bond
and Hammond, 1970), and water surface tension and water-solid contact angles (refer to
Chapter 9; Watson et al., 1971).
Principles of soil physics 600
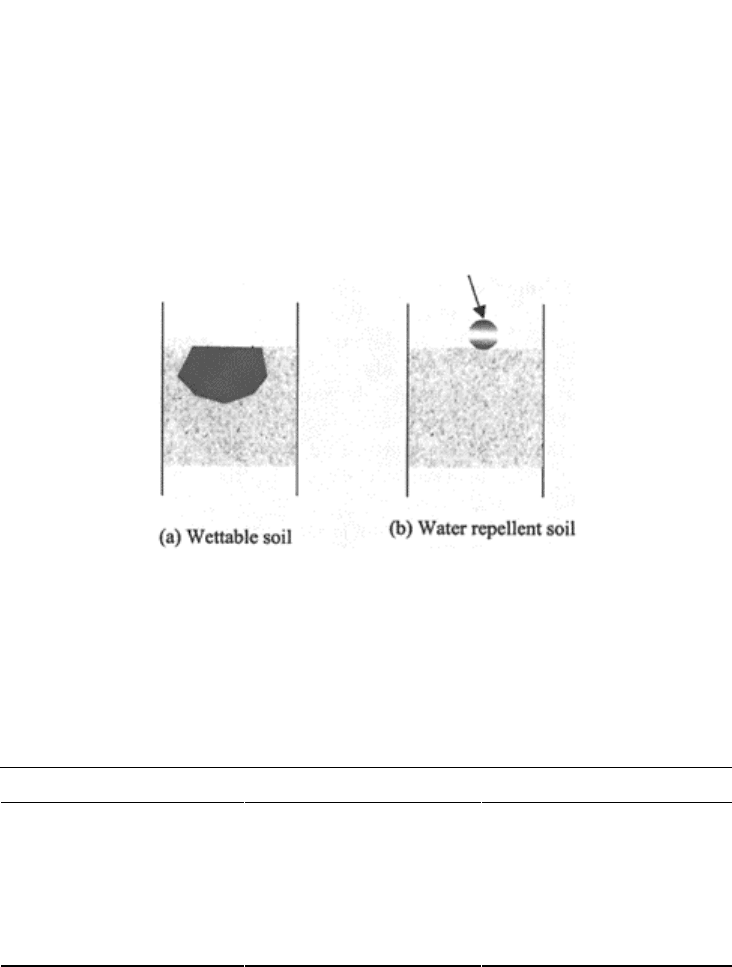
20.5.1 Wetting Pattern in Water-Repellent Soils
Dry soils are wetted when water is applied to them. A drop of water disappears and wets
soil because the force of attraction between soil particles and water results in loss of
cohesion in the latter, which lets it flow along the surfaces of particles. Once the
attractive forces between soil and water droplet are nonexistent, water remains as a
droplet and does not wet the soil. Before water starts infiltrating uniformly or percolating
inside the soil matrix, the presence of a continuous film of water over soil particle surface
is a prerequisite (Fig. 20.9).
The fundamental principle underlying the process of wetting shows that a reduction in
the surface tension of a solid (to be wetted) reduces its
FIGURE 20.9 Applied water droplets
make a film of water percolating in the
soil matrix due to suction gradients in
(a) wettable soil. It retains its shape as
a droplet in a (b) water-repellent soil.
TABLE 20.2 Water Repellency Classes
WDPT (seconds) Repellency class Water repellency
<5 0 Non-repellent
5–60 1 Slightly repellent
60–600 2 Strongly repellent
600–3600 3 Severely repellent
>3600 4 Extremely repellent
Source: Modified from Dekker and Ritsema, 2003.
Freezing and thawing effects 601
wettability, or a reduction in the surface tension of applied liquid increases the
wettability. The common method of classifying the water repellency is the empirical
water drop penetration time (WDPT). In this method, three drops of deionized water are
placed on a smoothened soil surface and the time over which drops are completely
absorbed is recorded. The time required for the drops to be absorbed depends on the
temperature of water and relative humidity of air. The increase in water temperature
reduces the surface tension and the time required for wetting. The increase in the relative
humidity of air increases the time for which the drops remain on the soil surface. The
water repellency classification given in Table 20.2 shows that soil is considered water-
repellent for WDPT >5 seconds, (Dekker and Jungerius, 1990; Dekker and Ritsema,
2003).
20.5.2 Effects of Water Repellency on Soil Processes
Water Infiltration
A water repellent soil does not get wet when water is applied under zero or negative
potential because contact angle is greater than 90°. Thus, a positive pressure must be
applied to force the entry of water into a soil. The value of the positive pressure depends
on the contact angle as well as pore dimension, and it increases with the contact angle
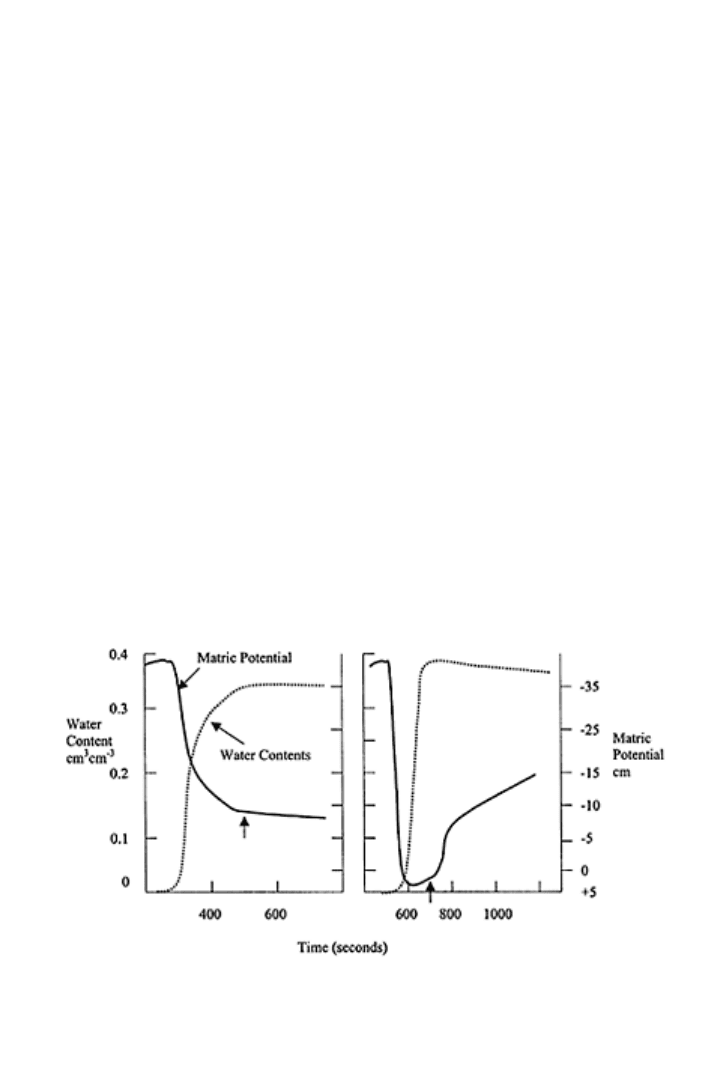
and decreases with the pore radius (Feng et al., 2001). The water content and the
attendant water pressure potential diagram (Fig. 20.10) with respect to time show that
non-water-repellent sand has a stable Richards-type imbibing front, slightly less saturated
than the total porosity of soil. The matric potential at the imbibing front for non-water-
repellent sand is negative. For a water-repellent soil, the matric potential behind the
imbibing front is slightly positive (Fig. 20.10b).
FIGURE 20.10 Schematic of matric
potential and moisture content for (a)
wettable and (b) water-repellent soil.
Principles of soil physics 602

The arrow points out to a negative
matric potential for wettable soil and
positive potential for repellent soil at
imbibing front. (Redrawn from Bauters
et al., 2003.)
In water-repellent soils, water movement is severely limited and the infiltration rates are
low. Most of the rainfall falling in a dry water-repellent soil may be lost as runoff.
However, as the dry water-repellent soil becomes wetter, the infiltration and water
movement gradually increases. The main difference between a hydrophilic and
hydrophobic soil is the shape of the wetting front. Infiltrating water in hydrophilic soils
forms an unconditionally stable horizontal Richards-type wetting front (refer to Chapter
13), whereas a hydrophobic soil forms an unstable front with fingers (Fig. 20.11).
Therefore, water distribution in the soil can have large variability with high water content
in the ectorganic layer (also known as “humus,” which protects the soil from erosion,
while enhancing aggregation) beneath which there can be a dry water-repellent layer,
which may be underlaid by a moist, less hydrophobic layer. The hydraulic conductivity
of water-repellent soil increases with depth of ponding (Carrillo et al., 2000).
Preferential Flow
The preferential transport of water and solutes can take place through soil matrix via
cracks formed in well-structured soils (such as clay or peat) due to shrink-swell
mechanism or biopores formed by soil fauna or the channels left behind by decayed
roots. In nonstructured sandy soils, the preferential
FIGURE 20.11 Irregular wetting in a
water-repellent soil.
flow can occur due to the formation of unstable wetting fronts, which can grow into
fingers because of the lateral diffusion (Ritsema et al., 1998).
Freezing and thawing effects 603
