Lin S.D. Water and Wastewater Calculations Manual
Подождите немного. Документ загружается.

Hypochlorous acid is a weak acid and subject to further dissociation
to hypochlorite ions (OCl
⫺
) and hydrogen ions:
HOCl ↔ OCl
⫺
⫹ H
⫹
(5.156)
and its acid dissociation constant K
a
is
(5.157)
The values of K
a
for hypochlorous acid is a function of temperature in
kelvins as follows (Morris, 1966):
ln K
a
⫽ 23.184 ⫺ 0.058T ⫺ 6908/T (5.158)
Example 1: The dissolved chlorine in the gas chlorinator is 3900 mg/L at pH
4.0. Determine the equilibrium vapor pressure of chlorine solution at 25°C
(use K
H
⫽ 4.5 ⫻ 10
⫺4
).
solution:
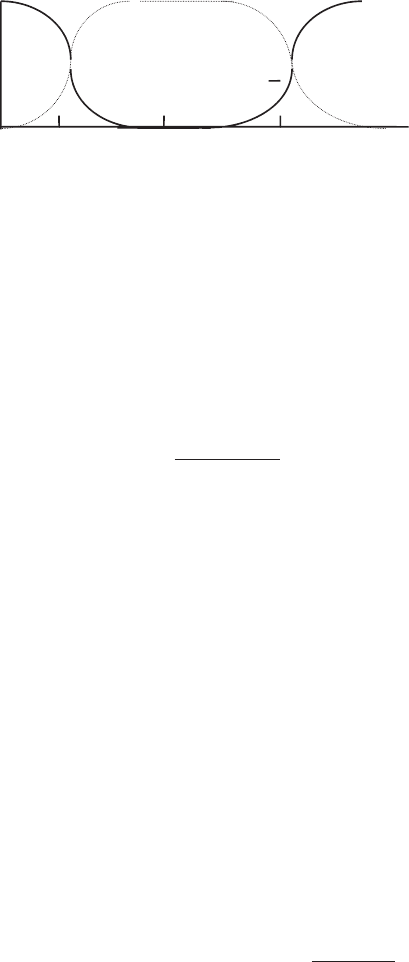
Step 1.
Since pH ⫽ 4.0 < 5, from Fig. 5.10, the dissociation of HOCl to OCl
⫺
is not
occurring. The available chlorine is [Cl
2
] ⫹ [HOCl]:
⫽ 0.055 mol/L
[HOCl] ⫽ 0.055 ⫺ [Cl
2
]
[Cl
2
] 1 [HOCl] 5 3900 mg/L 5
3.9 g/L
70.9 g/mol
5 2.61 3 10
28
at 208C
5 3.7 3 10
28
at 258C
K
a
5
[OCl
2
][H
1
]
[HOCl]
Public Water Supply 465
Figure 5.10 Distribution of chlorine species (Snoeyink and Jenkins, 1980).
pH
1.0
Cl
2
Fraction α
HOCl OCl
−
10−2024 6 8
Step 2. Compute [H
⫹
]
Step 3. Apply K
H
at 25°C
Substitute [H
⫹
] from Step 2
From Eq. (5.152), it can be expected that, for each mole of HOCl produced,
one mole of Cl
⫺
will also be produced. Assuming there is no chloride in the
feedwater, then
[Cl
⫺
] ⫽ [HOCl] ⫽ 0.055 ⫺ [Cl
2
]
Step 4. Solve the above two equation
Step 5. Compute H using Eq. (5.155)
Note: [HOCl] in negligible
5 0.062 mol/L
#
atm
H 5 4.805 3 10
26
expa
2818.48
25 1 273
b
[Cl
2
] 5 0.055 3 10
211
smol/Ld
5 0.2345 3 10
26
[Cl
2
]
1/2
5
20.0002/2 ± 20.000212
2
1 0.22
2
[Cl
2
] 1 0.0002/2[Cl
2
]
1/2
2 0.055 5 0
0.0002/2[Cl
2
]
1/2
5 0.055 2 [Cl
2
]
4.5 3 10
8
5
s0.055 2 [Cl
2
]d
2
[Cl
2
]
4.5 3 10
28
5
[Cl
2
][HOCl]
[Cl
2
]
K
H
5 4.5 3 10
24
5
[H
1
][Cl
2
][HOCl]
[Cl
2
]
[H
1
] 5 10
24
pH 5 4.0 5 log
1
[H
1
]
466 Chapter 5

Step 6. Compute the partial pressure of chlorine gas
P
Cl
2
⫽ [Cl
2
]/H ⫽ (0.055 ⫻ 10
⫺11
mol/L) (0.062 mol/(L ⋅ atm))
⫽ 0.89 ⫻ 10
⫺11
atm
or ⫽ 8.9 ⫻ 10
⫺12
atm ⫻ 101,325 Pa/atm
⫽ 9.0 ⫻ 10
⫺7
Pa
Example 2: A water treatment plant uses 110 lb/d (49.9 kg/d) of chlorine to
treat 10.0 MGD (37,850 m
3
/d or 0.438 m
3
/s) of water. The residual chlorine
after 30 min contact time is 0.55 mg/L. Determine the chlorine dosage and
chlorine demand of the water.
solution:
Step 1. Calculate chlorine demand
Since l mg/L ⫽ 8.341b/Mgal
or solve by SI units:
Since 1 mg/L ⫽ 1 g/m
3
Chlorine dosage ⫽ (49.9 kg/d) ⫻ (1000 g/kg)/(37,850 m
3
/d)
⫽ 1.32 g/m
3
⫽ 1.32 mg/L
Step 2. Calculate chlorine demand
Chlorine demand ⫽ chlorine dosage ⫺ chlorine residual
⫽ 1.32 mg/L ⫺ 0.55 mg/L
⫽ 0.77 mg/L
Example 3: Calcium hypochlorite (commercial high-test calcium hypochlo-
rite, HTH) containing 70% available chlorine is used for disinfection of a
new main. (a) Calculate the weight (kilograms) of dry hypochlorite powder
needed to prepare a 2.0% hypochlorite solution in a 190-L (50 gal) con-
tainer. (b) The volume of the new main is 60,600 L (16,000 gal). How much
of the 2 percent hypochlorite solution will be used with a feed rate of
55 mg/L of chlorine?
5 1.32 mg/L
Chlorine dosage 5
110 lb/d
10.0 Mgal/d
3
1 mg/L
8.34 lb/Mgal
Public Water Supply 467

solution:
Step 1. Compute kg of dry calcium hypochlorite powder needed
Step 2. Compute volume of 2% hypochlorite solution needed
2% solution ⫽ 20,000 mg/L ⫽20 g/L
Let X ⫽ 2% hypochlorite solution needed
Then (20,000 mg/L) (X L) ⫽ (55 mg/L) (60,600 L)
X ⫽ 167 L
Combined available chlorine.
Chlorine reacts with certain dissolved con-
stituents in water, such as ammonia and amino nitrogen compounds to
produce chloramines. These are referred to as combined chlorine. In
the presence of ammonium ions, free chlorine reacts in a stepwise
manner to form three species: monochloramine, NH
2
Cl; dichloramine,
NHCl
2
; and trichloramine or nitrogen trichloride, NCl
3
.
(5.159)
or (5.160)
(5.161)
(5.162)
At high pH, the reaction for forming dichloramine from monochlo-
ramine is not favored. At low pH, other reactions occur as follows:
(5.163)
(5.164)
Free residual chlorine is the sum of [HOCl] and [OCl
⫺
]. In practice,
free residual chlorine in water is 0.5 to 1.0 mg/L. The term “total avail-
able chlorine” is the sum of “free chlorine” and “combined chlorine.”
Chloramines are also disinfectants, but they are more slower to act
than that of hypochlorite.
Each chloramine molecule reacts with two chlorine atoms. Therefore,
each mole of mono-, di-, and trichloramine contains 71, 142, and 213 g
of available chlorine, respectively. The molecular weight of these three
NH
3
Cl
1
1 NH
2
Cl 4 NHCl
2
1 NH
1
4
NH
2
Cl 1 H
1
4 NH
3
Cl
1
NHCl
2
1 HOCl 4 NCl
3
1 H
2
O
NH
2
Cl 1 HOCl 4 NHCl
2
1 H
2
O
NH
3saqd
1 HOCl 4 NH
2
C1 1 H
2
O
NH
1
4
1 HOCl 4 NH
2
Cl 1 H
2
O 1 H
1
5 5.42 kg
Weight 5
190 L 3 1.0 kg/L 3 0.02
0.70
468 Chapter 5
chloramines are respectively 51.5, 85.9, and 120.4. Therefore, the chlo-
ramines contain 1.38, 1.65, and 1.85 g of available chlorine per gram of
chloramine, respectively.
The pH of the water is the most important factor on the formation of
chloramine species. In general, monochloramine is formed at pH above
7. The optimum pH for producing monochloramine is approximately 8.4.
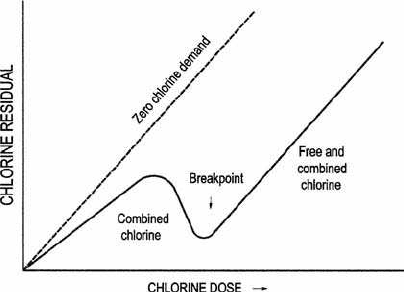
Breakpoint chlorination. When the molar ratio of chlorine to ammonia
is greater than 1.0, there is a reduction of chlorine and oxidation of
ammonia. A substantially complete oxidation—reduction process occurs
in ideal conditions by a 2:1 ratio and results in the disappearance of all
ammonium ions with excess free chlorine residual. This is called the
breakpoint phenomenon. As shown in Fig. 5.11, chlorine reacts with
easily oxidized constituents, such as iron, hydrogen sulfide, and some
organic matter. It then continues to oxidize ammonia to form chlo-
ramines and chloroorganic compound below a ratio of 5.0
(which is around the peak). The destruction of chloramines and chloroor-
ganic compounds are between the ratio of 5.0 and 7.6. The ratio at 7.6
is the breakpoint. All chloramines and other compounds are virtually
oxidized. Further addition of chlorine becomes free available chlorine,
HOCl, and OCl
⫺
. At this region, it is called breakpoint chlorination.
The breakpoint chlorination can be used as a means of ammonia
nitrogen removal from waters and wastewaters. The reaction is:
2NH
3
⫹ 3HOCl ↔ N
2
↑ ⫹ 3H
⫹
⫹ 3Cl
⫺
⫹ 3H
2
O (5.165)
or 2NH
3
⫹ 3Cl
2
↔ N
2
↑ ⫹ 6HCl (5.166)
NH
3
⫹ 4Cl
2
⫹ 3H
2
O ↔ NO
⫺
3
⫹ 8Cl
⫺
⫹ 9H
⫹
(5.167)
Cl
2
:NH
1
4
Public Water Supply 469
Figure 5.11 Theoretical drawing of breakpoint chlorination curve.

In practice, the breakpoint does not occur at a Cl
2
: NH
⫺
4
mass ratio of
around 10:1, and mass dose ratio of 15:1 or 20:1 is applied (White, 1972;
Hass, 1990).
McKee et al. (1960) developed the relationship of available chlorine
in the form of dichloramine to available chlorine in the form of mono-
chloramine as follows:
(5.168)
where A ⫽ ratio of available chlorine in di- to monochloramine
M ⫽ molar ratio of chlorine (as Cl
2
) added to ammonia-N present
B ⫽ 1 ⫺ 4K
eq
[H
⫹
] (5.169)
where the equilibrium constant k
eq
is from
H
⫹
⫹ 2NH
2
Cl ↔ NH
⫹
4
⫹ NHCl
2
(5.170)
(5.171)
⫽ 6.7 ⫻ 10
5
L/mol at 25°C
The relationship (Eq. (5.168)) is pH dependent. When pH decreases
and the Cl:N dose ratio increases, the relative amount of dichloramine
also increases.
Example: The treated water has pH of 7.4, a temperature of 25°C, and a free
chlorine residual of 1.2 mg/L.
Chloramine is planned to be used in the distribution system. How much
ammonia is required to keep the ratio of dichloramine to monochloramine of
0.15, assuming all residuals are not dissipated yet.
solution:
Step 1. Determine factor B by Eq. (5.171)
K
eq
⫽ 6.7 ⫻ 10
5
L/mol at 25°C
pH ⫽ 7.4, [H
⫹
] ⫽ 10
⫺7.4
From Eq. (5.169)
B ⫽ 1 ⫺ 4K
eq
[H
⫹
] ⫽ 1 ⫺ 4 (6.7 ⫻ 10
5
) (10
–7.4
)
⫽ 0.893
Step 2. Compute molar ratio M
A ⫽ 0.15
K
eq
5
[NH
1
4
][NHCl
2
]
[H
1
][NH
2
Cl]
2
A 5
BM
1 2 [1 2 BMs2 2 Md]
1/2
2 1
470 Chapter 5
Using Eq. (5.168)
Step 3. Determine the amount of ammonia nitrogen (N) to be added
Chlorine dioxide.
Chlorine dioxide (ClO
2
) is an effective disinfectant (1.4
times that of the oxidation power of chlorine). It is an alternative disin-
fectant of potable water, since it does not produce significant amounts of
trihalomethanes (THMs) which chlorine does. Chlorine dioxide has not
been widely used for water and wastewater disinfection in the United
States. In western Europe, the use of chlorine dioxide is increasing.
Chlorine dioxide is a neutral compound of chlorine in the ⫹IV oxida-
tion state, and each mole of chlorine dioxide yields five redox equivalents
(electrons) on being reduced to chlorine ions. The chemistry of chlorine
dioxide in water is relatively complex. Under acid conditions, it is
reduced to chloride:
ClO
2
⫹ 5e ⫹ 4H
⫹
↔ Cl
⫺
⫹ 2H
2
O (5.172)
5 0.30 mg/L as N
5 2.12 3 10
25
mol/L 3 14 g of N/mol
5 2.12 3 10
25
mol/L
NH
3
5 1.7 3 10
25
smol/Ld/0.80
M 5
1.7 3 10
25
mol/L
NH
3
5 1.7 3 10
25
mol/L
1.2 mg/L of Cl
2
5
1.2 mg/L
70.9 g/mol
3 0.001 g/mg
Mole wt. of Cl
2
5 70.9 g
M 5 0.80
0.29M
2
2 0.232M 5 0
s1 2 0.777Md
2
5 1 2 0.893Ms2 2 Md
1 2 [1 2 0893Ms2 2 M d]
1/2
5 0.777M
0.15 5
0.893M
1 2 [1 2 0.893M s2 2 Md]
21/2
2 1
A 5
BM
1 2 [1 2 BM s2 2 Md]
1/2
2 1
Public Water Supply 471
Under reactively neutral pH found in most natural water it is reduced
to chlorite:
ClO
2
⫹ e ↔ ClO
2
⫺
(5.173)
In alkaline solution, chlorine dioxide is disproportioned into chlorite,
ClO
⫺
2
, and chlorate, ClO
3
:
2ClO
2
⫹ 2OH
–
↔ ClO
⫺
2
⫹ ClO
⫺
3
⫹ H
2
O (5.174)
Chlorine dioxide is generally generated at the water treatment
plant prior to application. It is explosive at elevated temperatures and
on exposure of light or in the presence of organic substances. Chlorine
dioxide may be generated by either the oxygeneration of lower-valence
compounds (such as chlorites or chlorous acid) or the reduction of a
more oxidized compound of chlorine. Chlorine dioxide may be gener-
ated by oxidation of sodium chlorite with chlorine gas by the following
reaction.
2NaClO
2
⫹ Cl
2
↔ 2NaCl ⫹ 2ClO
2
(5.175)
The above equation suggests that 2 moles of sodium chlorite react with
1 mole of chlorine to produce 2 moles of chlorine dioxide.
Chlorine dioxide is only 60% to 70% pure (James M. Montgomery
Consulting Engineering, 1985). Recent technology has improved it to
in excess of 95%. It is a patented system that reacts gaseous chlorine
with a concentrated sodium chlorite solution under a vacuum. The
chlorine dioxide produced is then removed from the reaction chamber
by a gas ejector, which is very similar to the chlorine gas vacuum feed
system. The chlorine dioxide solution concentration is 200 to 1000 mg/L
and contains less than 5% excess chlorine (ASCE and AWWA, 1990).
Example: A water treatment plant has a chlorination capacity of 500 kg/d
(1100 lb/d) and is considering the switch to using chlorine dioxide for disin-
fection. The existing chlorinator would be employed to generate chlorine diox-
ide. Determine the theoretical amount of chlorine dioxide that can be
generated and the daily requirement of sodium chlorite.
solution:
Step 1. Find ClO
2
generated by Eq. (5.175)
2NaClO
2
⫹ Cl
2
↔ 2NaCl ⫹ 2ClO
2
MW 180.9 70.9 134.9
y 500 kg/d x
472 Chapter 5

Step 2. Determine NaClO
2
needed
18.2 Ozonation
Ozone (O
3
) is a blue (or colorless) unstable gas at the temperature and
pressure encountered in water and wastewater treatment processes. It
has a pungent odor. Ozone is one of the most powerful oxidizing agents.
It has been used for the purposes of disinfection of water and wastewater
and chemical oxidation and preparation for biological processing, such
as nitrification.
Ozonation of water for disinfection has been used in France, Germany,
and Canada. Interest in ozonation in water treatment is increasing in
the US due to THMs problems of chlorination. Ozone is an allotrope of
oxygen and is generated by an electrical discharge to split the stable
oxygen–oxygen covalent bond as follows:
3O
2
⫹ energy ↔ 2O
3
(5.176)
The solubility of ozone in water follows Henry’s law. The solubility con-
stant H is (ASCE and AWWA, 1990):
H ⫽ (1.29 ⫻ 10
6
/T ) ⫺ 3721 (5.177)
where T is temperature in Kelvin.
The maximum gaseous ozone concentration that can be generated
is approximately 50 mg/L (or 50 g/m
3
). In practice, the maximum solu-
tion of ozone in water is only 40mg/L (US EPA, 1986). Ozone produc-
tion in ozone generators from air is from 1% to 2 % by weight, and
from pure oxygen ranges from 2% to 10% by weight. One percent by
weight in air is 12.9 g/m
3
(⬵ 6000 ppm) and 1% by weight in oxygen
is 14.3 g/m
3
(⬵ 6700 ppm). Residual ozone in water is generally
5 2810 lb/d
5 1276 kg/d
y 5
500 kg/d
70.9 g
3 18.9 g
5 2100 lb/d
5 951 kg/d
x 5
500 kg/d
70.9 g
3 134.9 g
Public Water Supply 473
0.1 to 2 g/m
3
(mg/L) of water (Rice, 1986). The ozone residual present
in water decays rapidly
18.3 Disinfection kinetics
Although much research has been done on modeling disinfection kinet-
ics, the classical Chick’s law or Chick–Watson model is still commonly
used. Basic models describing the rate of microorganism destruction or
natural die-off is Chick’s law, a first-order chemical reaction:
ln(N/N
0
) ⫽⫺kt (5.178a)
or ⫺dN/dt ⫽ kt (5.178b)
where N ⫽ microbial density present at time t
N
0
⫽ microbial density present at time 0 (initial)
k ⫽ rate constant
t ⫽ time
⫺dN/dt ⫽ rate of decrease in microbial density
Watson refined Chick’s equation and develop an empirical equation
including changes in the concentration of disinfectant as the following
relationship:
k ⫽ k′C
n
(5.179)
where k′ ⫽ corrected die-off rate, presumably independent of
C and N
C ⫽ disinfection concentration
n ⫽ coefficient of dilution
The combination of Eqs. (5.178a) and (5.179) yields
ln(N/N
0
) ⫽⫺k′C
n
t (5.180a)
or ⫺dN/dt ⫽ k′tC
n
(5.180b)
The disinfection kinetics is influenced by temperature, and the
Arrhenius model is used for temperature correction as (US EPA, 1986):
k′
T
⫽ k′
20
(T⫺20)
(5.181)
where k′
T
⫽ rate constant at temperature T, °C
k′
20
⫽ rate constant at 20°C
⫽ empirical constant
The Chick–Watson relationship can plot the disinfection data of the
survival rate (N/N
0
) on a logarithmic scale against time, and a straight
474 Chapter 5
