Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

24.18 CHAPTER TWENTY-FOUR
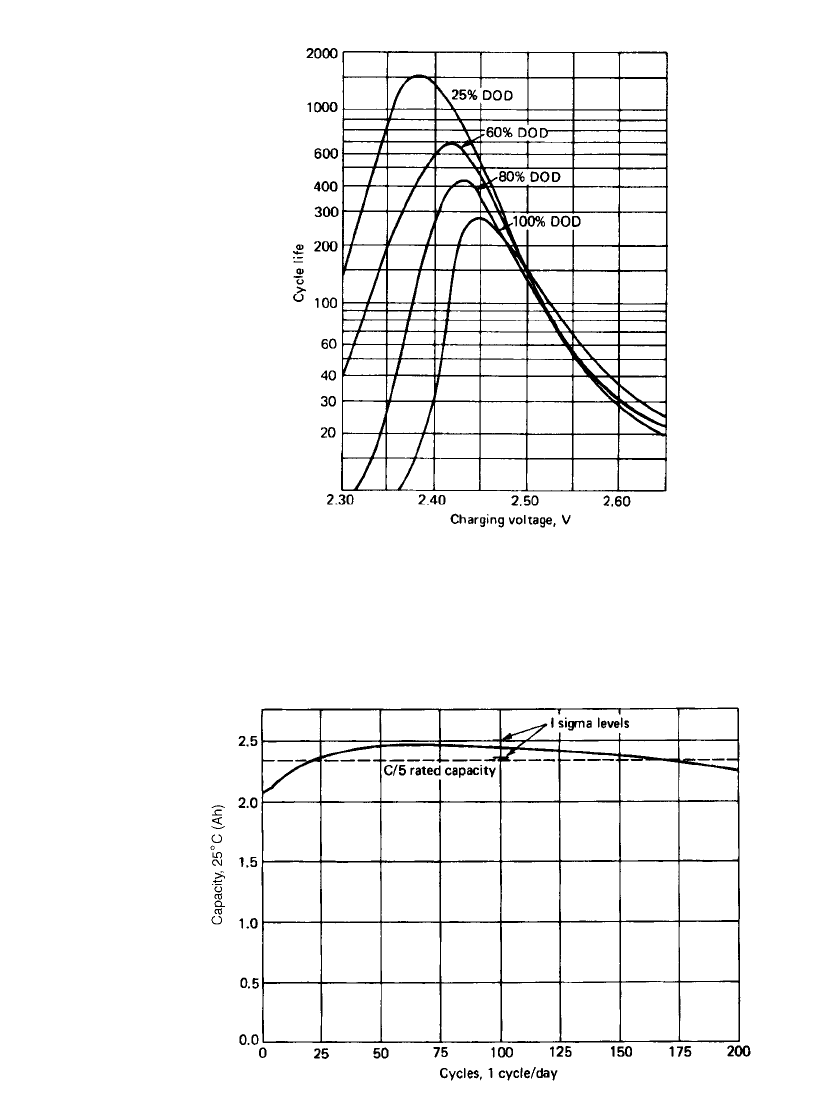
FIGURE 24.18 Effect of depth of discharge on cycle
life of VRLA batteries as a function of charging voltage
at 25⬚C, 16-h charge.
FIGURE 24.19 Effect of cycling on cell capacity, VRLA cell, C /5 discharge
rate. Rated at 2.35 Ah at C / 5 rate.
VALVE REGULATED LEAD-ACID BATTERIES 24.19
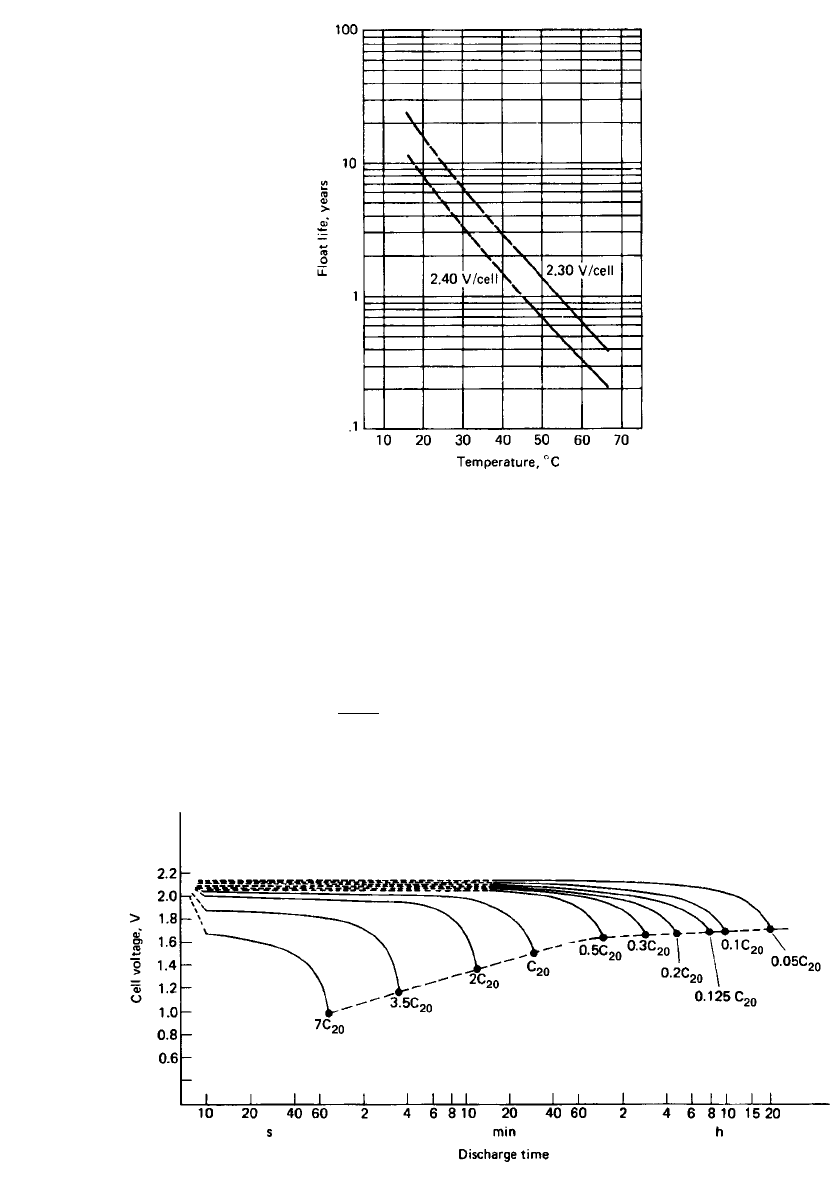
FIGURE 24.20 Float life of VRLA batteries.
24.4.2 Performance Characteristics—VRLA Prismatic Batteries
Typical discharge curves of the prismatic lead-acid battery at 20⬚C are shown in Fig. 24.21
which illustrates the high-rate capability and the flat discharge profile of this battery system.
The cells are usually rated at the 20-h or .05C rate, and the figure shows the performance
of the battery at discharge currents, expressed in terms of the C /20 rate, to various end
voltages. For example, for a cell rated at 5 Ah at the 20-h rate, the C /20 /5 rate is
C/20
⫽ 0.2 C/20 ⫽ (0.2)(5) ⫽ 1A
5
The C /20 /5 rate is 1.0 A. The C/20 rate is 0.25 A.
FIGURE 24.21 Typical discharge curves of prismatic lead-acid battery at various C / 20 discharge rates.
20⬚C. (Courtesy of Johnson Controls.)
24.20 CHAPTER TWENTY-FOUR
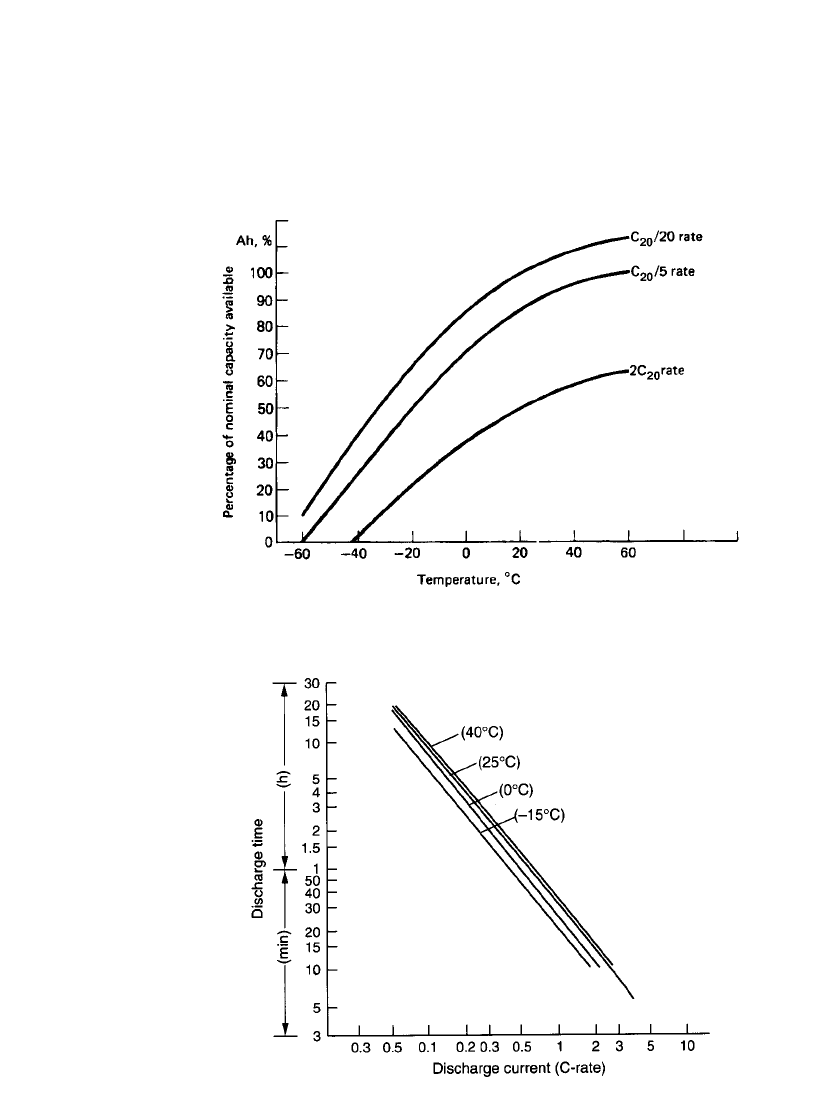
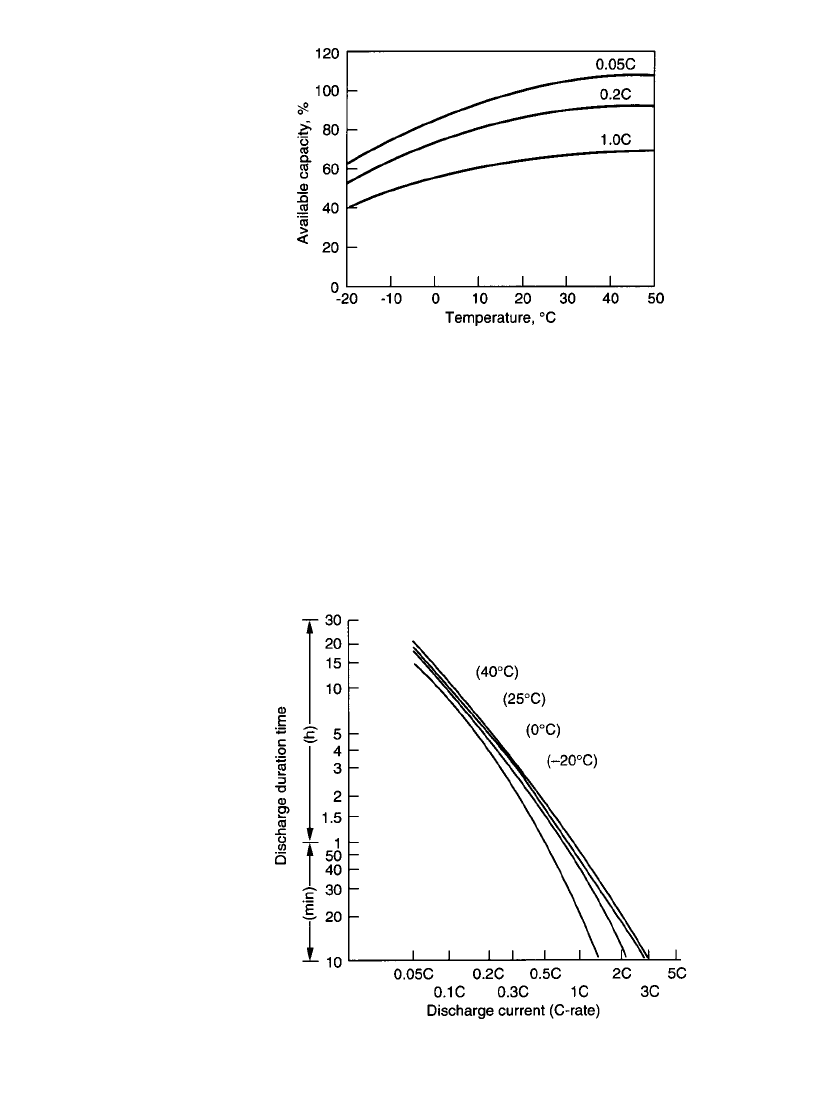
Figure 24.22 shows the effect of discharge rate and temperature on the delivered capacity
of the battery. A fully charged battery can operate over a very wide temperature range. These
data are summarized in Fig. 24.23 which plots the service life of the sealed prismatic lead-
acid battery at various temperatures and discharge rates (C-rates). Manufacturers’ data should
be obtained for specific performance characteristics as the characteristics may vary depending
on the size and design of the battery.
FIGURE 24.22 Effect of temperature and discharge rate on capacity of pris-
matic lead-acid battery.
FIGURE 24.23 Service life of sealed prismatic lead-acid batteries.
VALVE REGULATED LEAD-ACID BATTERIES 24.21
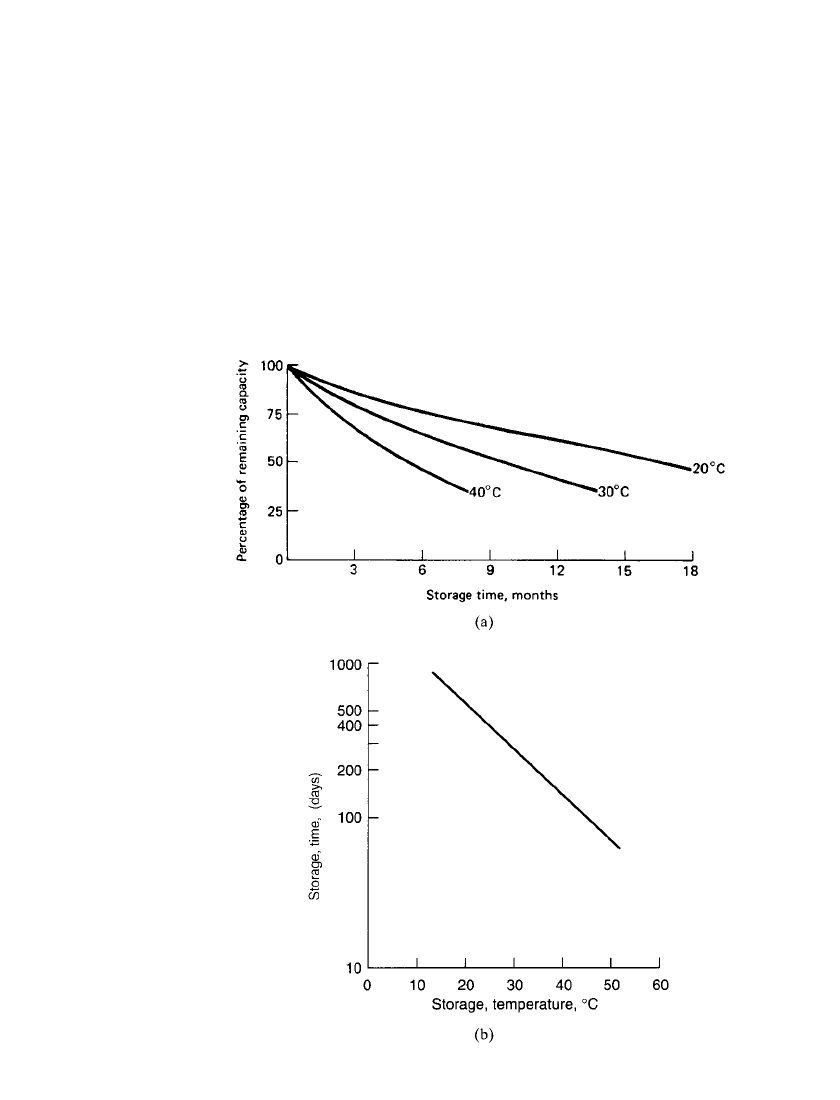
FIGURE 24.24 Characteristics of prismatic lead-acid battery. (a) Self-
discharge at various temperatures. (b) Storage time and temperature to 50%
of nominal capacity.
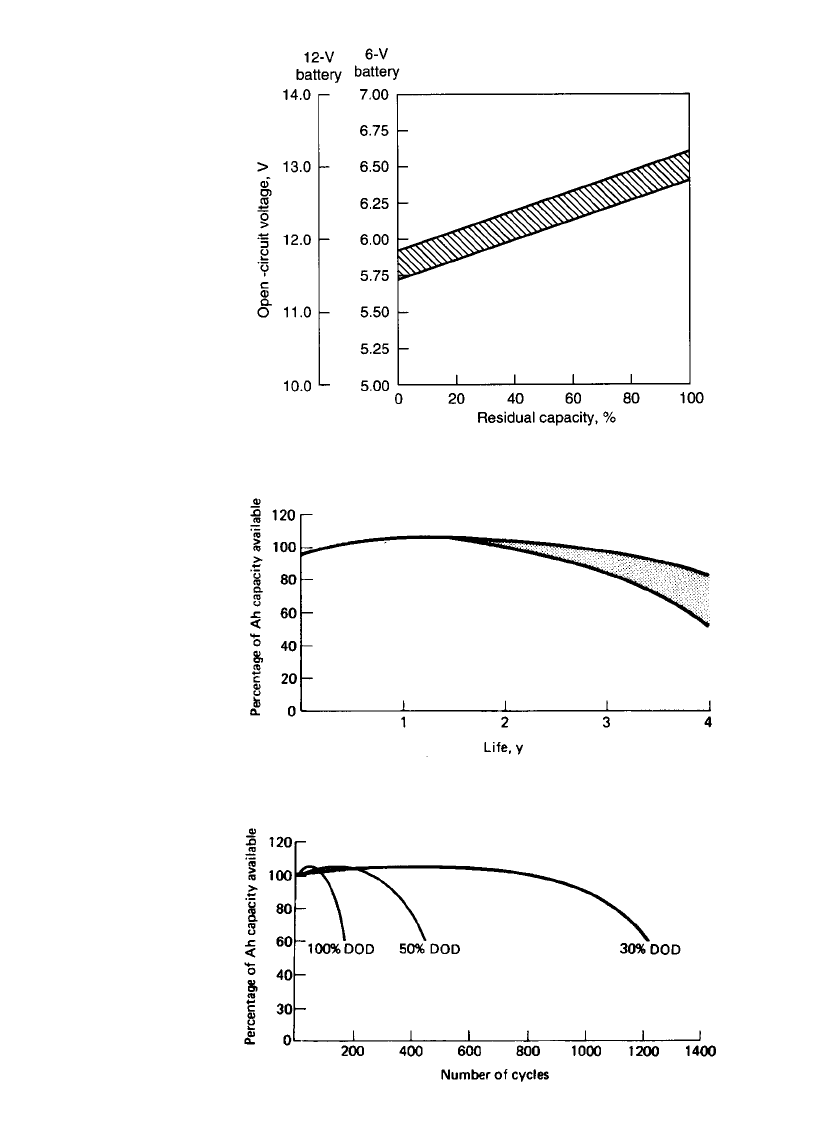
The typical self-discharge characteristics of the sealed prismatic lead-acid battery are
shown in Fig. 24.24. Figure 24.24a shows the capacity retention after storage for different
periods of time at several temperatures. Figure 24.24b shows the time it takes for the capacity
to decrease to 50% of the rated capacity throughout the temperature range. It loses relatively
little capacity in storage compared with the more conventional antimonial-lead grid battery.
The rate of self-discharge is about 4% per month. Once the battery has self-discharged to
the level where it has about a 50% state of charge, recharging the battery is advisable. The
residual capacity can be estimated by measuring the open-circuit voltage, as shown in Fig.
24.25.
The service-life characteristics of the gelled lead-acid battery on float service are shown
in Fig. 24.26. The cycle-life characteristics, to different depths of discharge (DOD), are
shown in Fig. 24.27. A characteristic of the battery is that the capacity increases in the early
stages of life and reaches a maximum at about 10 to 30 cycles. It will also gain in capacity
24.22 CHAPTER TWENTY-FOUR
FIGURE 24.25 Open-circuit voltage vs. residual capacity of sealed
prismatic lead-acid batteries at 25⬚C.
FIGURE 24.26 Performance of prismatic lead-acid battery on float
service at 20⬚C. Float voltage ⫽ 2.25 ⫺ 2.3 V per cell.
FIGURE 24.27 Cycle service life of prismatic lead-acid battery in re-
lation to depth of discharge (DOD) at 20⬚C.
VALVE REGULATED LEAD-ACID BATTERIES 24.23
while on extended charge, as in float service where discharges are infrequent. The battery
also does not exhibit a ‘‘memory’’ effect, as is the case with the sealed nickel-cadmium
battery which may become conditioned when used for short periods, and may not be able
to deliver full capacity when required.
The following are the recommended temperature ranges for the operation and storage of
the prismatic lead-acid battery: discharge,
⫺15 to 50⬚C; charge, 0 to 40⬚C; and storage, ⫺15
to 40
⬚C.
24.4.3 Performance Characteristics—Thin Prismatic Batteries
The performance characteristics of the thin prismatic batteries are similar to those of the
other sealed units. Their advantage becomes more apparent in the fabrication of battery packs
as the minimum of void space required in the pack enhances the energy density.
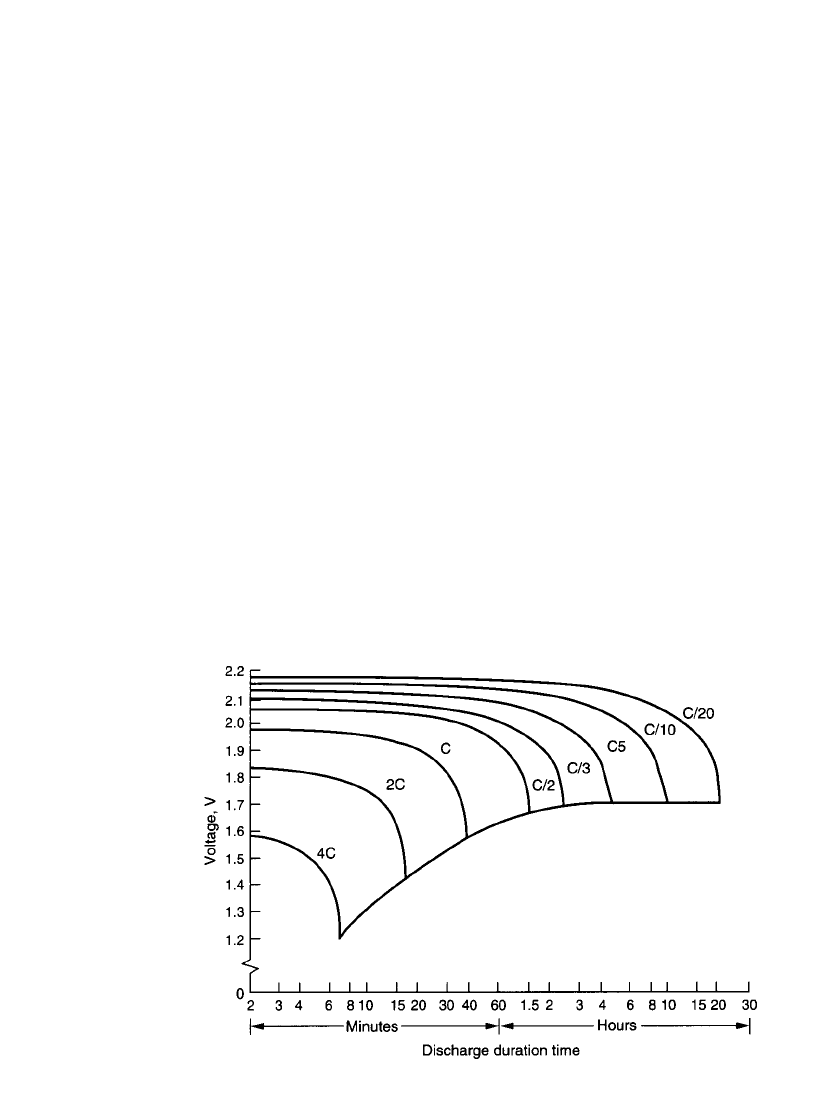
The characteristic discharge curves for the thin sealed lead-acid batteries are shown in
Fig. 24.28 and the effect of temperature on discharge capacity at various current drains is
plotted in Fig. 24.29. These data are summarized in Fig. 24.30, which plots the service hours
of a typical battery. The performance can be approximated from this monograph.
The loss of capacity with storage at different temperatures is also shown in Fig. 24.30.
As with all lead-acid batteries, they should be recharged when the charge retention is below
50%. The cycle life of the thin prismatic batteries is similar to the one shown for the VRLA
cells in Fig. 24.27.
The polarization characteristics of the thin prismatic VRLA batteries referred to in Fig.
24.28 are shown in Fig. 24.31a and b.
9
The data were taken with batteries in the horizontal
(pancake) position. This data illustrates the effect of state of charge on the current-voltage
relationship for both charge and discharge conditions.
FIGURE 24.28 Characteristic discharge curves for thin prismatic single-cell sealed
lead-acid batteries at 20⬚C. (Courtesy of Portable Energy Products, Inc.)
24.24 CHAPTER TWENTY-FOUR
FIGURE 24.29 Effect of temperature on discharge
performance of thin prismatic lead-acid batteries.
FIGURE 24.30 Service life of thin prismatic lead-acid bat-
teries.
VALVE REGULATED LEAD-ACID BATTERIES 24.25
2.2
2.1
2.0
1.9
1.8
1.7
0.1
1.0 10.0
2.5
2.4
2.3
2.2
2.1
2.0
1.0
0.1
1.0
10.0
Charge Current (A)
Cell State of Charge
Cell Potential (V)
Discharge Current (A)
Cell State of Charge
Cell Potential (V)
100%
67%
33%
0%
83%
50%
17%
100%
67%
33%
0%
83%
50%
17%
(a)
(b)
FIGURE 24.31 Polarization characteristics vs. state of charge for prismatic lead acid battery.
(a) during discharge; (b) during charge.
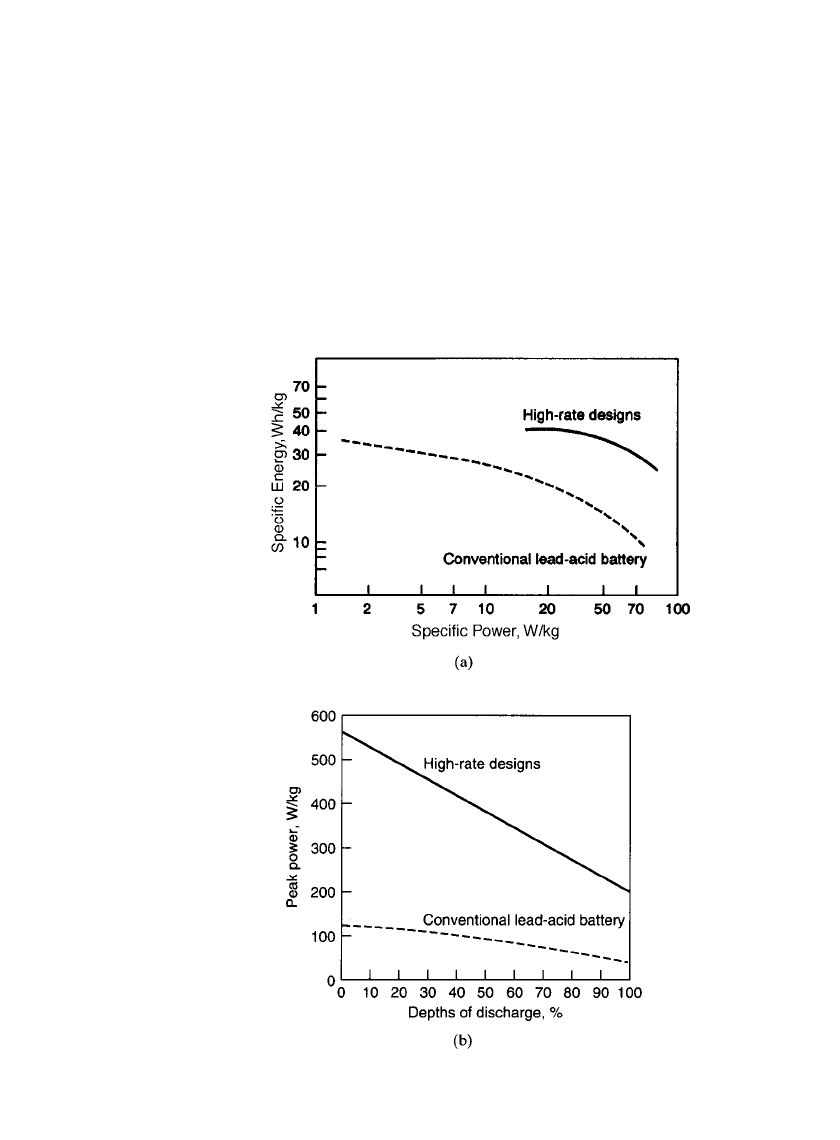
24.26 CHAPTER TWENTY-FOUR
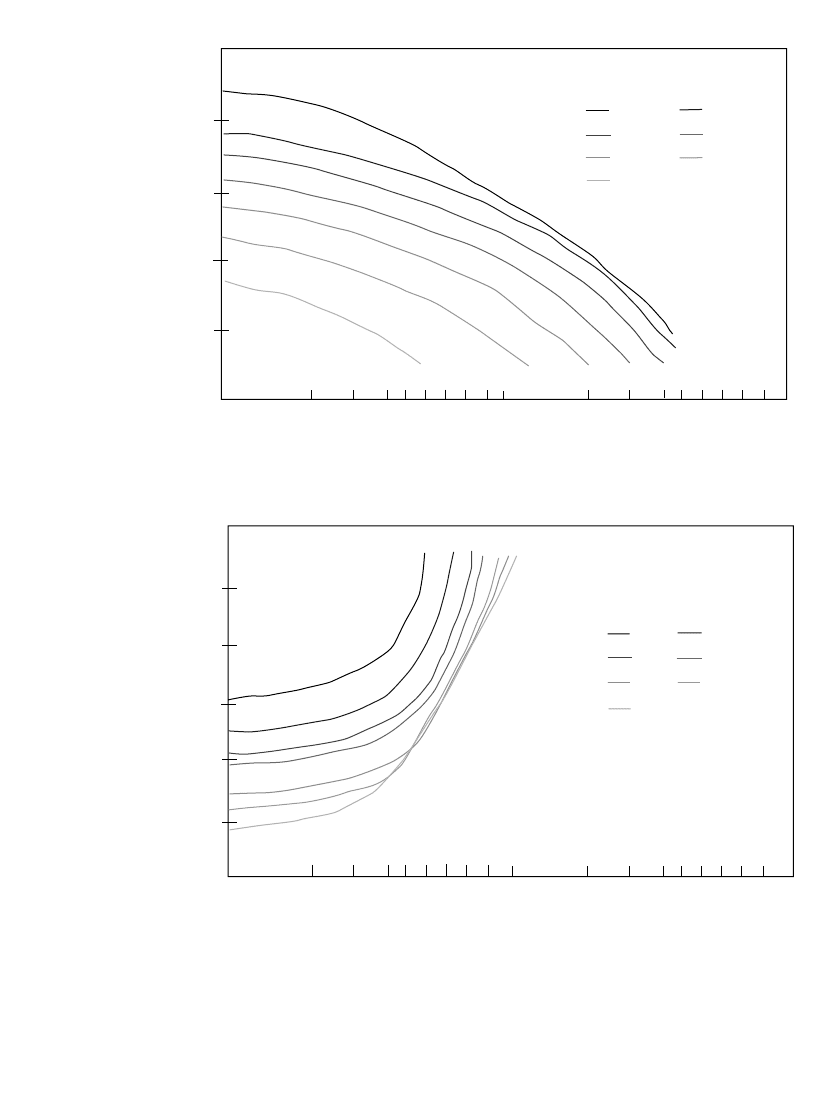
FIGURE 24.32 Comparison of characteristics of conventional
lead-acid and experimental high-rate design batteries. (a) Spe-
cific energy vs. specific power. (b) Peak power vs. depth of dis-
charge. (Proceedings of International Seminar on Primary and
Secondary Batteries, Boca Raton, Fla., 1994.)
24.4.4 Other Cell Designs
The lead-acid battery system still has a significant share of the portable rechargeable battery
market. Its low cost is one of its advantages, but its energy density and power density and
cycle life are inferior to the potential of the newer advanced systems.
New designs are being investigated to improve these characteristics. This work is directed
towards high surface area electrodes with thin active material layers using such materials
and designs as light-weight fiber-glass reinforced lead wire grids, thin metal foils, bipolar
plates, forced flow electrolyte systems, and unique cell assemblies. Improvement in specific
energy and energy density to 40 Wh/ kg and 100 Wh/L with the possibility of rapid recharge
have been attained.
13,14
Figure 24.32a compares the specific energy and specific power on a
cell basis, of the newer designs with conventional technology. Figure 24.32b shows the peak
power capability (W/kg) as a function of the depth-of-discharge.

VALVE REGULATED LEAD-ACID BATTERIES 24.27
24.5 CHARGING CHARACTERISTICS
24.5.1 General Considerations
15
Charging the VRLA battery, like charging other secondary systems, is a matter of replacing
the energy depleted during discharge. Because this process is inefficient, it is necessary to
return more than 100% of the energy removed during discharge. The amount of energy
necessary for recharge depends on how deeply the battery was discharged, the method of
recharge, the recharge time, and the temperature. The overcharge required in the lead-acid
battery is associated with the generation of gases and corrosion of the positive-grid materials.
In conventional flooded lead-acid batteries the gases generated are released from the system,
resulting in a loss of water, which is replenished by maintenance. The VRLA battery incor-
porates the gas recombination principle which allows the oxygen generated at normal over-
charge rates to be reduced at the negative plate, eliminating oxygen outgassing. Hydrogen
gas generation has been substantially reduced by the use of nonantimonial lead grid material.
The corrosion of the positive grid has been reduced by the use of pure or special alloy lead.
Also, the effects of corrosion of the positive grid have been minimized by the element
construction.
Charging can be accomplished by various methods. Constant-voltage charging is the con-
ventional method for lead-acid batteries and is also preferred for VRLA batteries. However,
constant current, taper current, and variations thereof can also be used.
VRLA batteries during cycling and float charge can become unbalanced in that the cor-
rosion rate of the positive becomes unequal to the self-discharge rate of the negative. A
suggested remedy
16
is to utilize a catalyst in the vent space of the cell to restore balance by
recombining hydrogen and oxygen.
24.5.2 Constant-Voltage Charging
Constant-voltage charging is the most efficient and fastest method of charging a VRLA
battery. Figure 24.33 shows the recharge times at various charge voltages for a cell discharged
to 100% of capacity. The charger required to achieve these times at given voltages must be
capable of at least the 2C rate. If the constant-voltage charger used has less than the 2C rate
of charge capability, the charge times should be lengthened by the hourly rate at which the
charger is limited; that is, if the charger is limited to the C /10 rate, then 10 h should be
added to each of the charge voltage-time relationships; if the charger is limited to the C/5
rate, then 5 h should be added, and so on. There are no limitations on the maximum current
imposed by the charging characteristics of the battery.
Figure 24.34 is a set of curves of charge current versus time for 2.5-Ah batteries charged
by a constant voltage of 2.45 V with chargers limited to 2-, 1-, and 0.3-A currents. As
shown, the only difference in these three charges is the length of time necessary to recharge
the battery.
Figure 24.35a shows the charge rate versus time and the percent of previous discharge
capacity returned versus time at 2.35-V constant voltage for a battery discharged at the
C/ 2.5 rate to 80% DOD. The time necessary to recharge the battery to 100% of the previous
discharge capacity is 1.5 h. The charger used was capable of an output in the 4C range and
of voltage regulation at the terminals of better than 0.1%. The initial high inrush of current
caused internal cell heating, which enhanced charge acceptance. If the battery had been more
deeply discharged, the length of time to reach 100% capacity would have increased. The
current decays exponentially with time, and after 3 h on charge, the current has decayed to
a low level. At 2.35 V, the cell will accept whatever current is necessary to maintain capacity.
Figure 24.35b is a similar plot but for a battery charged at 2.50 V constant voltage. All
of the capacity taken out on the previous discharge is returned in approximately one-half
hour.
