Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

23.76 CHAPTER TWENTY-THREE
Overdischarge. Overdischarging the battery should be avoided. The capacity of large bat-
teries, such as those used in industrial trucks, is generally rated in Ampere-hours at the 6-h
discharge rate to a final voltage of 1.75 V per cell. These batteries can usually deliver more
than rated capacity, but this should be done only in an emergency and not on a regular basis.
Discharging cells below the specified voltage reduces the electrolyte to a low concentration,
which has a deleterious effect on the pore structure of the battery. Battery life has been
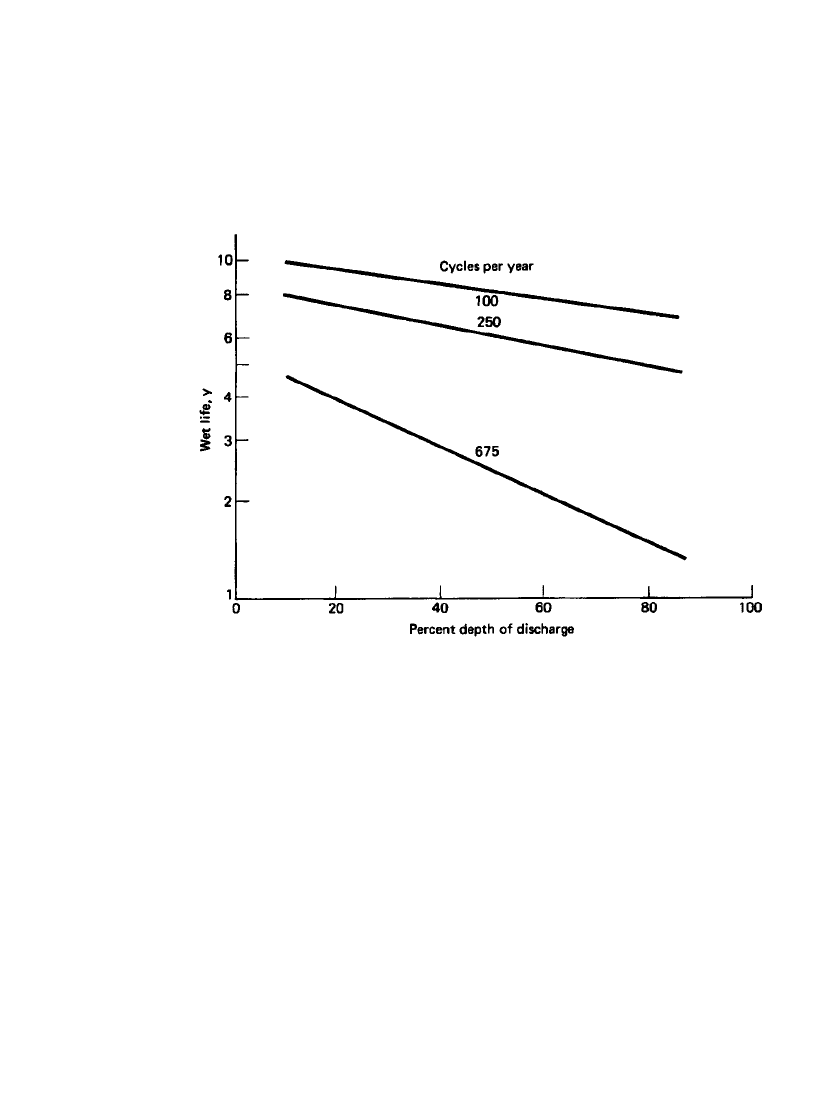
shown to be a direct function of the depth of discharge, as illustrated in Fig. 23.51.
30
FIGURE 23.51 Effect of depth of discharge and number of cycles per year on wet
life at 25⬚C. (From Ref. 30.)
Electrolyte Level. During normal operation, water is lost from a battery as the result of
evaporation and electrolysis into hydrogen and oxygen, which escape into the atmosphere.
Evaporation is a relatively small part of the loss, except in very hot, dry climates. With a
fully charged battery, electrolysis consumes water at a rate of 0.336 cm
3
per Ampere-hour
overcharge. A 500-Ah cell overcharged 10% can thus lose 16.8 cm
3
, or about 0.3% of its
water each cycle. It is important that the electrolyte be maintained at the proper level in the
battery. The electrolyte not only serves as the conductor of electricity but is a major factor
in the transfer of heat from the plates. If the electrolyte is below the plate level, then an area
of the plate is not electrochemically active; this causes a concentration of heat in other parts
of the cell. Periodic checking of water consumption can also serve as a rough check on
charging efficiency and may warn when adjustment of the charger is required.
Since replacing water can be a major maintenance cost, water loss can be reduced by
controlling the amount of overcharge and by using hydrogen and oxygen recombining de-
vices in each cell where possible. Addition of water is best accomplished after recharge and
before an equalization charge. Water is added at the end of the charge to reach the high acid
level line. Gassing during charge will stir the water into the acid uniformly. In freezing
weather, water should not be added without mixing, as it may freeze before gassing occurs.
Water added must be either distilled water, demineralized water, or local water which has
been approved for use in batteries. Automatic watering devices and reliability testing can
reduce maintenance labor costs further. Overfilling must be avoided because the resultant

LEAD-ACID BATTERIES 23.77
overflow of acid electrolyte will cause tray corrosion, ground paths, and loss of cell capacity.
A final check of specific gravity should be made after water has been added to ensure correct
acid concentration at the end of charge. A helpful approximation is
Specific gravity
⫽ cell open-circuit voltage ⫺ 0.845
which permits electrical monitoring of specific gravity on an occasional basis (see also Fig.
23.4). Although distilled water is no longer specified by most battery manufacturers, good-
quality water, low in minerals and heavy-metal ions such as iron, will help prolong battery
life.
Cleanliness. Keeping the battery clean will minimize corrosion of cell post connectors and
steel trays and avoid expensive repairs. Batteries commonly pick up dry dirt, which can be
readily blown off or brushed away. This dirt should be removed before moisture makes it a
conductor of stray currents. One problem is that the top of the battery can become wet with
electrolyte any time a cell is overfilled. The acid in this electrolyte does not evaporate and
should be neutralized by washing the battery with a solution of baking soda and hot water,
approximately 1 kg of baking soda to4Lofwater. After application of such a solution, the
area should be rinsed thoroughly with water.
High Temperature—Overheating. One of the most detrimental conditions for a battery is
high temperature, particularly above 55
⬚C, because the rates of corrosion, solubility of metal
components, and self-discharge increase with increasing temperature. High operating tem-
perature during cycle service requires higher charge input to restore discharge capacity and
local action (self-discharge) losses. More of the charge input is consumed by the electrolysis
reaction because of the reduction in the gassing voltage at the higher temperature (see Table
23.19). While a 10% overcharge per cycle maintains the state of charge at 25 to 35
⬚C, 35
to 40% overcharge may be required to maintain the state of charge at the higher (60 to 70
⬚C)
operating temperatures. On float service, float currents increase at the higher temperatures,
resulting in reduced life. Eleven days float at 75
⬚C is equivalent in life to 365 days at 25⬚C.
Batteries intended for high-temperature applications should use a lower initial specific
gravity electrolyte than those intended for use at normal temperatures (see Table 23.12).
Other design features, such as the use of more expander in the negative plate, are also
important to improve operation at high temperatures.
Cell Balancing. During cycling, a high-voltage battery having many cells in a series string
can become unbalanced, with certain cells limiting charge and discharge. Limiting cells
receive more overcharge than other cells in the string, have greater water consumption, and
thus require more maintenance. The equalization charge has the function of balancing cells
in the string at the top of charge. In an equalization charge, the normal recharge is extended
for 3 to 6 h at the finishing rate of 5 A per 100 Ah, 5-h rated capacity, allowing the battery
voltage to rise uncontrolled. The equalization charge should be continued until cell voltages
and specific gravities rise to a constant, acceptable value. Frequency of equalization charge
is normally a function of the accumulative discharge output and will be specified by the
manufacturer for each battery design and application.
23.8.2 Safety
Safety problems associated with lead-acid batteries include spills of sulfuric acid, potential
explosions from the generation of hydrogen and oxygen, and the generation of toxic gases
such as arsine and stibine. All these problems can be satisfactorily handled with proper
precautions. Wearing of face shields and plastic or rubber aprons and gloves when handling
acid is recommended to avoid chemical burns from sulfuric acid. Flush immediately and

23.78 CHAPTER TWENTY-THREE
thoroughly with clean water if acid gets into the eyes, skin, or clothing and obtain medical
attention when eyes are affected. A bicarbonate of soda solution (100 g per liter of water)
is commonly used to neutralize any acid accidentally spilled. After neutralization the area
should be rinsed with clear water.
Precautions must be routinely practiced to prevent explosions from ignition of the flam-
mable gas mixture of hydrogen and oxygen formed during overcharge of lead-acid batteries.
The maximum rate of formation is 0.42 L of hydrogen and 0.21 L of oxygen per Ampere-
hour overcharge at standard temperature and pressure. The gas mixture is explosive when
hydrogen in air exceeds 4% by volume. A standard practice is to set warning devices to ring
alarms at 20 to 25% of this lower explosive limit (LEL). Low-cost hydrogen detectors are
available commercially for this purpose.
With good air circulation around a battery, hydrogen accumulation is normally not a
problem. However, if relatively large batteries are confined in small rooms, exhaust fans
should be installed to vent the room constantly or to be turned on automatically when
hydrogen accumulation exceeds 20% of the lower explosive limit. Battery boxes should also
be vented to the atmosphere. Sparks or flame can ignite these hydrogen atmospheres above
the LEL. To prevent ignition, electrical sources of arcs, sparks, or flame must be mounted
in explosion-proof metal boxes. Battery cells can similarly be equipped with flame arrestors
in the vents to prevent outside sparks from igniting explosive gases inside the cell cases. It
is good practice to refrain from smoking, using open flames, or creating sparks in the vicinity
of the battery. A considerable number of the reported explosions of batteries come from
uncontrolled charging in nonautomotive applications. Often batteries will be charged, off the
vehicle, for long periods of time with an unregulated charger. In spite of the fact that the
charge currents can be low, fair volumes of gas can accumulate. When the battery is then
moved, this gas vents, and if a spark is present, explosions have been known to occur. The
introduction of the calcium alloy grids has minimized this problem, but the possibility of
explosion is still present.
Some types of batteries can release small quantities of the toxic gases stibine and arsine.
These batteries have positive or negative plates which contain small quantities of the metals
antimony and arsenic in the grid alloy to harden the grid and to reduce the rate of corrosion
of the grid during cycling. Arsine (AsH
3
) and stibine (SbH
3
) are generally formed when the
arsenic or antimony alloy material comes into contact with nascent hydrogen, usually during
overcharge of the battery, which then combines to form these colorless and essentially odor-
less gases. They are extremely dangerous and can cause serious illness and death. The OSHA
1978 concentration limits for SbH
3
and AsH
3
are 0.1 and 0.05 ppm, respectively, as a max-
imum allowable weighted average for any 8-h period. Ventilation of the battery area is very
important. Indications are that ventilation designed to maintain hydrogen below 20% LEL
(approximately 1% hydrogen) will also maintain stibine and arsine below their toxic limits.
The ordinary 12-V SLI automotive battery is a minor shock hazard. The hazard level
increases with higher-voltage systems, and systems in the range of 84 to 360 V are being
used for electric vehicles. Systems as high as 1000 V are under study for fixed-location
energy-storage systems for load leveling. Batteries are electrically alive even in the dis-
charged state, and the following precautions should be practiced:
1. Keep the top of the battery clean and dry to prevent ground short circuits and corrosion.
2. Do not lay metallic objects on the battery. Insulate all tools used in working on batteries.
3. Remove jewelry and any other electrical conductor before inspecting or servicing batter-
ies.
4. When lifting batteries, use insulated lifting tools to avoid risks or short circuits between
cell terminals by lifting chains or hooks.
5. Make sure gases do not accumulate in batteries before they are moved.
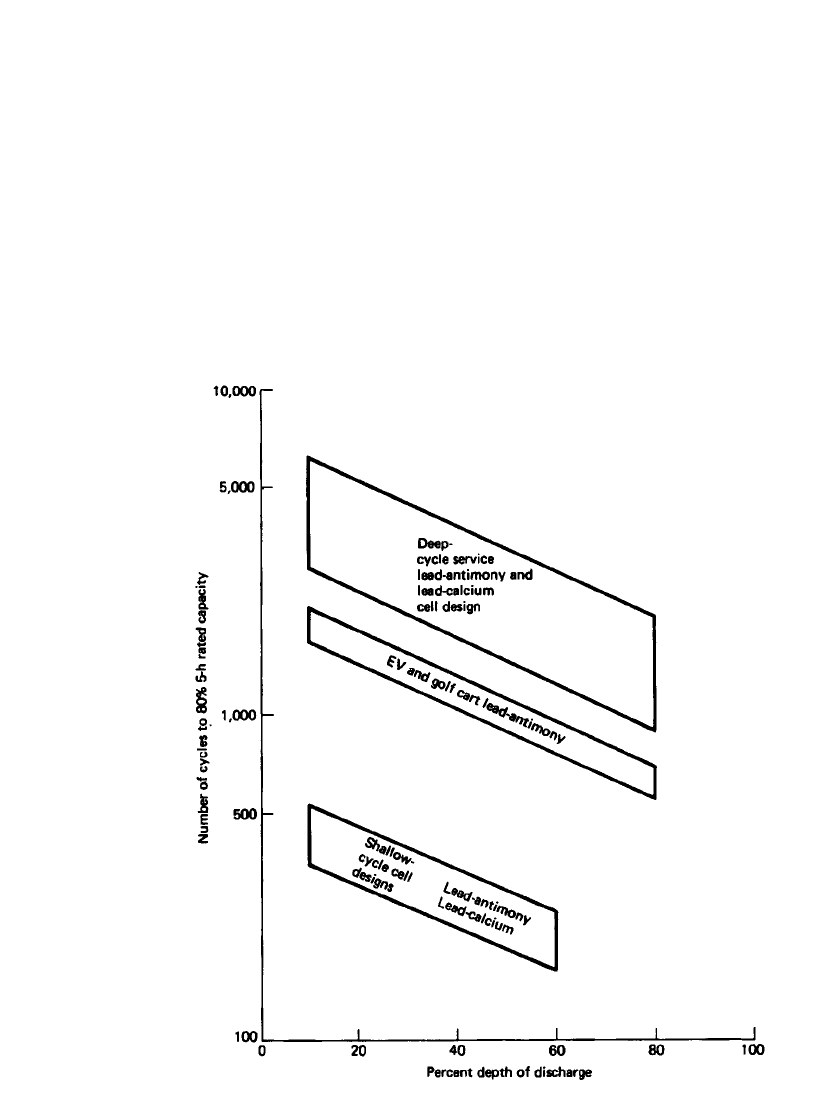
LEAD-ACID BATTERIES 23.79
FIGURE 23.52 Effect of cell design and depth of discharge on cycle life of various
types of lead-acid batteries at 25⬚C. (From Ref. 30.)
23.8.3 Effect of Operating Parameters on Battery Life
Operating parameters which have a strong influence on battery life are depth of discharge,
number of cycles used each year, charging control, type of storage, and operating tempera-
ture. In some cases the battery design features which increase life tend to decrease the initial
capacity, power, and energy output. It is important, therefore, that the design features of the
battery be selected to match the operating and life requirements of the application.
1. Increasing the depth of discharge decreases cycle life, as illustrated in Figs. 23.52
30
and
23.32.
2. Increasing the number of cycles performed per year decreases the wet life (Sec. 23.8.1
and Fig. 23.51).
3. Excessive overcharging leads to increasing positive grid corrosion, active material shed-
ding, and shorter wet life.

23.80 CHAPTER TWENTY-THREE
TABLE 23.19 Failure Modes of Lead-Acid Batteries
Battery type Normal life Normal failure mode
SLI Several years Grid corrosion
SLI (maintenance-free) Several years Lack of water, damage to positive
plates
Golf cart 300–600 cycles Positive shedding and grid
corrosion, sulfation
Stationary (industrial) 6–25 years Grid corosion
Traction (industrial) Minimum 1500 cycles Shedding, grid corrosion
4. Storing wet cells in a discharged condition promotes sulfation and decreases capacity and
life.
5. Proper charging operations with good equipment maintain the desired state of charge with
a minimum of overcharge and lead to optimum battery life.
6. Stratification of the electrolyte in large cells into levels of varying concentration can limit
charge acceptance, discharge output, and life unless controlled during the charge process.
During a recharge, sulfuric acid of higher concentration than the bulk electrolyte forms
in the pores of the plates. This higher-density acid settles to the bottom of the cell, giving
higher specific gravity acid near the bottom of the plates and lower specific gravity acid
near the top of the plates. This stratification accumulates during the nongassing periods
of charge. During the gassing periods of overcharge, partial stirring is accomplished by
gas bubbles formed at and rising along the surfaces of the plates and in the separator
system. During discharge, acid in the pores of the plates and near their surface is diluted;
however, concentration gradients set up by longer charge periods are seldom compensated
entirely, particularly if the discharge periods are shorter, as is usually the case. Diffusion
processes to eliminate these concentration gradients are very slow, and stratification during
repetitive cycling can become progressively greater. Two methods for stratification control
are by deliberate gassing of the plates during overcharge at the finishing rate and by
stirring of cell electrolyte by pumps (usually airlift pumps). The degree of success in
eliminating stratification is a function of cell design, the design of the pump accessory
system, and cell operating procedures.
23.8.4 Failure Modes
The failure modes of lead-acid batteries depend on the type of application and the particular
battery design. This is the rationale for the manufacture of different batteries since each one
is designed to give optimum performance in a specific type of use. The more prevalent
failure modes for the different types of lead-acid batteries are listed in Table 23.19.
31
Sig-
nificantly, if a battery is properly maintained, most of the inherent failures are due to the
degradation of the positive plate through either grid corrosion or paste shedding. These
failures are irreversible, and when they occur, the battery must be replaced. Details of the
failure modes of SLI batteries are given in Sec. 23.4.3.
The failure mode and the time to failure can be modified by changes in the inner para-
meters (I), such as battery materials, processing, and design, or by the conditions of use,
designated as the outer parameters (O). Some of these are listed in Table 23.20.
31

LEAD-ACID BATTERIES 23.81
TABLE 23.20 Modification of Lead-Acid Battery Failure Rate
Failure mechanism Rate of failure modification*
Shedding positives I: active mass structure, battery design
O: number of cycles, depth of discharge, charge factor
Sulfation/ leading of negatives I: active mass additives
O: temperature, charge factor, maintenance
Positive grid corrosion (overall,
localized, or positive grid
growth)
I: grid alloy, casting conditions, active mass
Separators I: electrolyte concentrations, battery design
O: temperature, charge factor, maintenance
Case, cover, vents, external
battery connections
I: battery materials and design
O: maintenance, abuse
*I—inner parameters; O—outer parameters.
23.9 APPLICATIONS AND MARKETS
The lead-acid battery is used in a wide variety of applications, and in the past few years
many new applications have arisen. The various types of lead-acid batteries and their appli-
cations are listed in Table 23.2. The new uses of lead-acid batteries are mainly associated
with the smaller sealed maintenance-free cells used in electronic and portable devices and
with the advanced designs for energy storage and electric vehicles.
23.9.1 Automotive Applications
Traditionally the most common use of the lead-acid battery is for starting, lighting, and
ignition in automobiles and other vehicles with internal combustion engines. Almost all of
these now have 12-V nominal electric systems. Most of the earlier generators have been
replaced by alternators and electromechanical regulators by electronic/solid-state controls.
High cranking ability at low temperatures is still the major design factor, but SLI batteries
today see more cycling-type service (compared with float service) because of the electrical
load of the auxiliaries. Size and weight reduction have also become important as well as the
battery geometry. Batteries are normally located in the cool air stream ahead of the engine
to prevent their overheating; thus their geometry can affect the profile of the vehicle. These
factors have led to the redesign of the lead-acid battery for SLI applications. The most
important changes were:
•
Change from high-antimony (4 to 5%) lead alloy grid to a low-antimony (1 to 2%) or
nonantimonial lead alloy grid, thus reducing hydrogen evolution
•
Use of thinner electrodes
•
Better separators with lower electrical resistance
•
Plate tabs located in from corners, and grids redesigned for high conductivity
•
Semisealed, maintenance-free construction
23.82 CHAPTER TWENTY-THREE
Automotive-type batteries are also used on trucks, aircraft, industrial equipment, and
motorcycles as well as in many other applications. They are used in off-road vehicles, such
as snowmobiles, in boats to crank inboard and outboard engines, and in various farm and
construction equipment. Military vehicles in the United States and NATO countries have
standardized on a 24-V electric system that is provided by a series connection of two 12-V
batteries.
The term ‘‘SLI battery’’ has evolved into something of a misnomer. In addition to starting,
lighting and ignition, the automotive SLI battery may provide the power for many other
functions. Although these features may not pose much of a burden individually, collectively
they add up to a significant drain on the SLI battery. Some of today’s automobiles require
up to 2 to 3 kW of power. This could more than double in the next few years. Table 23.21
shows some current or anticipated features requiring power exclusive of the SLI functions.
The typical SLI battery is not designed to handle the cycling demands prompted by some
of these items. As a result, the automotive industry is planning to go to a 36/ 42 volt system
in the near future.
32,33
The 42-V figure represents the nominal charge voltage for an eighteen
cell battery, while 36 V is the minimal operating voltage. Generally, only one of these figures
is mentioned as characterizing these new systems. A major advantage of going to the higher
voltage is, that at a given power level, the current required would be proportionately less
than with the conventional 12-V battery. The higher voltage will result in a substantial saving
in weight of the current-carrying distribution system in the car, i.e., less copper will be
required. In turn, this saving is projected to translate into a 5 to 10% increase in gas mileage.
Simply scaling up the current SLI lead-acid battery from 6 to 18 cells leads to a number
of problems, not the least of which is the significant increase in the weight of the battery.
In addition, with all the added drains on the battery and the possibility of automatic start-
stop, the battery will experience deeper and more numerous discharges than current SLI
batteries are designed to handle. Like VRLA batteries, today’s SLI batteries are maintenance
free in that they don’t require addition of water during their operating life. However, unlike
the starved electrolyte VRLA batteries, SLI batteries contain flooded cells. To address the
weight problem, VRLA batteries are an obvious choice. Unfortunately, as discussed in Chap.
24, the wide temperature swings encountered under the hood of an automobile present a
significant challenge to VRLA technology.
TABLE 23.21 Features Requiring Power for Present and Future Automobile Designs (Exclusive of
SLI Function).
Alarms (may include flashing LEDs) Audio-radio, tape or CD players
Computer Global positioning features (maps, routing, emergency location)
Electric suspension Electromagnetic valve trains
Automatic start-stop of engine Electric heating of catalysts
Air conditioning Sensing (e.g., for airbag deployment)
Electric heating of seats Anti-lock braking
Electric steering Electrochromic mirrors
Power windows Rear window deicer/ defogger
Rear seat entertainment center Cigarette lighter (other functions)
Electric door locks Cruise control
Clock

LEAD-ACID BATTERIES 23.83
Other potential problems associated with the higher voltage platform include such items
as electrical noise and more parasitic drains due to the higher voltage. Higher voltages are
undesirable due to potential shock hazard if the vehicle chasis is grounded. The higher costs
and the need for the semiconductor industry to modify their electronic circuits and devices
also present challenges. Counteracting the increased gas mileage anticipated from the reduced
amount of copper, the increased power requirements in future automobiles require more fuel.
An increase from a 2-kw to a 4-kw system will result in a reduction in gas mileage of up
to 6 miles per U.S. gallon. The timeframe for the introduction of a higher voltage automotive
battery system in volume production is currently a matter of controversy. Estimates range
from 2003 up to 2010 or later. A prime mover in the trend to higher voltage systems is the
MIT Consortium on Advanced Automotive Electrical/ Electronic Components and Systems,
a group that includes various auto manufacturers and suppliers.
Suggested approaches to a 36/42-V platform include a dual battery system combining
lead-acid with either a nickel-metal hydride or a lithium-ion battery. On the other hand, some
suggest abandoning the lead-acid battery altogether and going to a nickel-metal hydride
battery. Two commercially available Hybrid Electric Vehicles on the market in 2000 are
using the latter type of battery exclusively. The major advantage of lead-acid over the other
two systems remains cost. At this point, it seems likely that there will be a variety of
approaches to this 36/42-V regime. The role of lead-acid in this mix may depend critically
on the progress in VRLA technology, especially in the area of subduing the tendency for
thermal runaway under adverse conditions (see Chap. 24).
One example of a dual battery now being marketed is the so-called Gemini Twinpower
TM
battery manufactured in China. This system comprises two 12-V batteries in a single case
with an associated ‘‘Energy Management Controller’’ (EMC). One 12-V battery has thin
plates for the starter function; the other 12-V battery has thick plates and glass mat separators
designed for cycle life. The EMC maintains the starter battery fully charged and, during
starting, combines the two batteries for starting, while isolating the batteries when the engine
is turned off. This ‘‘smart’’ battery could be considered an intermediate step towards the 36/
42-V system.
23.9.2 Small Sealed Lead-Acid Cells
In recent years there has been a significant increase in the use of battery-operated consumer
equipment such as portable tools, lighting devices, instruments, photographic equipment,
calculators, radio and television, toys, and appliances. Batteries for these applications are
generally of low capacity, up to 25 Ah. Storage batteries are used frequently because of their
high power capability and rechargeability, but they have to be sealed or of the nonspill type
in order to function in all positions. Vented lead-acid batteries of the electrolyte-retaining
(ER) type and cylindrical (see Chap. 24) or prismatic cells are used in competition with
sealed nickel-cadmium cells. The lead-acid batteries offer lower initial cost, better float ser-
vice, higher cell voltage, and the absence of memory effect (loss of capacity on shallow
cycling). The nickel-cadmium cell has longer life and better cycling service.
The small sealed or semisealed lead-acid cells are available as single 2-V units or as
multiple-cell units, usually in 6-V monobloc constructions. They are an outgrowth of the
earlier ER-type batteries in which the electrolyte was absorbed in wood pulp separators. The
ER-type cells, while spill-proof, contained more electrolyte, did not recombine oxygen on
overcharge, and were vented.
A related small deep-discharge lead-acid battery is the one used for miner’s lamps and
similar equipment. These are 4-V units which are vented and can be watered. They are
designed to deliver 1 A for 12 h between charges.

23.84 CHAPTER TWENTY-THREE
23.9.3 Industrial Applications
Applications for lead-acid batteries, other than the SLI and small sealed power units, fall
into two categories, as shown in Table 23.22—those based on automotive-type constructions
and those based on industrial-type constructions. Often several designs can be used for a
single type of application.
TABLE 23.22 Major Applications of Lead-Acid Batteries (Non-SLI Types)
Automotive and small
energy storage designs
Traction Special
Industrial designs
Stationary
Traction
(motive power) Special
Golf cart
Off-road
vehicles
On-road
vehicles
Emergency lighting
Alarm signals
Photovoltaic
Sealed cells (for
tools,
instruments,
electronic
devices, etc.)
Switch gear
Emergency lighting
Telecommunication facilities
Railway signals
Uninterrupted power supply
Photovoltaics
Load leveling and energy
storage
Mine locomotives
Industrial trucks
Large electric
vehicles
Submarines
Ocean buoys
23.9.4 Electric Vehicle
Lead-acid batteries have powered off-the-road electric vehicles such as golf carts, forklift
trucks and airport baggage carriers for many decades. Most of these vehicles employ 36-V
systems utilizing six 6-V batteries in series. Many on-the-road designs have evolved based
on enhancing the mechanical properties of golf carts. These are traditionally used in retire-
ment villages and non-city applications. Lead-acid batteries have also been used for true on-
the-road vehicle designs, such as the General Motors EV1, and a number of delivery vans.
Small battery powered vans have been used in the UK for milk delivery for the past century
and 10–15,000 such vehicles are estimated to be in current use. Many of the modern electric
vehicles have utilized high voltage (AC or DC) motors, with batteries operating in the range
of 200 to 300 V.
Such high voltages complicate the battery design and also compromise life. One problem
is simply the large number of cells in series needed to attain the high voltage. Reliability
analyses in the battery industry have shown that statistically, the life of a battery decreases
as the number of cells in series increases. This is not a surprising conclusion but rather an
expected result of having units with individual failure rates in a series of such units. To
anticipate and accommodate the failure of individual cells without compromising the whole
battery requires the added complication of electronic circuitry to switch bad cells out of the
series connection. If full voltage is crucial, one might require standby cells to be switched
into the circuit at the same time. Additional problems associated with the high voltage
include higher leakage currents, ground short problems and corrosion. With the larger battery,
there is also a safety concern associated with the possible accumulation of greater amounts
of hydrogen than with conventional SLI batteries.
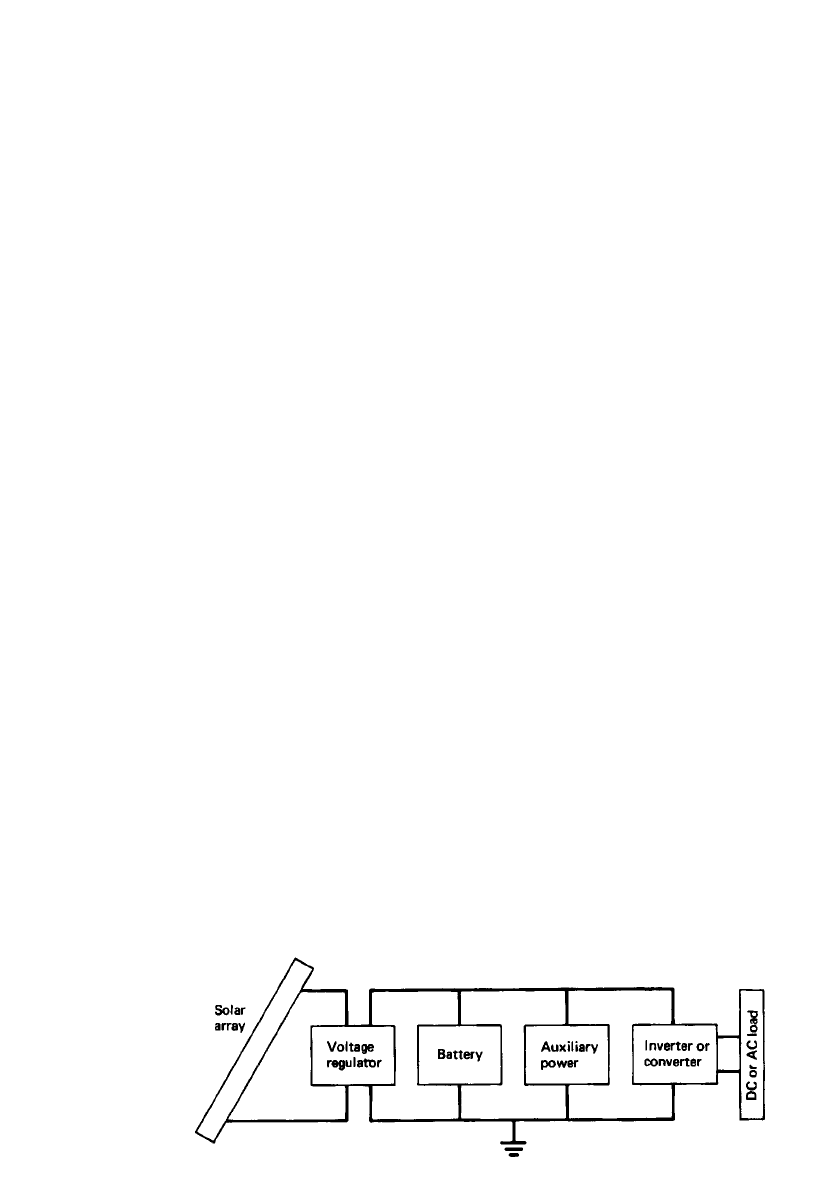
LEAD-ACID BATTERIES 23.85
FIGURE 23.53 Components of solar photovoltaic system. (From Ref. 30.)
The EV1, now in its second generation, is a limited production vehicle designed from
the ground up as an electric vehicle. It has an aerodynamic teardrop shape, regenerative
braking to charge the battery, together with an aluminum structure and composite body panels
for reduced weight. A battery of twenty-six 12-V valve-regulated lead-acid batteries powers
a 137 horsepower, 3-phase AC induction motor. The estimated driving range is between 55
and 95 miles between charges, depending on driving conditions and driver habits. An op-
tional nickel-metal hydride battery is estimated to extend the range from 75 to 130 miles.
With air conditioning, traction control, cruise control, anti-lock braking, speeds up to 80
miles per hour, and other features, the EV1 is not a stripped-down vehicle. Although such
a zero-emissions electric vehicle would appear to be the answer to environmental concerns
and the benefits have been widely publicized, consumer acceptance of a short range, more
expensive vehicle has not been widespread. On the other hand, the introduction of low-
emissions hybrid electric-internal combustion vehicles, notably the Toyota Prius, generated
immediate enthusiasm and sales, with favorable reviews in the automotive press. The Prius
employs an Ni-MH battery, as does the Honda Insight. In 1999, the costs for the major EV
candidate battery systems were roughly $200 to 400/kWh for lead-acid, $500 to 1,000/ kWh
for Ni-MH and
⬎$1,000/ kWh for lithium-ion batteries.
34
It is clear that mass production of
pure on-the-road electric vehicles will require lower cost batteries, increased range between
charges and an infrastructure of charging facilities to support the electric vehicles. Lead-acid
technology seems suitable only for those willing to accept a truly low-range, pure-electric
vehicle.
23.9.5 Energy-Storage Systems
Secondary batteries are now being considered for load leveling in electric utility systems as
an alternative to meet peak power demands currently provided with energy-expensive oil- or
gas-fueled turbines (see Chap. 37). Large batteries, on the order of 50 MWh at 1000 V, are
required. The lead-acid battery, again, is a major candidate for a near-term solution for this
application. The goal is to obtain in excess of 2000 cycles or 10 year of operation at a cost
of about $90 per kiloWatthour.
Smaller-sized batteries are used for energy storage in systems employing renewable but
interruptible energy sources, such as wind and solar (photovoltaic) energy. These systems
are usually located on the customer side of the utility power grid. The system generally
handles the following functions:
1. Converts solar, wind, or other such prime energy source to direct electric power
2. Regulates the electric power output
3. Feeds the electric energy into an external load circuit to perform work
4. Stores the electric energy in a battery subsystem for later use
A block diagram of a typical photovoltaic system is shown in Fig. 23.53.
30
