Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

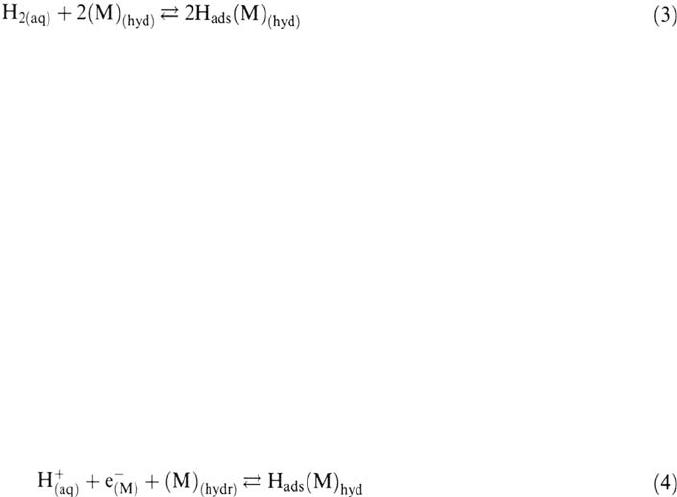
The Hydrogen Surface Reactions in Aqueous Electrolyte
The H entry into a metal from an aqueous electrolyte is believed to involve the same
surface-bulk transfer step as in the gas phase, but the preliminary adsorption step is a
more complex process because more H sources are involved in aqueous solution,
allowing more possible H surface reactions, and also because of the specificity of the
electrolyte-metal interface. Whereas H adsorption in the gas phase occurs by
dissociative adsorption of gaseous H
2
on the free sites of a bare metallic surface, H
adsorption in aqueous solution may occur either chemically by dissociation of
dissolved H
2
or electrochemically from solvated (hydrated) protons or water
molecules; it takes place on a hydrated surface and thus implies the displacement of
adsorbed water molecules or specifically adsorbed ions and local reorganization of
the double layer [20]; competition with the adsorption of oxygen species formed from
the dissociation of water may also occur [21–23]. The adsorbed H layer is also in
interaction with surrounding water molecules, i.e., it is hydrated [8c,24,25].
Elementary Surface Reactions
Chemical H
2
Dissociative Adsorption and H-H Combination (Tafel
Reaction) Under conditions in which there is no specific adsorption of ions or
adsorption of oxygen species, the reaction of adsorption from H
2(g)
in aqueous
electrolyte occurs through the following steps:
Dissolution of H
2
gas into solution, in equilibrium: H
2(g)
' H
2(aq)
Transport of the dissolved H
2
molecules to the surface
Direct dissociative adsorption:
56 Protopopoff and Marcus
where H
2(aq)
is a dissolved H
2
molecule, 2(M)
(hyd)
represents a pair of adjacent
hydrated sites available for adsorption of hydrogen, and H
ads
(M)
(hyd)
represents
an H atom adsorbed in a site (M) and hydrated.
The reverse reaction is the combinative desorption of H atoms, or chemical
combination, also known as the Tafel reaction.
H Electroadsorption/Electrodesorption (Volmer Reaction) In aqueous
electrolyte, because the metal substrate is an electrode with the electric potential
as an additional variable, H adsorption may occur electrochemically (i.e., assisted
by the potential) by reduction of hydrated protons or water molecules, depending
upon the pH [26].
1. Proton discharge in acid medium: it involves:
(a) Fast transport of hydrated protons from the bulk solution to the double
layer between the electrolyte and the cathode surface
(b) H electroadsorption by electron transfer from the electrode to the hydrated
protons in the outer Helmotz plane [26]:
where H
+
(aq)
represents a hydrated proton, and e
–
(M)
an electron supplied
by the metal M.
Copyright © 2002 Marcel Dekker, Inc.
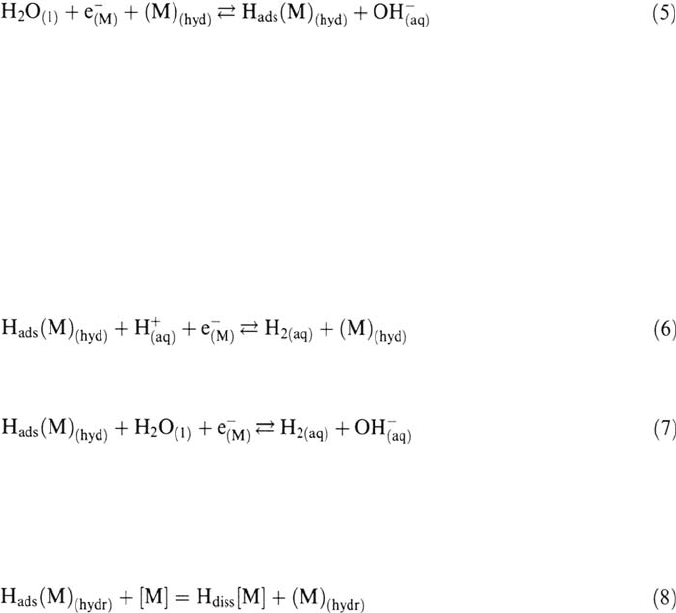
2. Reduction of water in neutral or basic medium:
Surface Effects on Hydrogen Entry into Metals 57
Although there is no limitation to the supply of water molecules in not too
concentrated electrolytes (the water activity at the surface is equal to unity), this
reaction has a lower rate constant than proton discharge for a given potential so
that it is predominant only when the proton concentration is very low.
Electrodissociation/Electrocombination (Heyrovsky Reaction) The H atoms
can be desorbed electrochemically by combination with hydrated protons or water
molecules (Heyrovsky reaction or electrocombination). Again, depending upon
the pH, two types of reaction occur:
1. Proton plus H atom reaction in acid medium:
2. Water plus H atom reaction in neutral or basic medium:
H Surface-Bulk Transfer The transfer step from the adsorbed to the absorbed
state is equivalent to the reaction described for the gas phase [Eq. (2)] except that
it occurs from adsorbed H atoms surrounded by water molecules or anions and it
liberates surface sites for hydratation, as follows:
Overall Electrode Reactions
The H sources in solution, i.e., hydrated protons, and water molecules can be
transformed through the preceding elementary adsorption and desorption steps
into H
2(g)
(HER) or H
abs
(HAR).
Hydrogen Evolution Reaction This overall reaction involves the following
two-step pathways proceeding alone or in parallel:
1. Volmer-Tafel pathway: electroadsorption followed by chemical combination
2. Volmer-Heyrovsky pathway: electroadsorption followed by electrocombination
These steps are followed by transport of dissolved H
2
molecules away from the
electrode via diffusion or gas evolution (formation of nuclei and phase separation).
On a noble metal, equilibrium of the HER (reversible H
+
/H
2
electrode) may
be attained provided that the partial H
2
pressure in solution is high enough because
the kinetics are fast; from thermodynamic arguments, the HER occurs with a net
rate if the potential is cathodic with respect to the equilibrium potential at the
given H
2
pressure and pH. Conversely, if the overpotential is anodic, the reverse
reaction, i.e., the H
2
oxidation reaction (HOR), prevails, involving H
2
dissociation
into adsorbed H followed by H electrodesorption [27].
Copyright © 2002 Marcel Dekker, Inc.
On a corroding metal surface, a mixed process occurs because the main
reverse reaction is dissolution or oxidation (passivation); consequently, the net
current is a function of the overpotential with respect to the mixed (corrosion) potential
(see Chap. 1). The anodic reaction and the H cathodic reaction proceed simultaneously
close to the corrosion potential, which implies that the HER may occur on a surface
covered by an oxide film (passivated) and that the detrimental effects of anodic
dissolution and H entry may be combined in the embrittlement process.
The mechanisms of the HER on the metals currently studied in H entry
experiments are relatively well known. At high overpotentials they all involve a
coupled electroadsorption-electrocombination mechanism where the reverse steps
are negligible.
On electrodes of noble metals of the Pt group, the mechanism of the HER
involves at low overpotentials electroadsorption in quasi-equilibrium, followed by a
rate-determining step (RDS) of chemical combination, then electrocombination at
higher overpotentials [26,28].
On Ni, the reported mechanisms are electroadsorption in quasi-equilibrium
followed by rate-determining electrocombination [26,29].
On Fe, early experiments indicated coupled mechanisms, with electroadsorption
followed by chemical combination at low overpotential (low θ
H
) or electrocombination
at high overpotential (higher θ
H
) [30–32]. However, more recent analyses reported
a parallel pathways mechanism with the chemical and electrochemical combination
steps occurring simultaneously, the former being predominant in 0.1 N H
2
SO
4
up
to high cathodic currents (current densities), whereas in 0.1 N NaOH or neutral
solutions the latter (involving water molecules) already predominates at low
cathodic currents [33,34]. The exact mechanism seems to depend on the structure
and purity of the iron electrode and its surface composition.
Underpotential and Overpotential H Electroadsorption The process of
reversible hydrogen electroadsorption occurs at the surface of metal electrodes of
the platinum group at potentials anodic to the reversible hydrogen electrode at the
given pH and at a pressure of 1 atm (half-standard reversible H
+
/H
2
electrode at
1 atm, denoted RHE1 here): H is electroadsorbed at equilibrium at E>0V
(RHE1)
up to a quasi-full monolayer. Because this process occurs, going cathodically, under
the equilibrium potential of the RHE1, by analogy with underpotential metallic
deposition, it is often called H underpotential deposition (UPD) and the adsorbed
H UPD H [35]. Contrary to the HER, which is a steady faradic reaction consuming
the H species electroadsorbed at overpotential, for which a stationary state is reached
when the net rate of electroadsorption of reactants is equal to twice the net rate
of H
2
formation, H UPD is a pseudocapacitive faradic process; i.e., at a given
potential the adsorption current goes to zero once the equilibrium coverage
is reached,corresponding to equality between the rate of adsorption and the rate
of desorption of H atoms. It can be characterized only by transient techniques such
as cyclic linear sweep voltammetry, giving for cathodic and anodic potential
sweeps the well-known H adsorption/desorption pseudocapacitance peaks
[35–39], symmetrical if the sweep rate is not too high [35]. By integration of a
voltammogram, the electroadsorption/desorption charge flowing through the
interface per unit area during a potential scan is obtained, which allows
determination of the H surface density at a given potential. If the metal atomic
58 Protopopoff and Marcus
Copyright © 2002 Marcel Dekker, Inc.

density is known (e.g., on a well-ordered single crystal surface), the coverage θ
H
(number of adsorbed H atoms per metal substrate atom) is obtained as a function
of the potential, giving electroadsorption isotherms [24,37,38,40–42].
UPD H detection is limited cathodically by the onset of the HER, producing a
steady current that is rapidly predominant; UPD is prevented on electrodes covered with
electronegative adorbates (see under Influence of the Surface Modifiers on H Entry),
and even on a clean electrode UP H adsorption may be hindered by competitive
anodic processes such as UP adsorption of O or OH and electrode oxidation
[23,29,43]. This explains why UPD H may be detected only on noble metals where the
anodic preoxidation processes occur at relatively high anodic potentials so that there is
a potential range where H atoms compete only with water molecules forming the metal
hydration layer and the specifically adsorbed anions [23,35,39,59]. However, H UPD
is not detected on Ag and Au, for which H adsorption from H
2(g)
is endothermic (see
later). On Pd, significant H absorption occurs at underpotential in bulk samples [44,45],
so equilibrium UP H adsorption may be characterized by cyclic voltammetry only on Pd
thin films in which the low number of bulk H sites limits the absorption reaction
[46,47]. On corroding or passivated metal electrodes, UPD H detection is impeded by
the metal dissolution and oxidation processes and the voltammograms are not easily
interpreted [43,48]; although some workers claimed that H electroadsorption occurs
in aqueous electrolytes above 0 V (RHE1) on Ni [49], Fe [50], or W [51], at present
there is no strong experimental evidence for this.
Conversely, the electroadsorption step involved in the HER at cathodic
overpotentials on all electrodes is called H overpotential deposition (OPD) and the
H
ads
intermediate involved in the HER is called OPD H [35]. Because overpotential
adsorption does not occur alone but is only a step in the steady HER process, it is
much more difficult to characterize. A technique of measurements of potential
relaxation transients (potential decay) could allow determining pseudocapacitance
versus potential curves and obtaining the OPD H coverage by integration [29], but
this is not straightforward. The OPD H fractional coverage is usually estimated from
analysis of the kinetics of the HER and HAR. OPD H is also likely to be the
intermediate of the HOR on Pt, in an anodic potential range overlapping that of UPD
H [27]. In principle, the electroadsorption of OPD H can occur under true
equilibrium only at the H
+
/H
2
equilibrium potential, and the OPD H coverage
versus potential variation depends upon the HER or the HOR mechanism (see later).
It seems likely that the OPD H atoms reacting in the HER on Pt are adsorbed on top
of Pt atoms, while the UPD H atoms are adsorbed in high coordination sites [52,53]
(see later). There are no similar data for non-noble metals.
Hydrogen Electroabsorption Reaction (HEAR) In aqueous solution, the
hydrogen absorption reaction occurs mainly by electrochemical reduction of pro-
tons or water molecules (electroabsorption). The overall H electroabsorption reac-
tion from protons is:
It was demonstrated for iron, from the analysis of the relation between the
Surface Effects on Hydrogen Entry into Metals 59
stationary cathodic and anodic currents measured on each side of a permeation
membrane, that the HER and the HEAR share a common adsorbed H intermediate
(H
ads
) and a common first step of electroadsorption [see Eqs. (4) and (5)] [31]; then H
Copyright © 2002 Marcel Dekker, Inc.
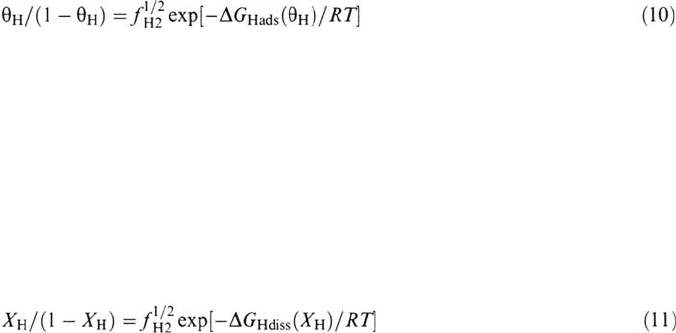
enters the metal lattice by the surface-bulk transfer step [Eq. (8)], assumed to be in
quasi-equilibrium because in most cases the permeation rate is found to be limited
only by H diffusion through the bulk. This mechanism has been verified to be valid
for permeation through iron and nickel and their alloys and it is the generally
accepted one [28].
However, it has been suggested by Russian workers [54,55] for explaining the
effects of adsorbed I
–
ions on H permeation into Pd membranes that the process of H
electroadsorption in the HER and the process of electroabsorption “should be
regarded as occurring simultaneously and to a certain extent independently of each
other” [55]. Thus, on Pd the HAR would not occur by the pathway described before,
but by what has been interpreted since as direct H entry from protons [31,56]; this
mechanism was invoked for explaining Pd membrane permeation data showing an
anomalous relationship between steady-state cathodic and permeation currents [57].
Actually, because a direct entry would involve a quantum tunneling effect not likely
at room temperature (see section on gas phase), a classical activation mechanism of
H entry through a surface intermediate state different from the adsorbed state
involved in the HER (OPD H) is more rational and has to be considered as a
possible alternative to the preceding mechanism, at least on Pd. The observation that
significant H electroabsorption into bulk Pd already occurs at positive potentials
versus RHE1, before the HER takes place, and is likely to involve H UPD [44,58,59]
could be consistent with the so-called direct entry mechanism as UPD H is an inter-
mediate surface state different from the HER intermediate (see following section).
THERMODYNAMICS OF METAL-HYDROGEN SYSTEMS
Thermodynamics of H Adsorption and Absorption in the Gas Phase
Isotherms of the Metal-Hydrogen Equilibria
Isotherm of H
2
Dissociative Adsorption The equation of the isotherm of
equilibrium adsorption from H
2(g)
[see Eq. (1)] is
60 Protopopoff and Marcus
where θ
H
is the H surface fractional coverage in the sites (M), f
H2
is the fugacity of
hydrogen gas expressed in atm, and ΔG
Hads
is the half-standard free energy of H
adsorption referred to ½H
2(g)
(ΔG
Hads
(θ
H
) = μ
Hads
(θ
H
)–½μ°
Η2
). ΔG
Hads
is in the
general case a function of the coverage θ
H
[60]. θ
H
is equal to the ratio Γ
H
/Γ
Hsat
of
the H
ads
surface density to the surface density at saturation. Γ
Hsat
is not necessarily
equal to the surface density of H sites on the given face because of H–H repulsive
interactions [8].
Isotherm of H
2
Dissociative Absorption (Dissolution) The equation of the
isotherm of equilibrium absorption (or dissolution) from H
2(g)
[see Eqs. (1) and
(2)] is the following:
where X
H
is the H bulk fractional solubility in the sites [M] and ΔG
Hdiss
is the
half-standard free energy of H dissolution from ½ H
2(g)
. ΔG
Hdiss
is the general
case a function of the fractional solubility X
H
.
Copyright © 2002 Marcel Dekker, Inc.
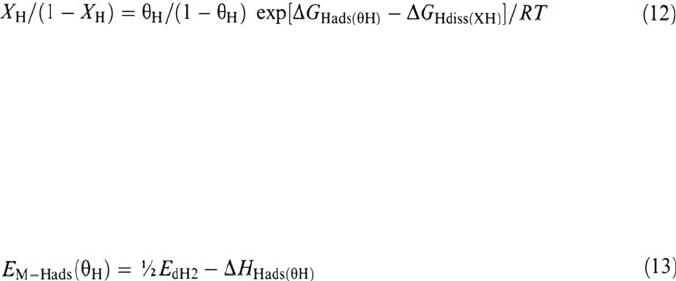
X
H
, the fractional solubility of H in the metal or fraction of the intersticial sites
occupied by H, may be expressed as the ratio c
H
/c
Hsat
of the H volumic concentration
to the concentration at saturation, or as r
H
/r
Hsat
where r
H
is the hydrogen/metal (H/M)
atomic ratio and r
Hsat
is the ratio at saturation. In a particular lattice, only one kind of
interstitial sites is populated by H at moderate pressures. In the ideal case where there
were no H–H interactions, r
Hsat
would be equal to the number of those interstitial sites
per metal atom ( = 1 for octahedral sites in face-centered cubic (fcc) metals, 2 for
tetrahedral sites in hexagonal close-packed (hcp) metals, 6 for tetrahedral sites in
body-centered cubic (bcc) metals) [5].
Isotherm of H Surface-Bulk Transfer The equation of the equilibrium
isotherm for the surface-bulk transfer step [Eq. (2)] is [53,61,62]
Surface Effects on Hydrogen Entry into Metals 61
where E
dH2
is the dissociation energy of H
2
, equal to 436 kJ mol
–1
at 25°C. In
consequence, the values of the M–H
ads
bond energy at zero coverage fall in the
range 240–290 kJ mol
–1
, i.e., 2.5 to 3.0 eV [8c] (see Chapter 2).
Experimental heats of adsorption of H obtained on polycrystalline transition
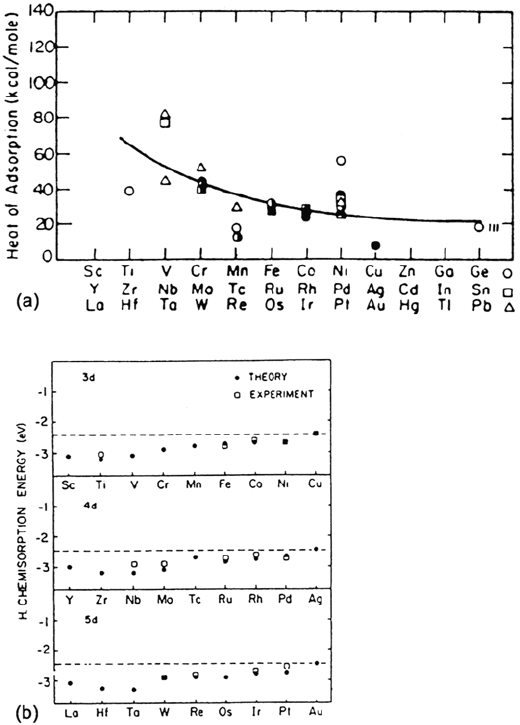
metal surfaces are shown in Figure 2a [63]. Calculated and experimental adsorption
bond energies for H on the most close-packed surfaces of the transition metals
are given in Figure 2b [64]. A diminution of the chemisorption bond strength is
observed from center left to the right in each metal series (3d, 4d, and 5d).
Experimental data obtained on single crystals for the three low-index faces of
Pd, Ni, and Fe are shown in Table 1 of Chapter 2. It is deduced that the initial heat
of adsorption and adsorption bond energy depend slightly on the nature of the metal
and the surface orientation.
H Absorption The solubility of H in metals varies over a great range, from
10
–10
to 10
–1
mol cm
–3
[28]. According to the sign of the enthalpy of dissolution from
H
2
(g), metals are exothermic H absorbers (ΔH
Hdiss
< 0; the H solubility decreases
with increasing temperature) or endothermic absorbers (ΔH
Hdiss
> 0; the H solubility
increases with temperature). Among the transition metals, the exothermic absorbers
most often undergo a transition to a hydride phase when the bulk H/M ratio
increases. These are metals of columns 3 to 5 [65]. Most other transition metals are
endothermic absorbers and have very low H solubility at 25°C and 1 atm, especially
Cu, Ag, and Au in column 11 [66]. The well-known exception is Pd, which is an
exothermic absorber. When the H
2
pressure (or cathodic overpotential) increases, Pd
forms with H an α solid solution up to the terminal solubility 0.03 H/Pd at 25°C,
above which a β hydride phase with 0.55 H/Pd precipitates; then the H-saturated
Thermochemical Data
H Adsorption All transition metals from column 3 to 10, plus Cu, are
exothermic H adsorbers (ΔH
Hads
< 0; θ
H
decreases when the temperature increases).
For these metals, the values of the initial H adsorption heat on single crystal faces
[–ΔH
Hads
(θ
H
= 0) = ½H°
H2
– H
Hads
(θH = θ)] fall in the range between 20 and
70 kJ mol
–1
[8]. The M–H
ads
bond energy at any coverage may be obtained from
the relation:
Copyright © 2002 Marcel Dekker, Inc.
metal and the H-deficient hydride coexist until the ratio H/Pd = 0.55 is passed;
for very high pressures or overpotentials, the hydride may be enriched in H up to
the stoichiometric compound PdH. Also a special case is Ni: (fcc), which exhibits
the highest H absorption capacity of any purely endothermic absorber (solubility
at 25°C and 1 atm: ~ 10
–6
mol cm
–3
[28], 1–5 × 10
–5
H/Ni [67]; it may form a
hydride out of equilibrium, i.e., under very high pressures or overpotentials, up
to NiH [68]; Cr (bcc) also forms hydrides in these conditions. Both Mn and Cr
exhibit a minimum in solubility as a function of temperature, which means that
they change from exothermic absorbers at low temperatures to endothermic at
high temperatures [68]. Fe (bcc) has low H solubility (about 3 × 10
–9
mol cm
–3
,
2–3 × 10
–8
H/Fe [67] at 25°C and 1 atm).
62 Protopopoff and Marcus
Figure 2 (a) Experimental heats of adsorption of hydrogen on polycnstalune transition metal
surfaces. (From Fig. 2a in Ref. 63.) (b) Calculated and experimental chemisorption bond
energies for H on the most close-packed surfaces of transitions metals (1 eV ≡ 96.5 kJ mol
–1
).
(From Fig. 2 in Ref. 64, with permission from Elsevier Science.)
Copyright © 2002 Marcel Dekker, Inc.
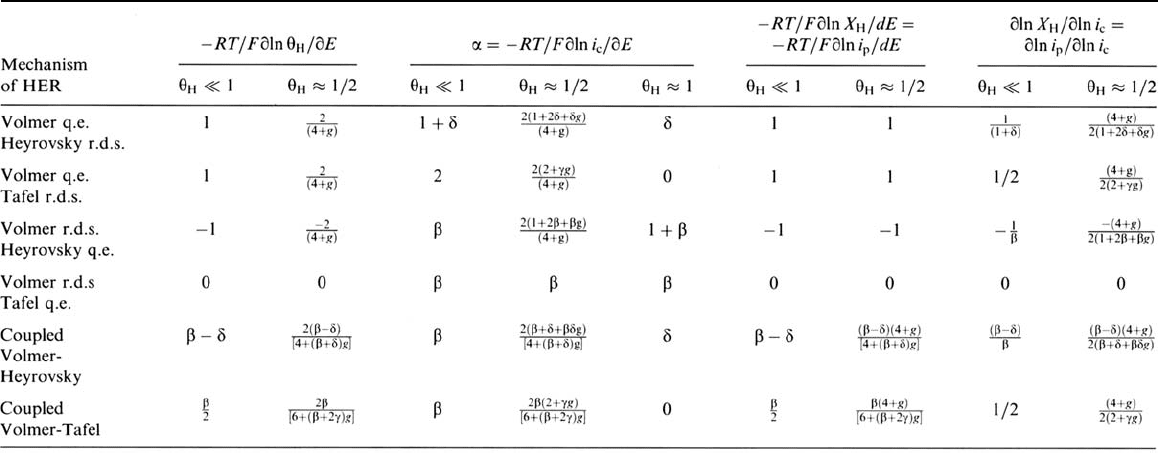
Table 1 Diagnostic Parameters for Various HER Mechanisms
Derivatives expressing the dependency of the H coverage, the HER current and the H bulk fractional concentration beneath the surface/steady permeation
current, on the potential and on the HER current, at given ΔG
ads
(given surface conditions), for the main HER mechanisms, and different values of the surface
coverage. The Tafel slope is related to the transfer coefficient α by the relation: ∂E/∂log i
c
= –RT ln 10/αF. Rigorously, the last two derivatives are to the
multiplied by the factor (1 – X
H
)/[1 + hX
H
(1 – X
H
)], which takes into account the limitation of bulk population due to possible interactions between dissolved
H atoms and saturation of the bulk sites. This factor drops to zero when θ
H
reaches unity. For endothermically absorbing metals, it can be considered as
practically equal to unity for moderate coverages (θ
H
≤ 1/2).
Copyright © 2002 Marcel Dekker, Inc.
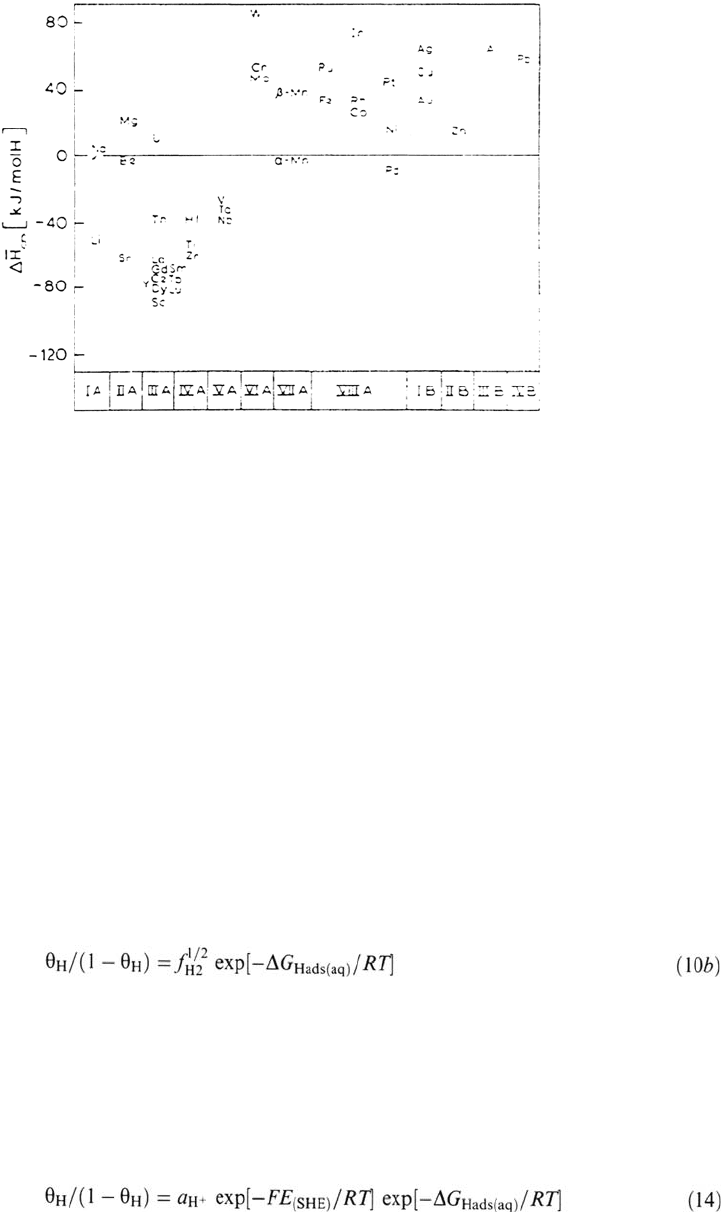
Figure 3 shows ΔH
Hdiss
at infinite dilution plotted for most metals [69].
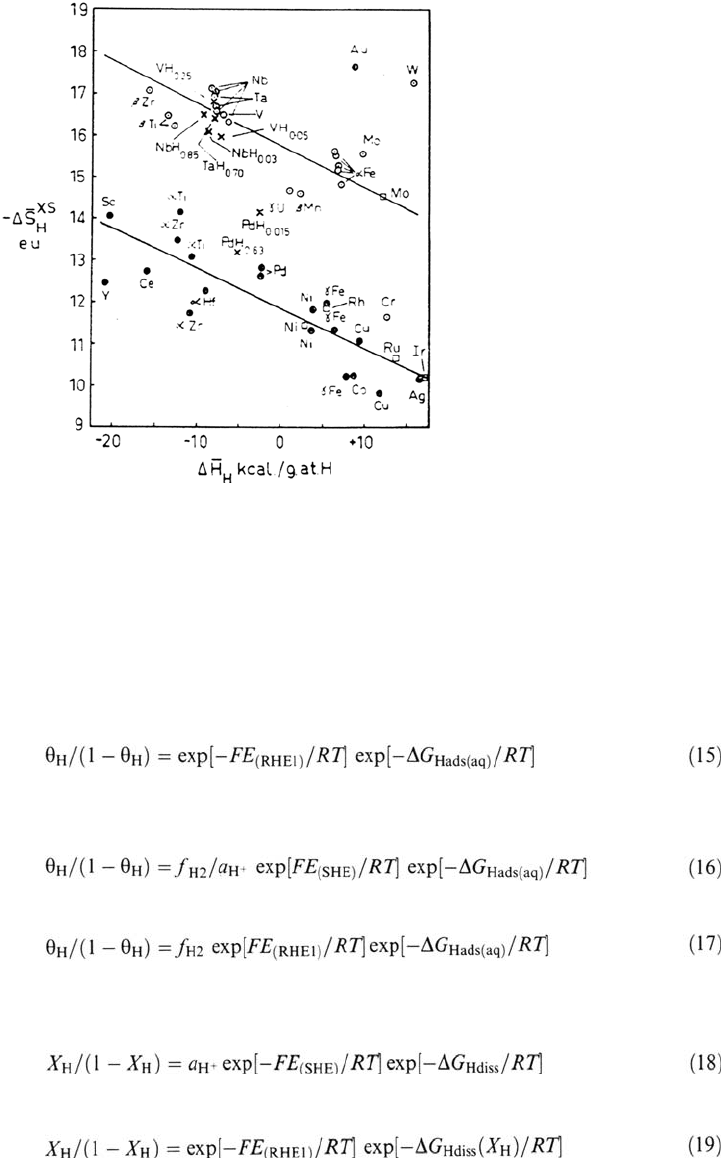
Figure 4 shows an approximately linear correlation between values of –ΔS
Hdiss
(cal mol
–1
K
–1
) and ΔH
Hdiss
for a given crystal structure [70], that is rather classic
(–ΔS
Hdiss
increases with –ΔH
Hdiss
): the larger the value of –ΔH
Hdiss
, the more tightly
bound the interstitial H and the lower its vibration frequency, hence the greater the
value of –ΔS
Hdiss,
the reduction in entropy from the gaseous state to the dissolved state.
The fcc and hcp metals give one correlation line and the bcc metals another one [70].
Thermodynamics of the M-H Equilibria in Aqueous Electrolyte
Isotherms of the Metal-Hydrogen Equilibria
Isotherm of H
2
Dissociative Adsorption/H-H Combination Under conditions
in which there is no competitive specific adsorption of ions or adsorption of oxygen
species in the H
ads
potential range, the equilibrium of the dissociative adsorption
from H
2(g)
in aqueous solution (or its reverse reaction, the chemical combination)
[see Eq. (3)] leads to the same adsorption isotherm as Eq. (10):
64 Protopopoff and Marcus
Figure 3 Enthalpies of solution of H at infinite dilution in metals; ΔH
∞
≡ΔH
Hdiss
in our
conventions. (From Fig. 6.3 in Ref. 69.)
where θ
H
, and f
H2
have already been defined and ΔG
Hads(aq)
is the half-standard
free energy of adsorption in aqueous solution from ½H
2(g)
[60] (which is a priori
different from ΔG
Hads
in the gas phase because adsorption in aqueous solution
implies displacement of asorbed water molecules and reorganization of the
double layer) [20,23].
Isotherm of H Electroadsorption/Electrodesorption The equation of the
equilibrium H electroadsorption isotherm [see Eqs. (4) and (5)] is [53,58,61,71]:
Copyright © 2002 Marcel Dekker, Inc.
where E
(SHE)
is the electrode potential referred to the reversible standard
hydrogen electrode; ΔG
Hads(aq)
has been defined. If the equation is rearranged to
involve the potential referred to the half-standard reversible hydrogen electrode in
the same electrolyte (a
H
+
, p
H2
= 1 atm)(RHE1), E
(RHE1)
= E
(SHE)
– RT/F ln a
H+
.
We obtain the simpler expression [41,53,58,71]:
Surface Effects on Hydrogen Entry into Metals 65
Figure 4 Correlation between excess entropy and enthalpy of solution of H in metals;
ΔS
XS
H
≡ΔS
Hdiss
= S
Hdiss
+ R ln X
H
/(1 – X
H
) – ½S°
H2
. (From Fig. 3 in Ref. 70, with
permission from Elsevier Science.)
Isotherm of Electrodissociation / Electrocombination Similarly, the isotherm of
equilibrium electrodissociation of H
2(g)
[see Eqs. (6) and (7)] can be expressed as [61]
or
Isotherm of H electroabsorption (HEAR) The equation of the equilibrium
electroabsorption isotherm [Eq. (9)] is [53,61,62]:
or
with ΔG
Hdiss
as defined before for the gas-phase reaction [Eq. (11)].
Copyright © 2002 Marcel Dekker, Inc.
