Martienssen W., Warlimont H. (Eds.). Handbook of Condensed Matter and Materials Data
Подождите немного. Документ загружается.

662 Part 4 Functional Materials
1.2
1.0
0.8
0.6
0.4
0.2
0
– 0.2
– 0.4
XL ΓΣΛ∆ KΓ
– 2.5
– 2.7
E (Ry)
– 0.9
– 1.1
– 1.3
– 1.5
Γ
15
X
5'
L
3'
Wave vector k
CaO
Λ
3
∆
1
Γ
15
Σ
1
Γ
1
XX
X
4'
L
2'
Λ
1
∆
5
Γ
1
K
4,1
Σ
4
X
1
Λ
1
∆
1
K
1
Σ
1
X
4'
L
2'
∆
1
K
3
Σ
3
L
1
Λ
1
∆
5
Σ
4
Λ
3
∆
1
K
1
Σ
1
X
5'
L
3
Λ
1
∆
2'
Σ
1
Γ
15
L
2'
Λ
1
∆
1
Γ
15
K
4
Σ
4
Γ
1
X
1
L
1
Λ
3
∆
5
Γ
1
K
1
Σ
3
Γ
25'
X
3
L
3'
Λ
1
∆
1
Γ
25'
K
4
Σ
1
Γ
2'
X
4'
L
2'
Λ
3
∆
2'
Γ
2'
K
1
Σ
1
Γ
12
X
5'
L
1
Λ
1
∆
5
Γ
12
K
3
Σ
4
Γ
15
X
1
L
3'
Λ
3
∆
1
Γ
15
K
1
Σ
3
Σ
1
Σ
3
K
3
K
3
K
3
K
1
∆
1
∆
5
Γ
15
Γ
1
Λ
3
Λ
1
Λ
1
X
2
X
5
Γ
1
Γ
15
L
3
L
3
∆
2
Σ
2
Σ
1
Σ
3
Σ
4
Σ
3
Σ
1
K
2
K
1
K
3
K
4
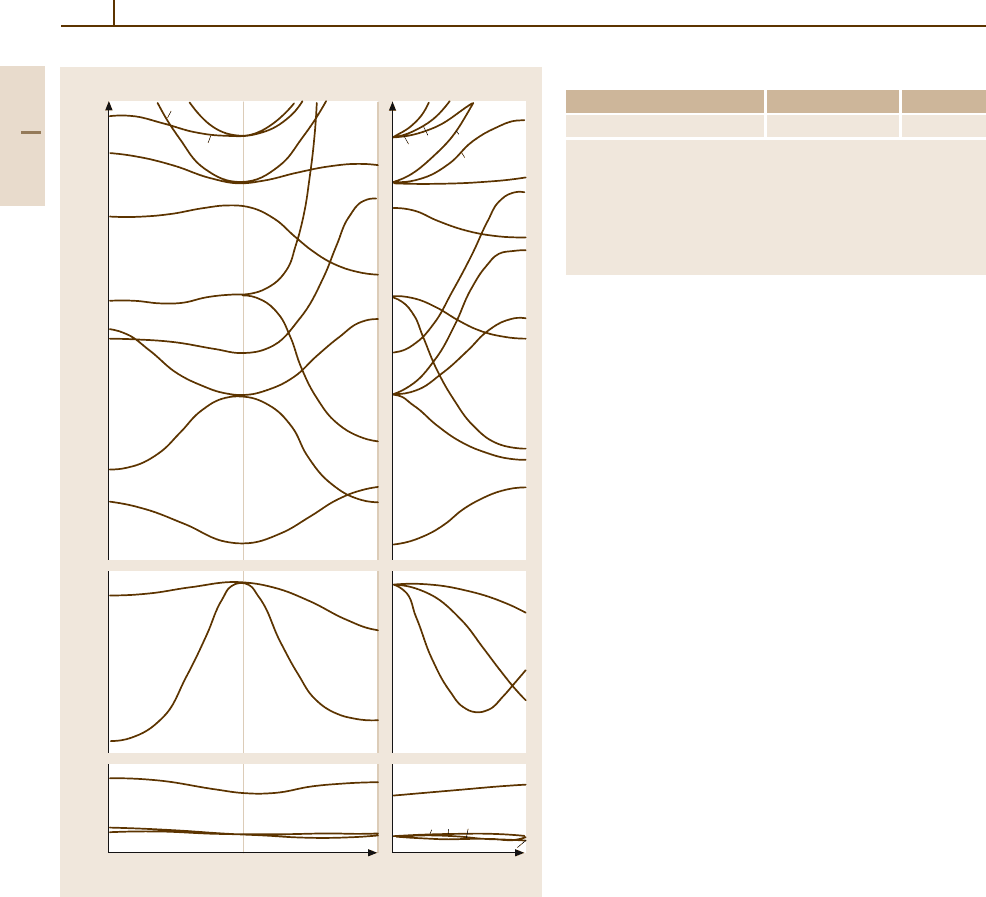
Fig. 4.1-146 Band structure of calcium oxide
Table 4.1-127 Band structure of calcium oxide
Crystal Figure Remarks
Calcium oxide CaO Fig. 4.1-146
a
a
Some controversy exists in the literature about the positions
of the lowest conduction band minima. One result shows the
lowest minimum at Γ
1
and the minimum of the d states at
X
3
, below some s states but above Γ
1
. Other results claim
the symmetry point of the conduction band bottom is at X
3
instead of Γ
1
, whereas according to other authors Γ
1
and X
3
are nearly degenerate.
Part 4 1.3

Semiconductors 1.3 II–VI Compounds 663
C. Transport Properties
Tables 4.1-128 – 4.1-130.
Table 4.1-128 Electronic transport in oxides of Ca, Sr, and Ba, general description
Crystal Transport properties
Calcium oxide CaO For general remarks, see the comments on the transport properties of MgO on p. 659. The transport of charge
carriers in CaO has been discussed in various models, e.g. polaron band conduction and hopping conduction
through compensated semiconducting samples, and also a considerable contribution from ionic conductivity
has been taken into account. From measurements of the oxygen pressure dependence of the conductivity of
undoped CaO, it is concluded that n-type conduction occurs at low pressures (< 10
−2
–10
−4
mbar) and that
p-type conduction occurs at higher pressures (> 10
−2
–1 mbar). In some cases this conclusion is confirmed
by Hall and thermoelectric measurements. For intermediate pressures, it is supposed that the lattice disorder
(presumably of Schottky type) gives rise to predominantly ionic conduction
Strontium oxide SrO The transport of charge carriers has been discussed in various models as in the case of CaO (see above). It is
supposed that the dominating scattering mechanism is optical-mode scattering
Barium oxide BaO The electrical transport has been discussed mainly on the assumption that it is due to electronic conduction.
The ionic contribution is small. Polaron conduction and predominant optical-mode scattering are assumed
Table 4.1-129 Electrical conductivity σ and electron mobility µ
n
of oxides of Ca, Sr, and Ba
Crystal Conductivity Electron mobility Remarks
µ
n
at temperature at O
2
pressure
(cm
2
/Vs) (K) (mbar)
Calcium oxide CaO 8 700 < 10
−6
Single crystals, Hall mobility
Strontium oxide SrO 5 700 < 10
−6
Single crystals, Hall mobility
Barium oxide BaO Fig. 4.1-148 5 600 < 10
−6
Polycrystals, Hall mobility
Table 4.1-130 Thermal conductivity κ of oxides of Ca, Sr, and Ba
Crystal Temperature (K) κ(W/cmK) Remarks
Calcium oxide CaO 300 0.3 See Fig. 4.1-147 for temperature dependence
Strontium oxide SrO 300 0.1 See Fig. 4.1-147 for temperature dependence
Barium oxide BaO 300 0.03 See Fig. 4.1-147 for temperature dependence
Part 4 1.3
664 Part 4 Functional Materials
80
4
6
82
10
2
10
3
10
–1
10
10
–2
1
T (K)
κ
(W/cm K)
MgO
CaO
SrO
BaO
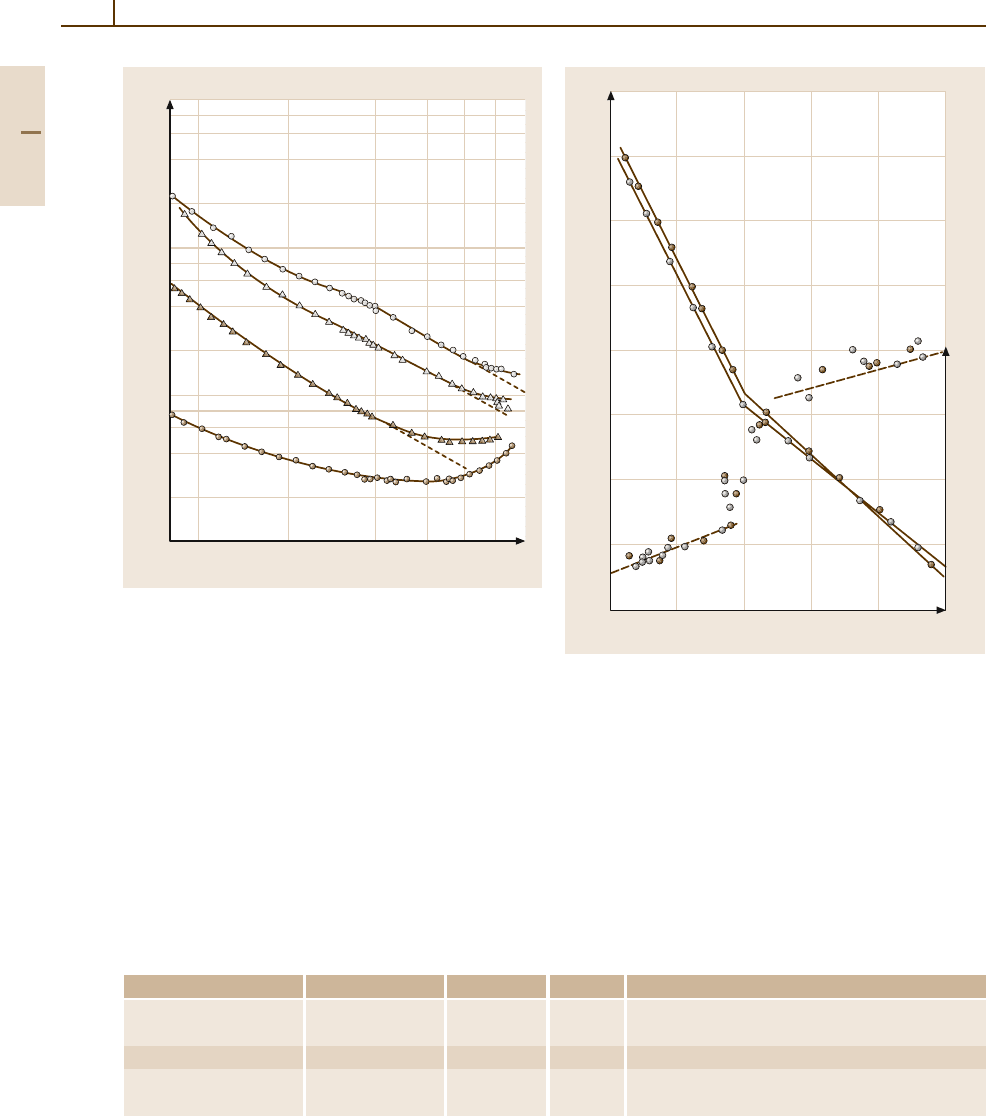
Fig. 4.1-147 MgO, CaO, SrO, BaO. Temperature de-
pendence of the thermal conductivity κ of single
crystals [1.124]. Dashed lines, κ ∝ T
−1
D. Electromagnetic and Optical Properties
Table 4.1-131.
Table 4.1-131 Dielectric constant ε of oxides of Ca, Sr, and Ba
Crystal Temperature (K) ε(0) ε(∞) Remarks
Calcium oxide CaO 273 12.01 (10) Capacitance measurement at 1 kHz and 10 kHz
3.27 Compilation
Strontium oxide SrO
Barium oxide BaO 296 3.903–4.197 Calculated from refractive-index values in the spectral
range 624–436 nm
σ (Ω
–1
cm
–1
)
1
10
–1
10
–2
10
–3
10
–4
10
–5
10
–6
10
–7
10
–8
0.5 0.7 0.9 1.1 1.3 1.5
2.0
1.5
1.0
0.5
0
T
–1
(10
–3
K
–1
)
S (mV K
–1
)
BaO
S
S
σ
σ
Fig. 4.1-148 BaO. Electrical conductivity σ (solid lines)
and thermoelectric power S (dashed lines) of two crystals
vs. 1/T [1.125]. p
O
2
= 3×10
−5
mbar. n-type conduction
at temperatures below the discontinuity
Part 4 1.3
Semiconductors 1.3 II–VI Compounds 665
4.1.3.4 Zinc Compounds
A. Crystal Structure, Mechanical and Thermal Properties
Tables 4.1-132 – 4.1-139.
Table 4.1-132 Crystal structures of zinc compounds
Crystal Space group Crystal system Structure type Figure
Zinc oxide ZnO C
4
6v
P6
3
mc hex Wurtzite Fig. 4.1-130
Zinc sulfide ZnS T
2
d
F
4
3m
fcc Zinc blende Fig. 4.1-131
a
Zinc selenide ZnSe T
2
d
F43m fcc Zinc blende Fig.4.1-131
Zinc telluride ZnTe T
2
d
F
4
3m
fcc Zinc blende Fig. 4.1-131
a
At room temperature, ZnS bulk material consists predominantly of the cubic phase, often with hexagonal inclusions, leading to
polytypic material. Epitaxial ZnS has mostly been grown on GaAs and thus has pseudomorphically assumed the cubic structure of
the substrate material. In this section, all data refer to the cubic modification unless explicitly stated otherwise.
Table 4.1-133 Lattice parameters of zinc compounds
Crystal Lattice parameters (nm) Temperature Remarks
Zinc oxide ZnO a =0.3249 (6) X-ray diffraction
c =0.52042 (20)
Zinc sulfide ZnS a = 0.54053 RT X-ray diffraction
Zinc selenide ZnSe a = 0.5667 (4) 300 K X-ray diffraction
Zinc telluride ZnTe a =0.60882 RT X-ray diffraction
Table 4.1-134 Densities of zinc compounds
Crystal Density (g/cm
3
) Temperature Remarks
Zinc oxide ZnO 5.67526 (19) 293 K Hydrostatic weighing
Zinc sulfide ZnS Cubic: 4.088 RT
Hexagonal: 4.087 RT
Zinc selenide ZnSe 5.266 Shock wave experiment
Zinc telluride ZnTe 5.636 298 K
Table 4.1-135 Elastic constants c
ik
(in GPa) of zinc compounds
Crystal c
11
(GPa) c
13
(GPa) c
33
(GPa) c
44
(GPa) c
66
(GPa) Remarks
Zinc oxide ZnO 206 (4) 118 (10) 211 (4) 44.3 (10) 44.0 (10) ZnO film on Si substrate, Brillouin
scattering
Table 4.1-135 Elastic constants c
ik
(in GPa) of zinc compounds, cont.
Crystal c
11
(GPa) c
12
(GPa) c
44
(GPa) Temperature Remarks
Zinc sulfide ZnS 103.2 (5) 64.6 (5) 46.2 (4) 293 K Resonance method
Zinc selenide ZnSe 90.3 (19) 53.6 (23) 39.4 (12) Brillouin scattering
Zinc telluride ZnTe 72.2 (2) 40.9 (6) 30.8 (3) RT Brillouin scattering
Part 4 1.3
666 Part 4 Functional Materials
ZnS (hex)
3.823
3.821
3.819
3.817
T (K)
V
m
(cm
3
)
23.84
23.80
23.76
23.72
6.264
6.256
6.248
1.640
1.638
1.636
0 70 140 210 280 350
c (Å)a (Å)
c/a
3.825
a)
b)
Two points
With silicone grease
c
a
c/a
V
m
Table 4.1-136 Melting point T
m
of zinc compounds
Crystal Melting point T
m
(K) Remarks
Zinc oxide ZnO 2242 (5)
Zinc sulfide ZnS 1991 Under normal pressure, ZnS sublimes before melting
Zinc selenide ZnSe 1799
Zinc telluride ZnTe 1563 (8)
Table 4.1-137 Linear thermal expansion coefficient α of zinc compounds
Crystal Expansion coefficient α(K
−1
) Temperature (K) Remarks
Zinc oxide ZnO α
parall c
:2.92× 10
−6
399 Interferometric and capacitance methods
α
perpend c
:4.75× 10
−6
300
Zinc sulfide ZnS See Fig. 4.1-149
Zinc selenide ZnSe 7.4×10
−6
300 Polycrystal, capacitance dilatometer
Zinc telluride ZnTe 0.083×10
−6
300
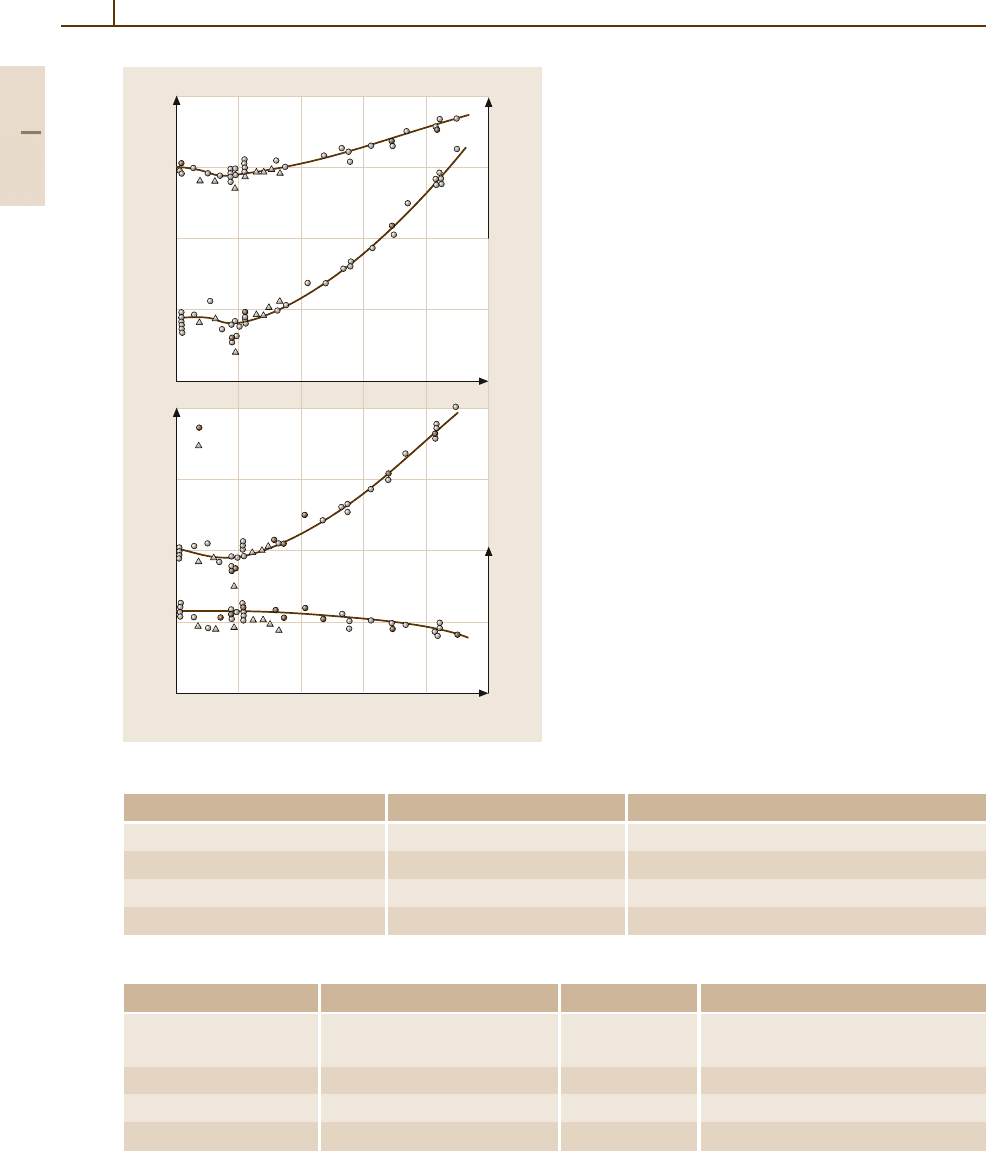
Fig. 4.1-149a,b ZnS, hexagonal. (a) Lattice parameters a
and c vs. temperature;
(b) molar volume and c/a ratio vs.
temperature [1.126]
Part 4 1.3
Semiconductors 1.3 II–VI Compounds 667
Table 4.1-138 Heat capacities and Debye temperatures of zinc compounds
Crystal Heat capacity Debye temperature Θ
D
Temperature Remarks
c
p
(J/mol K) (K) (K)
Zinc oxide ZnO 440 (25) 300 Calorimetric data
Zinc sulfide ZnS 45.358 352 77 Zinc blende lattice, Θ
D
calculated from c
p
45.882 351 298 Wurtzite lattice; Θ
D
calculated from c
p
Zinc selenide ZnSe 51.88 339 (2) 298
Zinc telluride ZnTe 180 (6) 93–298 Θ
D
from X-ray intensities from powders
Table 4.1-139 Phonon frequencies/wavenumbers at symmetry points for zinc compounds. Zinc oxide (ZnO), fundamental
optical modes, T = 300 K, from Raman spectroscopy; zinc sulfide (ZnS), T = 300 K; zinc selenide (ZnSe), from Raman
spectroscopy and luminescence; zinc telluride (ZnTe), RT, from neutron scattering
ZnO ZnS
Mode
Symmetry Wavenumber Mode Symmetry Wavenumber
point (cm
−1
) point (cm
−1
)
˜
ν
E
2
101
˜
ν
LO
Γ 350
˜
ν
E
2
437
˜
ν
TO
Γ 274
˜
ν
TO
E
1, perp c
407
˜
ν
LO
X 332 (1)
˜
ν
TO
A
1, parall c
380
˜
ν
TO
X 318 (1)
˜
ν
LO
E
1, perp c
583
˜
ν
LA
X 212 (1)
˜
ν
LO
A
1, parall c
574
˜
ν
TA
X 88 (1)
˜
ν
LO
L 334 (1)
˜
ν
TO
L 298 (1)
˜
ν
LA
L 192 (1)
˜
ν
TA
L 72 (1)
ZnSe ZnTe
Mode
Symmetry Phonon energy Mode Symmetry Frequency
point (meV) point (THz)
hν
LO
Γ
1
30.99 ν
LO
Γ 6.20 (5)
hν
TO
Γ
15
25.17 ν
TO
Γ 5.30 (7)
hν
LA
Γ 19.8 ν
LO
X 5.51 (10)
hν
TA
Γ 8.0 ν
TO
X 5.21 (10)
hν
LO
X 27.64 ν
LA
X 4.29 (5)
hν
TO
25.54 ν
TA
X 1.62 (2)
hν
LA
23.55 ν
LO
L 5.39 (5)
hν
LO
L 27.77 ν
TO
L 5.20 (9)
hν
TO
25.54 ν
LA
L 4.06 (5)
hν W
3
24.9 ν
TA
L 1.25 (2)
hν W
1
18.59
hν W
2
11.53
hν W
2
26.53
hν W
4
14.26
hν W
4
26.41
Part 4 1.3
668 Part 4 Functional Materials
B. Electronic Properties
Tables 4.1-140 – 4.1-145.
Band Structures of Zinc Compounds.
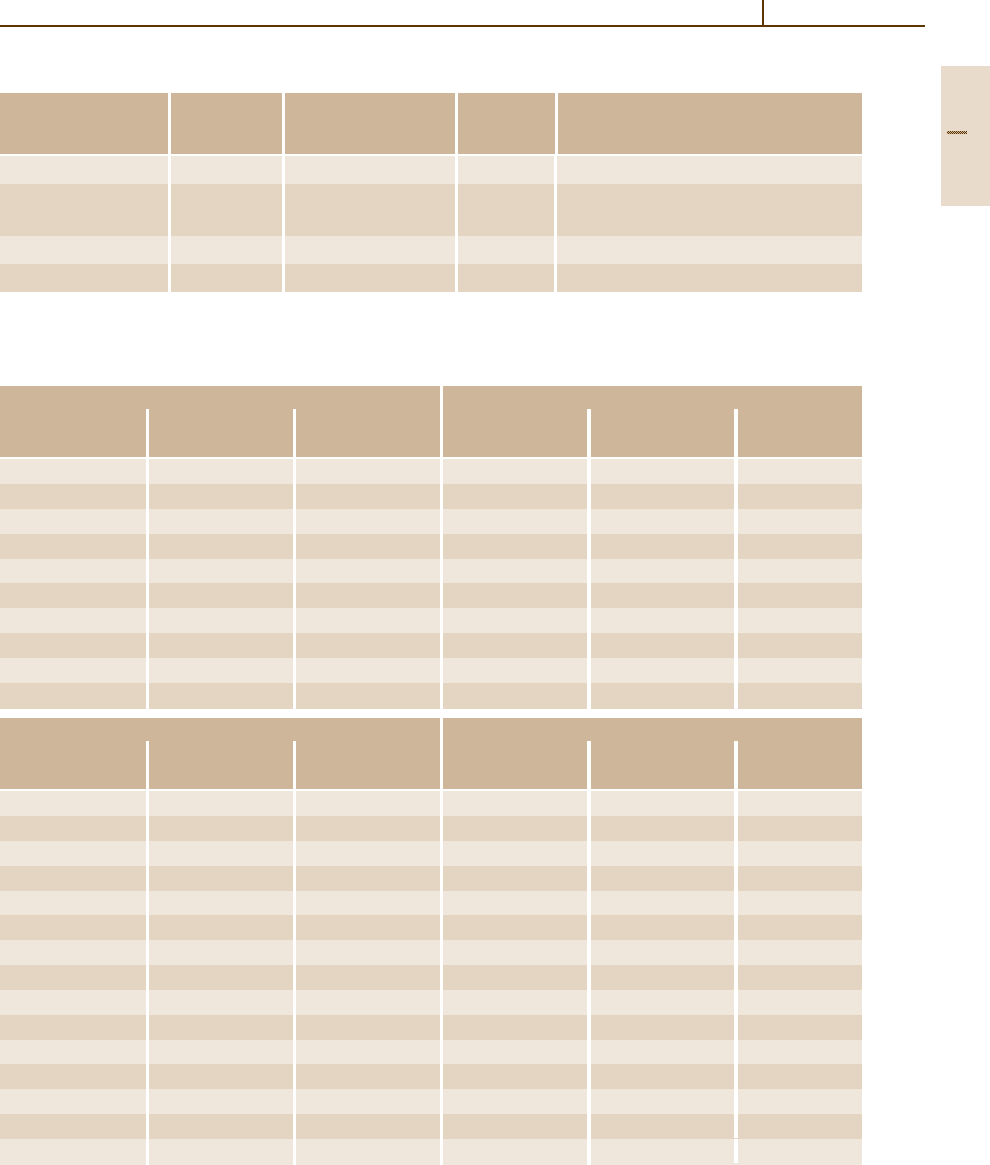
Zinc oxide (ZnO) (Fig. 4.1-150)
The topmost valence band (Γ
5
+Γ
1
) is split owing to
crystal-field splitting and spin–orbit coupling into three
spin-degenerate states (Γ
7
, Γ
9
, Γ
7
). The exciton states
formed with holes in these valence band states are re-
ferred to as the A, B, and C excitons, respectively. Owing
to the negative spin–orbit coupling (there is a contribu-
tion from Zn 3d states), the positions of the edges of the
two highest valence band states are reversed ascompared
with other II–VI compounds with the wurtzite structure.
The conduction band edge, originating from the 4s
states of Zn, possesses Γ
7
symmetry.
Zinc sulfide ZnS (Fig. 4.1-150)
Cubic ZnS is a direct-gap semiconductor with the small-
est energy gap at the center of the Brillouin zone (Γ).
When spin–orbit splitting is taken into account, the top-
most valence band state Γ
15v
splits into Γ
8v
and Γ
7v
;
further splitting into A, B, and C levels is caused by the
crystal field.
E (eV)
Γ
12.5
10.0
7.5
5.0
2.5
0
–2.5
–5.0
–7.5
–10.0
–12.5
E (eV)
Γ
10.0
7.5
5.0
2.5
0
–2.5
–5.0
–7.5
–10.0
–12.5
–15.0
Wave vector k
ALMΓ ASH KRU Σ∆ PT
ΓLXWK
M
6
ZnO
M
4
M
3
M
3
M
1
A
1,3
A
5,6
Γ
1
Γ
6
Γ
5
Γ
3
Γ
1
A
1,3
Γ
5
Γ
1
Γ
6
Γ
3
L
1,3
L
1,3
H
1,2
H
3
K
3
K
1
K
2
L
1,3
M
1
M
3
M
4
M
2
M
3
M
1
L
2,4
L
1,3
A
5,6
A
1,3
H
3
K
3
K
2
K
1
H
1,2
K
3
H
3
ZnS (cubic)
E
g
S
H
K
P
M
Γ
∆
Σ
A
Fig. 4.1-150 Band structure of zinc oxide and cubic zinc sulfide
Table 4.1-140 First Brillouin zones of zinc compounds
Crystal Figure
Zinc oxide ZnO Fig. 4.1-132
Zinc sulfide ZnS Fig. 4.1-133
Zinc selenide ZnSe Fig. 4.1-133
Zinc telluride ZnTe Fig. 4.1-133
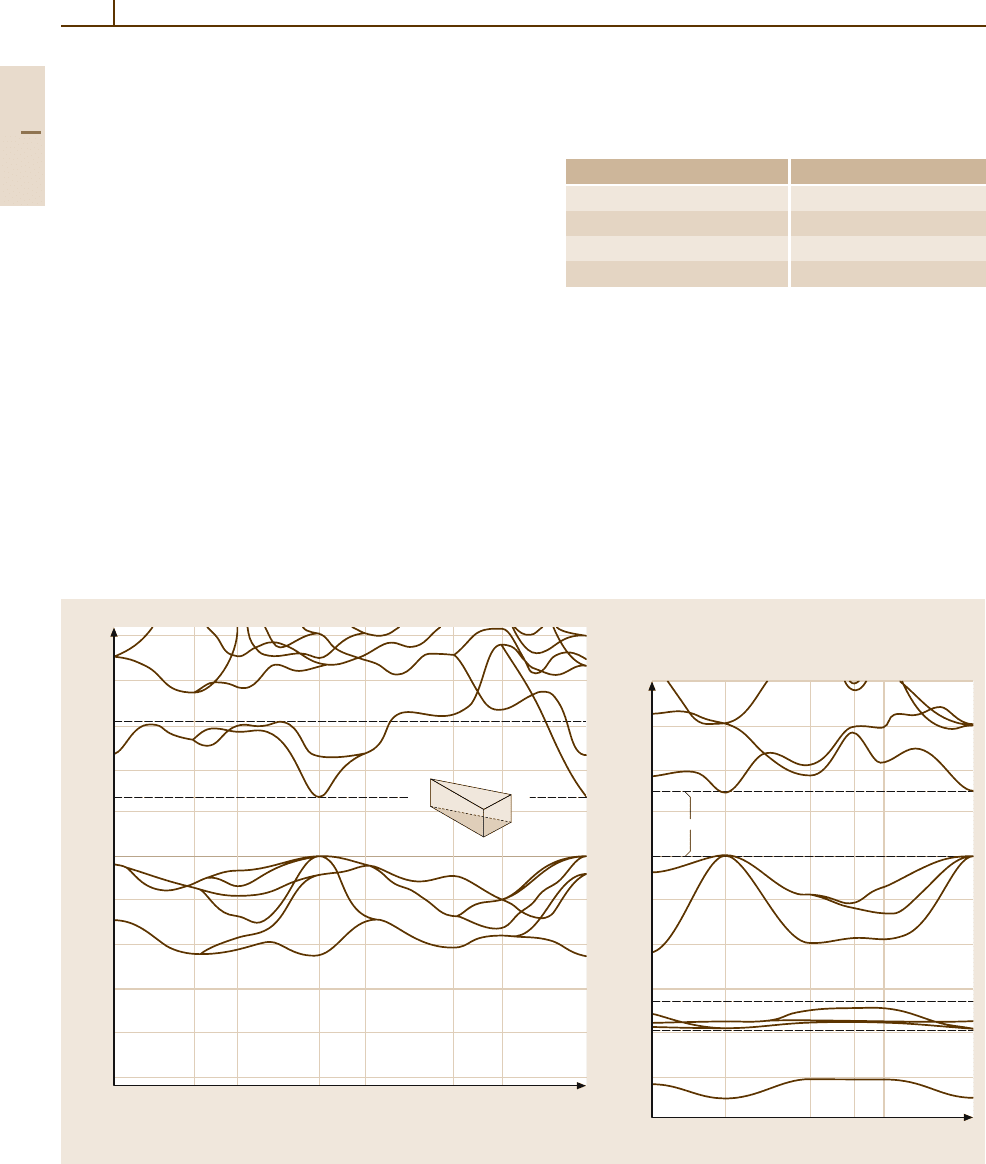
Zinc selenide ZnSe (Fig. 4.1-151)
Zinc selenide is a direct-gap semiconductor with the
smallest energy gap at the center of the Brillouin zone
(Γ). The topmost valence band (Γ
15
) is split owing to
spin–orbit coupling into a fourfold (Γ
8
)andatwofold
(Γ
7
) state.
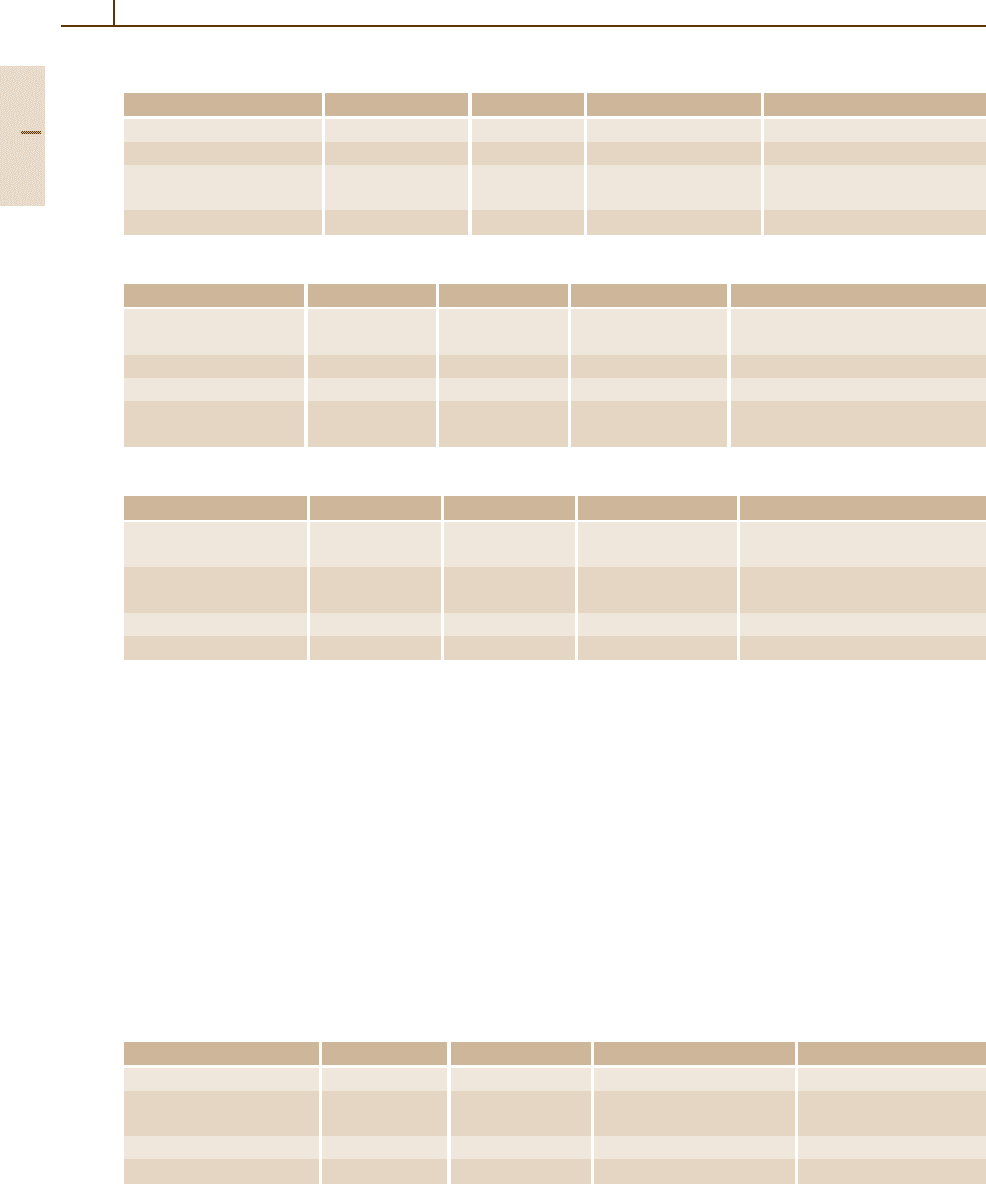
Zinc telluride ZnTe (Fig. 4.1-152)
Zinc telluride is a direct-gap semiconductor with the
smallest energy gap at the center of the Brillouin zone
(Γ). The topmost valence band (Γ
15
) is split owing to
spin–orbit coupling into a fourfold (Γ
8
)andatwofold
(Γ
7
) state.
Part 4 1.3

Semiconductors 1.3 II–VI Compounds 669
9
6
3
0
–3
–6
–9
–12
XL ΓΣΛ∆ Γ
E (eV)
U,K
Wave vector k
ZnSe
X
6
L
6
Γ
6
L
6
L
6
Γ
7
Γ
8
Γ
6
L
4,5
L
6
L
6
L
4,5
Γ
7
Γ
8
X
7
X
7
X
6
X
6
X
6
Γ
6
Γ
7
Γ
8
Γ
6
Γ
7
Γ
8
Fig. 4.1-151 Band structure of zinc selenide
Table 4.1-141 Energy gaps of zinc compounds
Crystal Band gap (eV) Between bands Temperature (K) Remarks
Zinc oxide ZnO E
A
g
3.4410 (1) 6 Two-photon absorption,
E
B
g
3.4434 (1) distance from A, B, and C valence bands
E
C
g
3.4817 (2) to conduction band
Zinc sulfide ZnS E
g, dir
3.723 (1) Γ
15v
and Γ
1c
300 Luminescence;
E
g, dir
3.78 Γ
8v
and Γ
6c
295 For temperature dependence
E
g, dir
3.76 Γ
7v
and Γ
6c
298 see Fig. 4.1-162
Zinc selenide ZnSe E
g, dir
2.8222 (1) Γ
8v
and Γ
6c
6 Two-photon spectroscopy
Zinc telluride ZnTe E
g, dir
2.3945 Γ
8v
and Γ
6c
< 2 Transmission spectroscopy
2.35 Γ
8v
and Γ
6c
300 Reflectivity
Table 4.1-142 Exciton binding energies of zinc compounds
Crystal Binding energy (meV) Temperature (K) Remarks
Zinc oxide ZnO E
b
(A) 63.1 6 Two-photon absorption,
E
b
(B) 50.4
E
b
(C) 48.9
Zinc sulfide ZnS E
b
(1S) 38 (1) 10 Absorption,
E
b
(2S) 10 (2) Heavy-hole exciton
Zinc selenide ZnSe E
b
(1S) 20.8 1.8 Absorption, strained layers
E
b
(2P
1/2
)4.19 1.6 Two-photon absorption
E
b
(2P
5/2
,Γ
7
)4.80
E
b
(2P
5/2
,Γ
8
)5.15
a = 6.089 Å
10
8
6
4
2
0
–2
–4
–6
–8
–10
–12
Γ KΓ XW L
E (eV)
Wave vector k
ZnTe
Γ
12
X
5
K
2
L
1
∆
2
Λ
3
Σ
2
V
1
Γ
12
Γ
1
K
1
∆
1
Λ
1
V
2
Γ
15
K
2
L
1
∆
1
Λ
1
Σ
1
Γ
1
K
1
∆
1
Λ
3
Σ
1
K
1
L
3
∆
1
Σ
1
Γ
15
Γ
1
X
1
K
1
Λ
1
V
1
X
3
K
2
L
1
Σ
2
V
1
Γ
1
X
5
K
1
L
3
∆
3
+∆
4
Λ
3
Σ
1
V
2
Γ
15
X
3
K
1
L
1
∆
1
Λ
1
Σ
1
Γ
15
Γ
1
X
1
K
1
L
1
∆
1
Λ
1
Σ
1
V
1
Γ
1
∆
3
+∆
4
Σ
1
Zn 3d
Fig. 4.1-152 Band structure of zinc telluride
Part 4 1.3
670 Part 4 Functional Materials
Table 4.1-143 Spin–orbit splitting energy ∆
so
of zinc compounds
Crystal Splitting energy Bands Temperature (K) Remarks
Zinc oxide ZnO −3.5 (2) meV 6 Two-photon absorption
Zinc sulfide ZnS 64 meV Γ
8v
to Γ
7v
293 Reflectivity
Zinc selenide ZnSe 0.42 eV Γ
8v
to Γ
7v
295 Reflectivity
0.20 eV Γ
4−5v
to Γ
6v
300
Zinc telluride ZnTe 0.97 eV Γ
8v
to Γ
7v
80 Reflectivity
Table 4.1-144 Effective masses of electrons (in units of the electron mass m
0
) for zinc compounds
Crystal Quantity Mass Temperature (K) Remarks
Zinc oxide ZnO m
n
0.275 6 Cyclotron resonance
m
n, polaron
0.3 80 Polaron mass
Zinc sulfide ZnS m
n, polaron
0.22 Polaron mass, cyclotron resonance
Zinc selenide ZnSe m
n
0.160 (2) 4.2 Photoluminescence
Zinc telluride ZnTe m
n
0.122 (2) 3.5 Cyclotron resonance
m
n, polaron
0.124 (2) 1.5 Polaron mass
Table 4.1-145 Effective masses of holes (in units of the electron mass m
0
) for zinc compounds
Crystal Quantity Mass Temperature (K) Remarks
Zinc oxide ZnO m
p, parall
0.59 1.6 Magnetoreflection
m
p, perpend
0.59 Polaron mass
Zinc sulfide ZnS m
p, light
0.23 Calculated
m
p, heavy
1.76
Zinc selenide ZnSe m
p
0.75 2.1–200 Phonon-assisted exciton absorption
Zinc telluride ZnTe m
p
0.6 Estimated from hole mobility
C. Transport Properties
Table 4.1-146.
Electronic Transport, General Description.
Zinc oxide (ZnO)
The electronic conductivity of pure, stoichiometric
ZnO is still unknown. The concentration of foreign
admixtures in undoped crystals is of the order of
10
15
–10
16
cm
−3
. Since E
g, opt
= 3.2 eV and impurity
ionization energies are about 0.01–0.1 eV at tempera-
tures below 900 K, impurity conduction is always
observed. At temperatures above 900 K, dissociation of
the intrinsic material occurs.
Table 4.1-146 Thermal conductivity κ of zinc compounds
Crystal κ(W/cm K) Temperature (K) Temperature dependence Remarks
Zinc oxide ZnO 0.54 300 Fig. 4.1-154 Steady-state heat flow
Zinc sulfide ZnS 0.27 300 Pulse method
3.6 30
Zinc selenide ZnSe 0.19 300 Fig. 4.1-155
Zinc telluride ZnTe 0.18 300
The conductivity depends on the surrounding atmo-
sphere (which my be O
2
, Zn, or Ar). Nevertheless, it
is possible to investigate the influence of intentional
admixtures on the conductivity, the charge-carrier con-
centration, and the mobility as a function of temperature
and current direction.
Data for the electrical resistivityvary between values
of the order of 10
8
–10
9
Ω cm for ultrapure bulk single
crystals to values of the order of 10
−2
–10
−4
Ω cm for
doping concentrations of up to 10
20
cm
−3
. Data for the
Part 4 1.3
Semiconductors 1.3 II–VI Compounds 671
electron mobility µ
n
at room temperature range between
0.5 and 200 cm
2
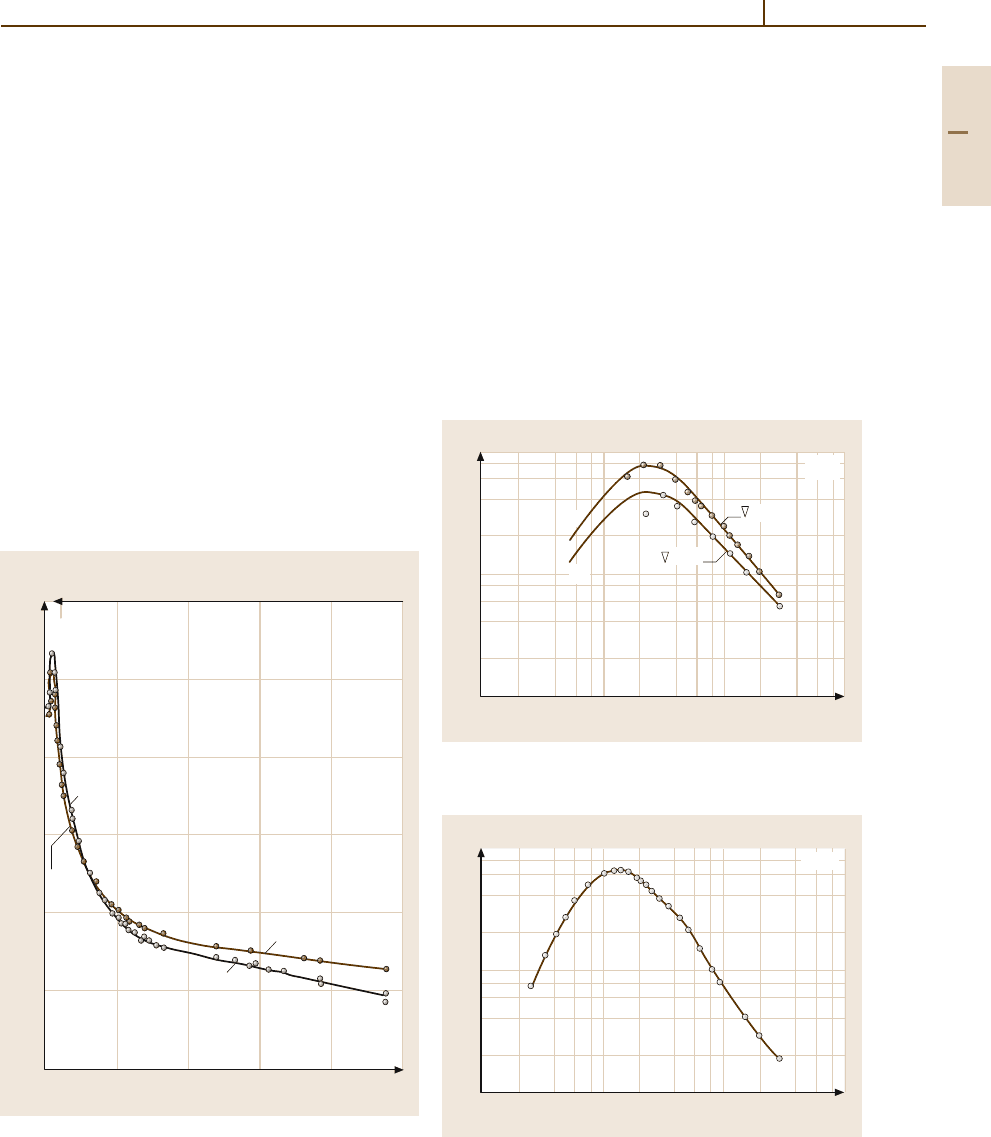
/V s. Figure 4.1-153shows theelectrical
conductivity of a crystal without intentional admixtures.
Zinc sulfide (ZnS)
The (photo)conductivity of ZnS depends strongly on
the growth conditions, dopants, doping characteristics,
etc. Data for the electrical resistivity vary between 10
−3
and 10
2
Ω cm depending on sample preparation. Val-
ues of the electron mobility around 100 cm
2
/V s and
600 cm
2
/V s have been found at room temperature, and
values around 3000 cm
2
/V s have been found at 77 K.
A value of 40 cm
2
/V s has been given for the hole mo-
bility.
Zinc selenide (ZnSe)
The (photo)conductivity of ZnSe depends strongly on
the growth conditions, dopants, doping characteristics,
etc. In undoped material, values of the electrical resistiv-
ity between 10
5
andafewΩ cm have been found. Values
of the electron mobility at room temperature reach up to
400 cm
2
/V s; for the hole mobility, µ
p
= 110 cm
2
/Vs
has been calculated as an upper limit.
10
1
10
–1
10
–2
10
–3
10
–4
10
–5
T
–1
(10
–3
K
–1
)
σ
(Ω
–1
cm
–1
)
T (K)
50 10 3.35 2.5 2
0 100 200 300 400 500
I ⊥ c
ZnO
I ⊥ c
I⏐⏐ c
I⏐⏐ c
Fig. 4.1-153 ZnO. Electronic conductivity parallel and per-
pendicular to the c axis vs. temperature. Crystal grown in
Al
2
O
3
ceramic without intentional admixtures [1.127]
Zinc telluride (ZnTe)
ZnTe is a p-type conductor owing to deviations from
stoichiometry when it is not intentionally doped; n-type
doping is extremely difficult. The transport properties
of ZnTe depend strongly on the growth conditions,
dopants, doping characteristics, etc.
Typical data for the electrical resistivity at room
temperature are of the order of a few times 10
4
Ω cm;
at temperatures around 77 K, values of a few times
10
6
Ω cm have been found. The experimental values of
the electron and hole mobilities at room temperature
range from 300 to 350 cm
2
/Vs.
10
3
10
2
10
110
10
2
10
3
T (K)
κ
33
κ
(W/m K)
ZnO
κ
11
T⊥c
T ⎢⎢ c
ii
Fig. 4.1-154 ZnO. Thermal conductivity (lattice contribu-
tion) vs. temperature. Solid lines, calculated values [1.128]
T (K)
κ
(W/cm K)
10
1
10
–1
11010
2
10
3
ZnSe
Fig. 4.1-155 ZnSe. Thermal conductivity vs. tempera-
ture [1.129]
Part 4 1.3
