Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

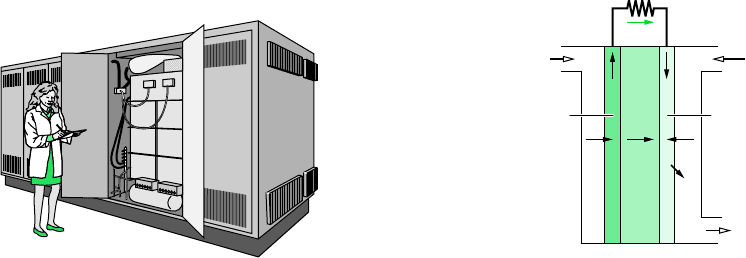
646 Chapter 13 Reacting Mixtures and Combustion
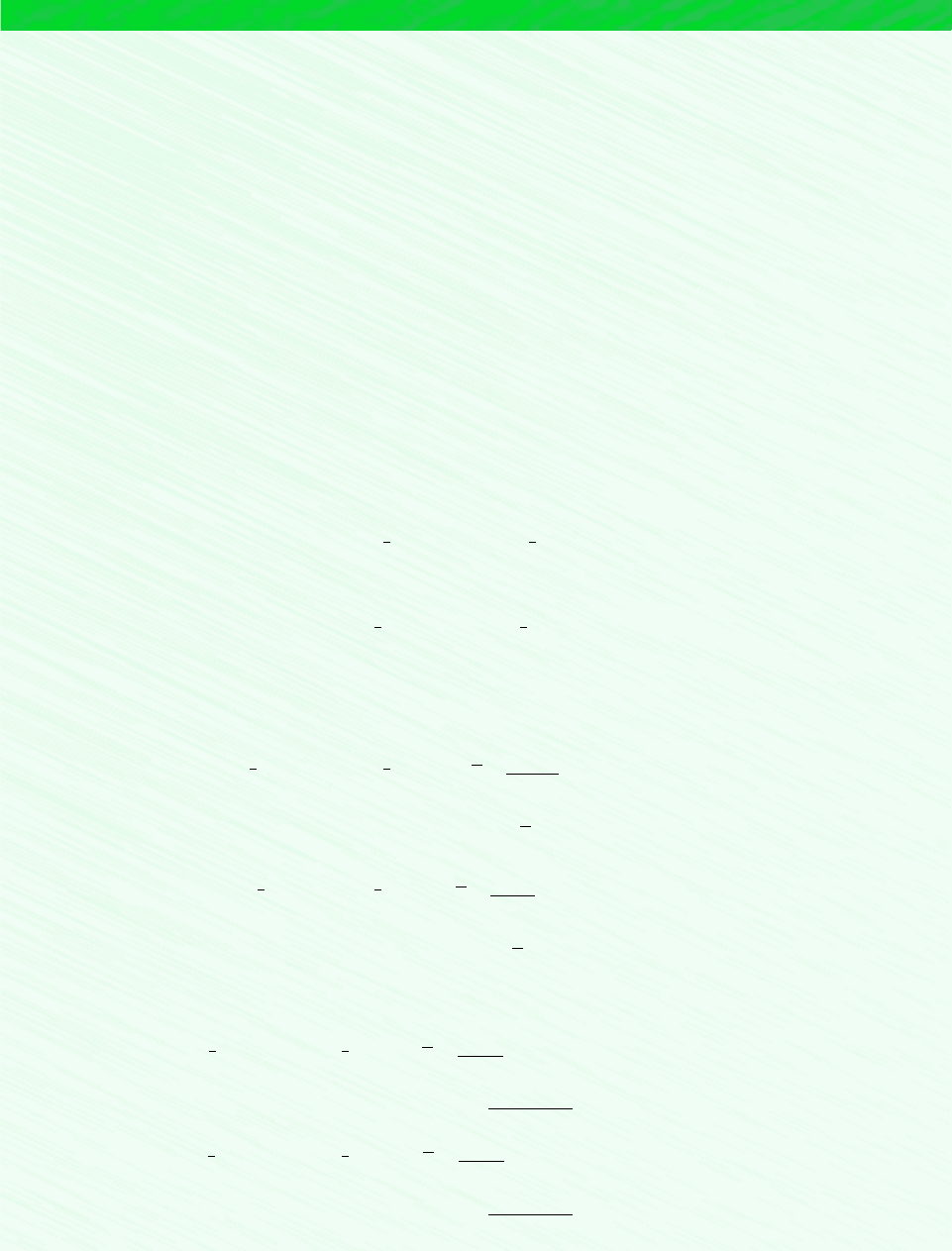
PROTON EXCHANGE MEMBRANE FUEL CELL.
The fuel cell shown in Fig. 13.3b operates
with hydrogen (H
2
) as the fuel and oxygen (O
2
) as the oxidizer. This type of cell is known
as a proton exchange membrane cell (PEMFC). At the anode, hydrogen ions (H
) and elec-
trons are produced. At the cathode, ions and electrons are consumed. The reactions at these
electrodes and the overall cell reaction are labeled on the figure. The only products of this
fuel cell are water and the power generated.
Charge-carrying hydrogen ions are conducted through the electrolytic membrane. For ac-
ceptable ion conductivity, a high membrane water content is required. This requirement restricts
the fuel cell to operating in the range 60–100C, which is below the boiling point of water.
Cooling is generally needed to maintain the fuel cell at the operating temperature. Due to such
relatively low temperature operation, costly platinum catalysts are required at both the anode
and cathode to increase ionization reaction rates. Catalytic activity is more important in lower
temperature fuel cells because reaction rates tend to decrease with decreasing temperature.
Major automakers are beginning to introduce vehicles powered by proton exchange mem-
brane fuel cells. Fuel cell stacks formed from several hundred individual fuel cells are required
to meet automotive needs. These fuel cell stacks are integrated with several components
necessary to support fuel cell operation, including components that provide fuel, oxidizer,
and coolant. Power requirements, irreversibilities, and losses associated with the support
components conspire to give a much lower net power output for a given fuel input than
obtainable from the stand-alone fuel cell.
Proton exchange membrane fuel cells also have potential to replace batteries in portable
devices such as cellular phones, laptop computers, and video players.
FUELS. With today’s technology, hydrogen is preferred for fuel cell applications because of its
exceptional ability to produce electrons when suitable catalysts are used, while in principle pro-
ducing no harmful emissions from the fuel cell itself. Methanol (CH
3
OH) and carbon monoxide
can be used directly as fuels in some applications, but often with performance penalties.
Since hydrogen is not naturally occurring, it must be produced chemically from hydro-
carbons, including natural gas, by electrolysis of water, or by other means. Hydrogen pro-
duction technologies involving chemical processes can generate undesirable emissions.
Irreversibilities and losses inherent in hydrogen production also result in a lower overall con-
version of primary input to fuel cell-generated power than reported for fuel cells directly
fueled with hydrogen.
In some applications, hydrogen is provided directly to the fuel cell from storage as a com-
pressed gas, a cryogenic liquid, or atoms absorbed within metallic structures. Hydrogen also
Fuel
H
2
Product
H
2
O
Anode
Electrolyte
Cathode
Oxidizer
O
2
1
–
2
H
2
O
H
2
2H
+
1
–
2
O
2
2e
–
2e
–
External electric circuit
Overall cell reaction: H
2
+ O
2
→ H
2
O
1
–
2
H
2
→ 2H
+
+ 2e
–
O
2
+ 2H
+
+ 2e
–
→
H
2
O
1
–
2
(b)(a)
2e
–
Figure 13.3 Fuel cell power systems. (a) 25-kW solid oxide fuel cell module. (b) Schematic of
a proton exchange membrane fuel cell.
13.4 Fuel Cells 647
stakes are high in the
consumer electronics
market.
Like the fuel cells
being developed to
power cars and gen-
erate electricity for
homes and offices, the
pocket-size versions
face stiff challenges
on the way to market.
They rely on costly
precious-metal cata-
lysts to operate and are difficult to manufacture. They run on
combustible fuels, typically hydrogen or methanol, and this
brings concerns over safety. Still, many think fuel cells for
portable electronics will be the first fuel cells most of us will
see because of strong consumer demand for cost-competitive,
longer lasting, instantly rechargeable power.
Goodbye Batteries, Hello Fuel Cells?
Thermodynamics in the News...
Power needs of cellular phones, laptops, and other portable
electronic devices are increasing so rapidly that the battery in-
dustry is struggling to keep up. Some observers say that
today’s batteries won’t be able to provide enough power, are
too heavy, and don’t last long enough to meet the needs of
quickly evolving electronics. Pocket-size fuel cells might prove
to be a viable alternative.
To meet consumer needs, companies are rushing to develop
small fuel cells that promise to provide power up to 10 times
longer on a single charge than conventional batteries. These cells
can be charged instantly just by adding more fuel. Battery com-
panies are fighting back with a new generation of batteries,
known as lithium ion batteries, already used in watches, flash
cameras, and rechargeable power packs. Lithium ion batteries
provide several times the output of similar-size alkaline batter-
ies and can be recharged numerous times. To compete, fuel cells
must prove themselves as reliable and versatile as batteries, and
can be produced at the point of use. Internal reforming refers to applications where hydrogen
production is integrated with the fuel cell. When hydrogen is produced separately from the
fuel cell itself, this is known as external reforming. Owing to thermal limitations of current
technology, internal reforming is feasible only in higher-temperature molten carbonate and
solid oxide fuel cells.
Table 13.1 summarizes the most promising fuel cell technologies currently under investi-
gation. Included are potential applications and other characteristics. For more detailed
discussions, see the sources listed in Table 13.1.
TABLE 13.1 Characteristics of Major Fuel Cell Types
Proton Phosphoric Acid Molten Solid Oxide Fuel
Exchange Fuel Cell (PAFC) Carbonate Fuel Cell (SOFC)
Membrane Fuel Cell (MCFC)
Cell (PEMFC)
Transportation - automotive power - large vehicle power - vehicle auxiliary
application power
- heavy vehicle
propulsion
Other applications - portable power - on-site cogeneration - on-site cogeneration - on-site cogeneration
- small-scale - electric power - electric power - electric power
stationary power generation generation generation
Electrolyte ion exchange membrane liquid phosphoric acid liquid molten carbonate solid oxide ceramic
Charge carrier H
H
CO
3
O
Operating 60–100C 150–220C 600–700C 800–1000C
temperature
Fuel reforming external external internal or external internal or external
Source: Appleby, A. J., and Foulkes, F. R., 1993, Fuel Cell Handbook, Krieger Publishing Company, Malabar, Florida. Hirschenhofer, J. H.,
Stauffer, D. B., Engleman, R. R., and Klett, M. G., 1998, Fuel Cell Handbook, Fourth Edition, DOE/FETC-99/1076.

648 Chapter 13 Reacting Mixtures and Combustion
Thus far our analyses of reacting systems have been conducted using the conservation of
mass and conservation of energy principles. In the present section some of the implications
of the second law of thermodynamics for reacting systems are considered. The discussion
continues in the second part of this chapter dealing with the exergy concept, and in the next
chapter where the subject of chemical equilibrium is taken up.
13.5.1 Evaluating Entropy for Reacting Systems
The property entropy plays an important part in quantitative evaluations using the second law
of thermodynamics. When reacting systems are under consideration, the same problem arises
for entropy as for enthalpy and internal energy: A common datum must be used to assign
entropy values for each substance involved in the reaction. This is accomplished using the
third law of thermodynamics and the absolute entropy concept.
The third law deals with the entropy of substances at the absolute zero of temperature. Based
on empirical evidence, this law states that the entropy of a pure crystalline substance is zero at
the absolute zero of temperature, 0 K. Substances not having a pure crystalline structure at
absolute zero have a nonzero value of entropy at absolute zero. The experimental evidence
on which the third law is based is obtained primarily from studies of chemical reactions at low
temperatures and specific heat measurements at temperatures approaching absolute zero.
ABSOLUTE ENTROPY. For present considerations, the importance of the third law is that it
provides a datum relative to which the entropy of each substance participating in a reaction can
be evaluated so that no ambiguities or conflicts arise. The entropy relative to this datum is called
the absolute entropy. The change in entropy of a substance between absolute zero and any
given state can be determined from precise measurements of energy transfers and specific heat
data or from procedures based on statistical thermodynamics and observed molecular data.
Table A-25 give the value of the absolute entropy for selected substances at the
standard reference state, T
ref
298.15 K, p
ref
1 atm. Two values of absolute entropy for
water are provided. One is for liquid water and the other is for water vapor. As for the
case of the enthalpy of formation of water considered previously, the vapor value listed
is for a hypothetical ideal gas state in which water is a vapor at 25C and 1 atm.
Tables A-22 and A-23 give tabulations of absolute entropy versus temperature at a pressure
of 1 atm for selected gases. The absolute entropy at 1 atm and temperature T is designated
as s(T) or depending on whether the value is on a unit mass or per mole basis. In
all these tables, ideal gas behavior is assumed for the gases.
When the absolute entropy is known at the standard state, the specific entropy at any other
state can be found by adding the specific entropy change between the two states to the absolute
entropy at the standard state. Similarly, when the absolute entropy is known at the pressure
p
ref
and temperature T, the absolute entropy at the same temperature and any pressure p can
be found from
The second term on the right side of this equation can be evaluated for an ideal gas by
using Eq. 6.21b, giving
(13.22)
where is the absolute entropy at temperature T and pressure p
ref
.s
°(T)
s
1T, p2 s °1T 2 R ln
p
p
ref
1ideal gas2
s 1T, p2 s 1T, p
ref
2 3s 1T, p2 s 1T, p
ref
24
s
°(T),
13.5 Absolute Entropy and the
Third Law of Thermodynamics
third law of
thermodynamics
absolute entropy
13.5 Absolute Entropy and the Third Law of Thermodynamics 649
The entropy of the ith component of an ideal gas mixture is evaluated at the mixture tem-
perature T and the partial pressure The partial pressure is given by p
i
y
i
p,
where y
i
is the mole fraction of component i and p is the mixture pressure. Thus, Eq. 13.22
takes the form
or
(13.23)
where is the absolute entropy of component i at temperature T and p
ref
.
13.5.2 Entropy Balances for Reacting Systems
Many of the considerations that enter when energy balances are written for reacting systems
also apply to entropy balances. The writing of entropy balances for reacting systems will be
illustrated by referring to special cases of broad interest.
CONTROL VOLUMES AT STEADY STATE. Let us begin by reconsidering the steady-state re-
actor of Fig. 13.2, for which the combustion reaction is given by Eq. 13.11. The combustion air
and the products of combustion are each assumed to form ideal gas mixtures. The entropy rate
balance for the two-inlet, single-exit reactor can be expressed on a per mole of fuel basis as
(13.24)
where is the molar flow rate of the fuel and the coefficients appearing in the underlined
terms are the same as those for the corresponding substances in the reaction equation.
The specific entropies of Eq. 13.24 are absolute entropies. Let us consider how the en-
tropies are evaluated for the combustion products and the combustion air. The entropies of
the combustion products would be evaluated from Eq. 13.23, using the temperature, pres-
sure, and composition of the products. The entropies of the entering oxygen and nitrogen
would be evaluated similarly, using the temperature, pressure, and composition of the com-
bustion air. If the fuel and air entered the reactor as an ideal gas mixture, the entropies of
the mixture components would be evaluated from Eq. 13.23 using the appropriate partial
pressures. Such considerations are illustrated in Example 13.9.
n
#
F
cas
CO
2
b
2
s
H
2
O
aa
b
4
b 3.76 s
N
2
d
s
#
cv
n
#
F
0
a
j
Q
#
j
T
j
n
#
F
s
F
caa
b
4
b s
O
2
aa
b
4
b 3.76 s
N
2
d
s°
i
(T )
s
i
1T, p
i
2 s
i
°1T 2 R ln
y
i
p
p
ref
a
component i of an
ideal gas mixture
b
s
i
1T, p
i
2 s°
i
1T 2 R ln
p
i
p
ref
p
i
: s
i
(T, p
i
).
EXAMPLE 13.9 Evaluating Entropy Production for a Reactor
Liquid octane at 25C, 1 atm enters a well-insulated reactor and reacts with air entering at the same temperature and pressure.
The products of combustion exit at 1 atm pressure. For steady-state operation and negligible effects of kinetic and potential
energy, determine the rate of entropy production, in kJ/K per kmol of fuel, for complete combustion with (a) the theoretical
amount of air, (b) 400% theoretical air.
SOLUTION
Known: Liquid octane and air, each at 25C and 1 atm, burn completely within a well-insulated reactor operating at steady
state. The products of combustion exit at 1 atm pressure.
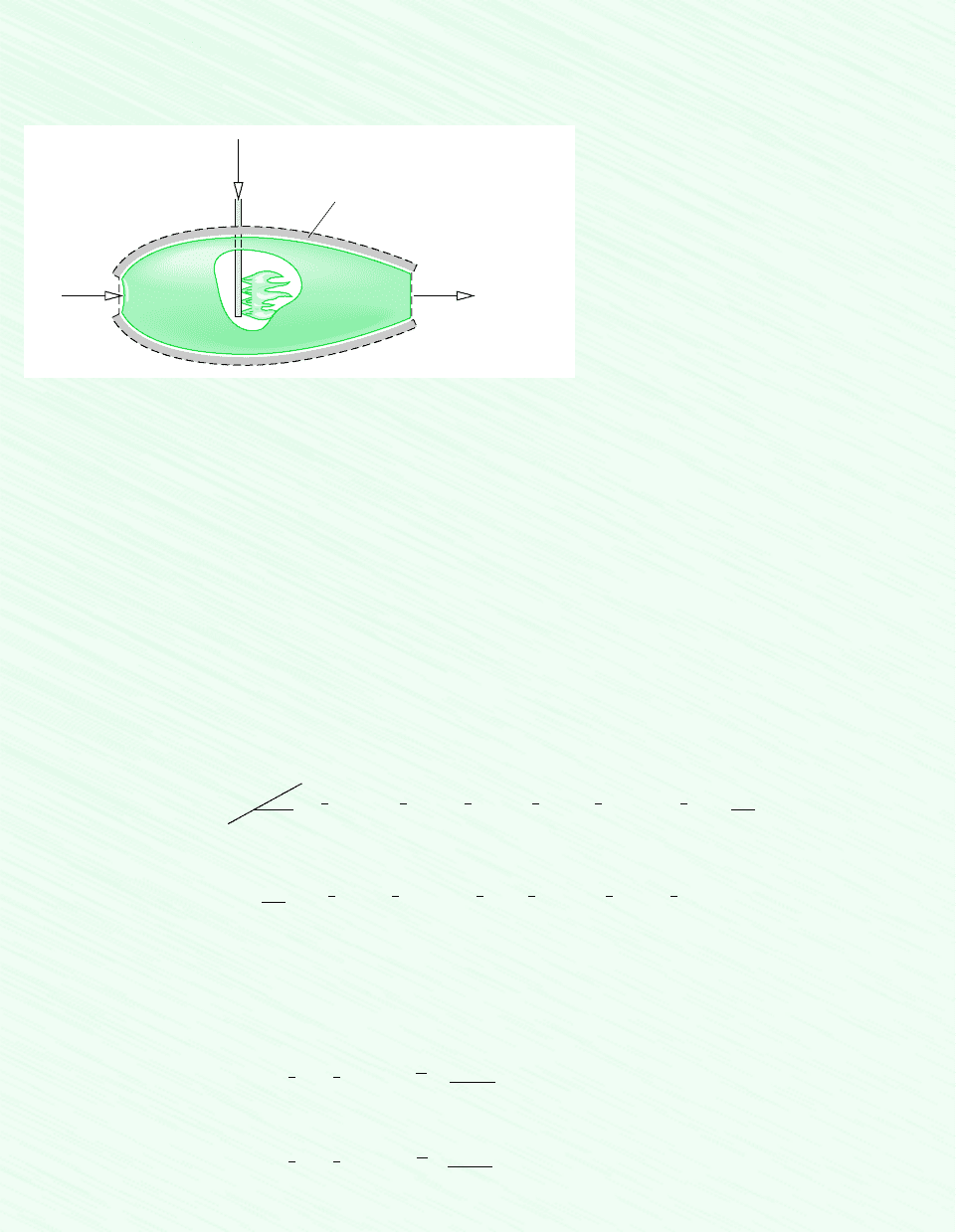
650 Chapter 13 Reacting Mixtures and Combustion
Find: Determine the rate of entropy production, in kJ/K per kmol of fuel, for combustion with (a) the theoretical amount of
air, (b) 400% theoretical air.
Schematic and Given Data:
Air
25°C, 1 atm
Combustion products,
1 atm
C
8
H
18
(l)
25°C, 1 atm
T
P
= 2395 K (part a)
T
P
= 962 K (part b)
Insulation
Figure E13.9
Assumptions:
1. The control volume shown on the accompanying figure by a dashed line operates at steady state and without heat transfer
with its surroundings.
2. Combustion is complete. Each mole of oxygen in the combustion air is accompanied by 3.76 moles of nitrogen, which is
inert.
3. The combustion air can be modeled as an ideal gas mixture, as can the products of combustion.
4. The reactants enter at 25C, 1 atm. The products exit at a pressure of 1 atm.
Analysis: The temperature of the exiting products of combustion T
P
was evaluated in Example 13.8 for each of the two cases.
For combustion with the theoretical amount of air, T
P
2395 K. For complete combustion with 400% theoretical air, T
P
962 K.
(a) For combustion of liquid octane with the theoretical amount of air, the chemical equation is
With assumptions 1 and 3, the entropy rate balance on a per mole of fuel basis, Eq. 13.24, takes the form
or on rearrangement
(1)
Each coefficient of this equation is the same as for the corresponding term of the balanced chemical equation.
The fuel enters the reactor separately at T
ref
, p
ref
. The absolute entropy of liquid octane required by the entropy balance is
obtained from Table A-25 as 360.79 kJ/kmol K.
The oxygen and nitrogen in the combustion air enter the reactor as components of an ideal gas mixture at T
ref
, p
ref
. With
Eq. 13.23 and absolute entropy data from Table A-23
191.5 8.314 ln 0.79 193.46 kJ/kmol
#
K
s
N
2
s °
N
2
1T
ref
2 R ln
y
N
2
p
ref
p
ref
205.03 8.314 ln 0.21 218.01 kJ/kmol
#
K
s
O
2
s °
O
2
1T
ref
2 R ln
y
O
2
p
ref
p
ref
#
s
#
cv
n
#
F
18 s
CO
2
9 s
H
2
O1g2
47 s
N
2
2 s
F
112.5 s
O
2
47 s
N
2
2
0
a
j
Q
#
j
T
j
n
#
F
0
s
F
112.5 s
O
2
47 s
N
2
2 18 s
CO
2
9 s
H
2
O1g2
47 s
N
2
2
s
#
cv
n
#
F
C
8
H
18
1l2 12.5O
2
47N
2
S 8CO
2
9H
2
O1g2 47N
2

13.5 Absolute Entropy and the Third Law of Thermodynamics 651
The product gas exits as an ideal gas mixture at 1 atm, 2395 K with the following composition:
With Eq. 13.23 and absolute entropy data at 2395 K from Tables A-23
Inserting values into Eq.1, the expression for the rate of entropy production
Alternative Solution:
As an alternative, the following IT code can be used to determine the entropy production per mole of fuel entering, where
sigma denotes , and sN2_R and sN2_P denote the entropy of N
2
in the reactants and products, respectively, and so
on. In the Units menu, select temperature in K, pressure in bar, and amount of substance in moles.
TR = 25 + 273.15 // K
p = 1.01325 // bar
TP = 2394 // K (Value from the IT alternative solution to Example 13.8)
// Determine the partial pressures
pO2_R = 0.21 * p
pN2_R = 0.79 * p
pCO2_P = (8/64) * p
pH2O_P = (9/64) * p
pN2_P = (47/64) * p
// Evaluate the absolute entropies
sC8H18 = 360.79 // kJ/kmol K (from Table A-25)
sO2_R = s_TP(“O2’’, TR, pO2_R)
sN2_R = s_TP(“N2’’, TR, pN2_R)
sCO2_P = s_TP(“CO2’’, TP, pCO2_P)
sH2O_P = s_TP(“H2O’’, TP, pH2O_P)
sN2_P = s_TP(“N2’’, TP, pN2_P)
// Evaluate the reactant and product entropies, sR and sP, respectively
sR = sC8H18 + 12.5 * sO2_R + 47 * sN2_R
sP = 8 * sCO2_P + 9 * sH2O_P + 47 * sN2_P
// Entropy balance, Eq. (1)
sigma = sP – sR
Using the Solve button, the result is sigma = 5404 kJ/kmol (octane) K, which agrees with the result obtained above.
(b) The complete combustion of liquid octane with 400% theoretical air is described by the following chemical equation:
The entropy rate balance on a per mole of fuel basis takes the form
s
#
cv
n
#
F
18 s
CO
2
9 s
H
2
O 1g2
37.5 s
O
2
188 s
N
2
2 s
F
150 s
O
2
188 s
N
2
2
C
8
H
18
1l2 50O
2
188N
2
S 8CO
2
9H
2
O1g2 37.5O
2
188N
2
#
s
#
cv
n
#
F
5404 kJ/kmol 1octane2
#
K
360.79 12.51218.012 471193.462
s
#
cv
n
#
F
81337.462 91290.302 471261.072
s
N
2
258.503 8.314 ln 0.7344 261.07 kJ/kmol
#
K
s
H
2
O
273.986 8.314 ln 0.1406 290.30 kJ/kmol
#
K
320.173 8.314 ln
0.125 337.46 kJ/kmol
#
K
s
CO
2
s °
CO
2
R ln y
CO
2
y
H
2
O 1g2
9
64 0.1406, y
N
2
47
64 0.7344.
y
CO
2
8
64 0.125,
❶

652 Chapter 13 Reacting Mixtures and Combustion
The specific entropies of the reactants have the same values as in part (a). The product gas exits as an ideal gas mixture at 1 atm,
962 K with the following composition:
With the same approach as in part (a)
Inserting values into the expression for the rate of entropy production
The use of IT to solve part (b) is left as an exercise.
For several gases modeled as ideal gases, IT directly returns the absolute entropies required by entropy balances for re-
acting systems. The entropy data obtained from IT agree with values calculated from Eq. 13.23 using table data.
Comparing the results of parts (a) and (b), note that once enough oxygen has been provided for complete combustion,
mixing a greater amount of air with the fuel prior to combustion results in a lower product gas temperature and a greater
rate of entropy production.
Although the rates of entropy production calculated in this example are positive, as required by the second law, this does
not mean that the proposed reactions necessarily would occur, for the results are based on the assumption of complete
combustion. The possibility of achieving complete combustion with specified reactants at a given temperature and pres-
sure can be investigated with the methods of Chap. 14, dealing with chemical equilibrium. For further discussion, see
Sec. 14.4.1.
9754 kJ/kmol 1octane2
#
K
360.79 501218.012 1881193.462
s
#
cv
n
#
F
81295.4812 91258.3972 37.51257.6422 1881228.9112
s
N
2
226.795 8.314 ln 0.7753 228.911 kJ/kmol
#
K
s
O
2
242.12 8.314 ln 0.1546 257.642 kJ/kmol
#
K
s
H
2
O
231.01 8.314 ln 0.0371 258.397 kJ/kmol
#
K
s
CO
2
267.12 8.314 ln 0.033 295.481 kJ/kmol
#
K
y
N
2
0.7753.
y
O
2
37.5
242.5 0.1546,y
H
2
O1g2
9
242.5 0.0371,y
CO
2
8
242.5 0.033,
❶
❷
❸
❷
❸
CLOSED SYSTEMS. Next consider an entropy balance for a process of a closed system dur-
ing which a chemical reaction occurs
(13.25)
S
R
and S
P
denote, respectively, the entropy of the reactants and the entropy of the products.
When the reactants and products form ideal gas mixtures, the entropy balance can be ex-
pressed on a per mole of fuel basis as
(13.26)
where the coefficients n on the left are the coefficients of the reaction equation giving the
moles of each reactant or product per mole of fuel. The entropy terms would be evalu-
ated from Eq. 13.23 using the temperature, pressure, and composition of the reactants or
products, as appropriate. The fuel would be mixed with the oxidizer, so this must be taken
into account when determining the partial pressures of the reactants. Example 13.10
provides an illustration of the evaluation of entropy change for combustion at constant
volume.
a
P
ns
a
R
ns
1
n
F
a
dQ
T
b
b
s
n
F
S
P
S
R
a
dQ
T
b
b
s
13.5 Absolute Entropy and the Third Law of Thermodynamics 653
EXAMPLE 13.10 Entropy Change for Combustion at Constant Volume
Determine the change in entropy of the system of Example 13.6 in kJ/K.
SOLUTION
Known: A mixture of gaseous methane and oxygen, initially at 25C and 1 atm, burns completely within a closed rigid con-
tainer. The products are cooled to 900 K, 3.02 atm.
Find: Determine the change in entropy for the process in kJ/K.
Schematic and Given Data: See Fig. E13.6.
Assumptions:
1. The contents of the container are taken as the system.
2. The initial mixture can be modeled as an ideal gas mixture, as can the products of combustion.
3. Combustion is complete.
Analysis: The chemical equation for the complete combustion of methane with oxygen is
The change in entropy for the process of the closed system is S S
P
S
R
, where S
R
and S
P
denote, respectively, the
initial and final entropies of the system. Since the initial mixture forms an ideal gas mixture (assumption 2), the entropy of
the reactants can be expressed as the sum of the contributions of the components, each evaluated at the mixture temperature
and the partial pressure of the component. That is
where and denote, respectively, the mole fractions of the methane and oxygen in the initial mixture. Sim-
ilarly, since the products of combustion form an ideal gas mixture (assumption 2)
where and denote, respectively, the mole fractions of the carbon dioxide and water vapor in the prod-
ucts of combustion. In these equations, p
1
and p
2
denote the pressure at the initial and final states, respectively.
The specific entropies required to determine S
R
can be calculated from Eq. 13.23. Since T
1
T
ref
and p
1
p
ref
, absolute
entropy data from Table A-25 can be used as follows
Similarly
At the final state, the products are at T
2
900 K and p
2
3.02 atm. With Eq. 13.23 and absolute entropy data from
Tables A-23
228.321 8.314 ln
12
32 13.022
1
222.503 kJ/kmol
#
K
s
H
2
O
1T
2
, y
H
2
O
p
2
2 s°
H
2
O
1T
2
2 R ln
y
H
2
O
p
2
p
ref
263.559 8.314 ln
11
32 13.022
1
263.504 kJ/kmol
#
K
s
CO
2
1T
2
, y
CO
2
p
2
2 s°
CO
2
1T
2
2 R ln
y
CO
2
p
2
p
ref
205.03 8.314 ln
2
3
208.401 kJ/kmol
#
K
s
O
2
1T
1
, y
O
2
p
1
2 s°
O
2
1T
ref
2 R ln
y
O
2
p
ref
p
ref
186.16 8.314 ln
1
3
195.294 kJ/kmol
#
K
s
CH
4
1T
1
, y
CH
4
p
1
2 s°
CH
4
1T
ref
2 R ln
y
CH
4
p
ref
p
ref
y
H
2
O
2
3y
CO
2
1
3
S
p
1 s
CO
2
1T
2
, y
CO
2
p
2
2 2 s
H
2
O
1T
2
, y
H
2
O
p
2
2
y
O
2
2
3y
CH
4
1
3
S
R
1 s
CH
4
1T
1
, y
CH
4
p
1
2 2 s
O
2
1T
1
, y
O
2
p
1
2
CH
4
2O
2
S CO
2
2H
2
O

654 Chapter 13 Reacting Mixtures and Combustion
Finally, the entropy change for the process is
96.414 kJ/K
3263.504 21222.50324 3195.294 21208.40124
¢S S
P
S
R
13.5.3 Evaluating Gibbs Function for Reacting Systems
The thermodynamic property known as the Gibbs function plays a role in the second part of this
chapter dealing with exergy analysis. The specific Gibbs function introduced in Sec. 11.3, is
(13.27)
The procedure followed in setting a datum for the Gibbs function closely parallels that used
in defining the enthalpy of formation: To each stable element at the standard state is assigned
a zero value of the Gibbs function. The Gibbs function of formation of a compound equals
the change in the Gibbs function for the reaction in which the compound is formed from its
elements, the compound and the elements all being at T
ref
and p
ref
. Table A-25 gives the Gibbs
function of formation, at 25C and 1 atm for selected substances.
The Gibbs function at a state other than the standard state is found by adding to the Gibbs
function of formation the change in the specific Gibbs function between the standard
state and the state of interest
(13.28)
With Eq. 13.27, can be written as
The Gibbs function of component i in an ideal gas mixture is evaluated at the partial pres-
sure of component i and the mixture temperature.
The procedure for determining the Gibbs function of formation is illustrated in the next
example.
¢g
3h1T, p2 h1T
ref
, p
ref
24 3T s 1T, p2 T
ref
s 1T
ref
, p
ref
24
¢g
g1T, p2 g °
f
3g1T, p2 g1T
ref
, p
ref
24 g °
f
¢g
¢g
g
°
f
,
g h T s
g,
Gibbs function of
formation
EXAMPLE 13.11 Determining the Gibbs Function of Formation
Determine the Gibbs function of formation of methane at the standard state, 25C and 1 atm, in kJ/kmol, and compare with
the value given in Table A-25.
SOLUTION
Known: The compound is methane.
Find: Determine the Gibbs function of formation at the standard state, in kJ/kmol, and compare with the Table A-25 value.
Assumptions: In the formation of methane from carbon and hydrogen (H
2
), the carbon and hydrogen are each initially at
25C and 1 atm. The methane formed is also at 25C and 1 atm.
Analysis: Methane is formed from carbon and hydrogen according to The change in the Gibbs function
for this reaction is
where and denote, respectively, the Gibbs functions of the reactants and products, each per kmol of methane.g
R
g
P
1h
CH
4
h
C
2h
H
2
2 T 1s
CH
4
s
C
2s
H
2
2
g
P
g
R
1h T s2
CH
4
1h T s2
C
21h T s2
H
2
C 2H
2
S CH
4
.
13.6 Introducing Chemical Exergy 655
In the present case, all substances are at the same temperature and pressure, 25C and 1 atm, which correspond to the stan-
dard reference state values. At the standard reference state, the enthalpies and Gibbs functions for carbon and hydrogen are
zero by definition. Thus, in the above equation Also, giving
where the superscript denotes properties at T
ref
, p
ref
. With enthalpy of formation and absolute entropy data from Table A-25
The slight difference between the calculated value for the Gibbs function of formation of methane and the value from Table A-25
can be attributed to round-off.
1g°
f
2
CH
4
74,850 298.153186.16 5.74 21130.572450,783 kJ/kmol
1g°
f
2
CH
4
1h°
f
2
CH
4
T
ref
1s°
CH
4
s°
C
2 s°
H
2
2
g
P
1g°
f
2
CH
4
,g
R
h
C
h
H
2
0.
CHEMICAL EXERGY
1
The objective of this part of the chapter is to extend the exergy concept introduced in Chap. 7
by considering the role of chemical composition. To distinguish the current considerations
from those introduced previously, let the system be a specified amount of a hydrocarbon fuel
C
a
H
b
at temperature T
0
and pressure p
0
and let the exergy reference environment consist of
a gas phase at T
0
, p
0
involving nitrogen, oxygen, water vapor, and carbon dioxide. Since the
system is in thermal and mechanical equilibrium with the environment, the value of exergy as
defined in Sec. 7.2 would be zero. More precisely, we should say that the thermomechanical
contribution to the exergy magnitude has a value of zero, for a chemical contribution related
to composition can be defined that has a nonzero value. This aspect of the exergy concept is
the subject of the present section.
13.6 Introducing Chemical Exergy
Exergy is introduced in Chap. 7 through study of a combined system consisting of a system
of interest and an exergy reference environment. The object of the development of Sec. 7.2.3
is an expression for the maximum theoretical work obtainable from the combined system
as the system comes into thermal and mechanical equilibrium with the environment. The
thermomechanical exergy is this value for work. We begin the present section by studying
a combined system formed by an environment and an amount of a hydrocarbon fuel at T
0
,
p
0
. The object is to evaluate the work obtainable by allowing the fuel to react with oxygen
from the environment to produce the environmental components carbon dioxide and water,
each at its respective state in the environment. The chemical exergy is, by definition, the
maximum theoretical work that could be developed by the combined system. The sum of the
thermomechanical and chemical exergies is the total exergy associated with a given system
at a specified state, relative to a specified exergy reference environment.
CHEMICAL EXERGY OF A HYDROCARBON: C
a
H
b
Consider a combined system formed by an environment and an amount of a hydrocarbon
fuel, C
a
H
b
. To help us visualize how work might be obtained through the reaction of the fuel
with environmental components, a fuel cell operating at steady state is included in the
combined system, as shown in Fig. 13.4. Referring to this figure, fuel enters the cell at tem-
perature T
0
and pressure p
0
. At another location, oxygen enters from the environment.
1
Study of Chap. 7 is a prerequisite for this part of the chapter.
thermomechanical
exergy
chemical exergy
